#CHEMISTRY
Text








Photo dump! My roommate and I tried out a new cafe in town, finals are coming up, final chem lab for the year had been done, etc. Man I am tired
#college#student#chemistry#studyblr#biology#biochemistry#study aesthetic#study desk#study motivation#romanticizing school#romanticizing stem#romanticizing science#romanticizing college#stem academia#stem#stem aesthetic#stem studyblr#stemblr#stem student#study blog#studyspo#studying#finals#romanticizing finals
20 notes
·
View notes
Text
LAST Day Till Med School Entries
Heyaaa, just here for a quick update.
Last 2 days were not so productive, even tho I studied for about 6h a day. My mind was all over the place, I couldn't concentrate at all and that's pretty much it.
Today's plan is to just chill and maybe revise the things I'm most unsure of.
I hope for the best tomorrow and I know that God has a plan! 😇

P.S (off topic rant) Am I the only one who's getting s3xuall stuff on my fy page? Wtf Tumblr 💀
Even tho I've blocked that kind of posts they still come up occasionally. Why is this even allowed on here 😭
#studyblr#studying#study blog#study motivation#notes#study aesthetic#chemistry#biology#grades#organic chemistry
14 notes
·
View notes
Text
look, I know I've talked about this essay (?) before but like,
If you ever needed a good demonstration of the quote "Any sufficiently advanced technology is indistinguishable from magic", have I got an exercise for you.
Somebody made a small article explaining the basics of atomic theory but it's written in Anglish. Anglish is basically a made-up version of English where they remove any elements (words, prefixes, etc) that were originally borrowed from romance languages like french and latin, as well as greek and other foreign loanwords, keeping only those of germanic origin.
What happens is an english which is for the most part intelligible, but since a lot everyday english, and especially the scientific vocabulary, has has heavy latin and greek influence, they have to make up new words from the existing germanic-english vocabulary. For me it kind of reads super viking-ey.
Anyway when you read this article on atomic theory, in Anglish called Uncleftish Beholding, you get this text which kind of reads like a fantasy novel. Like in my mind it feels like it recontextualizes advanced scientific concepts to explain it to a viking audience from ancient times.
Even though you're familiar with the scientific ideas, because it bypasses the normal language we use for these concepts, you get a chance to examine these ideas as if you were a visitor from another civilization - and guess what, it does feel like it's about magic. It has a mythical quality to it, like it feels like a book about magic written during viking times. For me this has the same vibe as reading deep magic lore from a Robert Jordan book.
#off topic#literature#language#linguistics#science#science history#science fiction#fantasy#physics#atomic theory#anglish#chemistry#robert jordan#the wheel of time#uncleftish beholding
42K notes
·
View notes
Text
Cesium-133, let it be. Cesium-134, let it be even more.
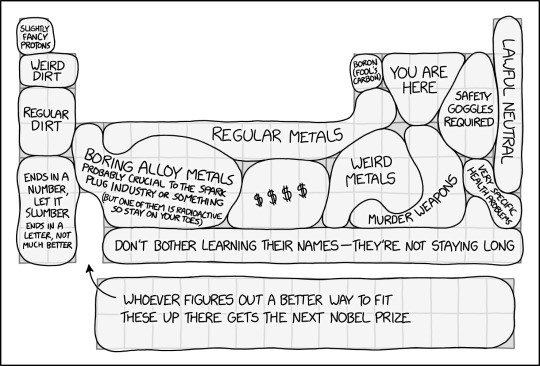
Periodic Table Regions [Explained]
Transcript
[A periodic table with regions labeled.]
[Hydrogen:] Slightly fancy protons
[Lithium and Beryllium:] Weird dirt
[Group 1 & 2 metals, Periods 3-4:] Regular dirt
[Group 1 & 2 metals, Periods 5-7:] Ends in a number, let it slumber ends in a letter, not much better
[Left side of the transition metals group:] Boring alloy metals Probably critical to the spark plug industry or something (but one of them is radioactive so stay on your toes)
[Most of the top row of the transition metals + aluminum:] Regular metals
[Below the rightmost "regular metals" - the "ordinary metals" and some transition metals:] Weird metals
[The platinum group:] $$$$
[Boron:] Boron (fool's carbon)
[Carbon, Nitrogen, Oxygen, and Phosphorus:] You are here
[The Halogens:] Safety goggles required
[Noble Gases:] Lawful neutral
[Iodine and Radon:] Very specific health problems
[Ordinary metals and metalloids - Arsenic, Antimony, Tellurium, Thallium, Lead, Bismuth, Polonium] Murder weapons
[Astatine and Period 7 from Rutherfordium onwards:] Don't bother learning their names - they're not staying long
[Lanthanides and Actinides:] Whoever figures out a better way to fit these up there gets the next Nobel Prize
7K notes
·
View notes
Text
#i have full exam season next week and coming up with the names gave me some extra fun#personally i’m more of a#chemistry#person myself#(though my recent grades are working hard to prove me wrong)#anyways#how does one tag again?#poll#!!#science#stem#science side of tumblr#never mind#nadirants
9K notes
·
View notes
Text
With the fast fashion industry… how it is… finding sustainable ways to make fabric is super important. Fibers from synthetic fabrics make up 35% of the microplastics that make their way to the ocean. Natural fibers sourced from plants or animals are much more environmentally sound options, including silk.
Currently, the only way to get natural silk on a large scale is to harvest it from silkworms. You’ve probably heard about the strength and durability of spider silk (it is 6x stronger than Kevlar!) but as of yet there hasn’t been a good way of getting it. Raising spiders the way people do silkworms isn’t really an option. Spiders need a lot of room to build their webs compared to silkworms, and individual spiders don’t produce that much silk. Plus, when you put a whole bunch of spiders in captivity together, they tend to start eating each other.
Attempts to artificially recreate spider silk have also been less than successful. Spider silk has a surface layer of glycoproteins and lipids on it that works as a sort of anti-aging “skin”- allowing the silk to withstand conditions such as sunlight and humidity. But this layer has been very tricky to reproduce.
However, as scientists in China realized, silkworms produce that same kind of layer on their silk. So what if we just genetically modified silkworms to produce spider silk?
That is exactly what the researchers at Donghua University in Shanghai did. A team of researchers introduced spider silk protein genes to silkworms using CRISPR-Cas9 gene editing and microinjections in silkworm eggs. In addition to this, they altered the spider silk proteins so that they would interact properly with the other proteins in silkworm glands. And it worked! This is the first study ever to produce full length spider silk proteins from silkworms.
The applications of this are incredibly exciting. In addition to producing comfortable textiles and new, innovative bulletproof vests, silkworm generated spider silk could be used in cutting edge smart materials or even just to create better performing sutures. In the future, this team intends to research how to modify this new spider silk to be even stronger, and they are confident that “large-scale commercialization is on the horizon."
#science#chemistry#biology#sustainability#fashion#bugs#spiders#silkworms#nature#biochemistry#stemblr#genetics
4K notes
·
View notes
Text
Paleontologist: I became a paleontologist because dinosaurs are cool
Astronomer: I became an astronomer because space is cool
Chemist: I became a chemist because explosions are cool
Archeologist: I became an archeologist because Indiana Jones is cool
Mycologist: I. Fucking. LOVE. Mushrooms.
Paleontologist: Uh…
Mycologist: IWillLiterallyMurderYouJustSoICanWatchFungiBreakDownYourDecayingRemainsDon’tTestMeBoneBoy
#Science#Mycology#Chemistry#biology#Paleontology#scientists sitcom#scientists specializing in their obscure special interest is my favorite gender#I am one of those#Astronomy#Archeology
4K notes
·
View notes
Text
The Chemical Structure of Redstone
So I was curious about what the chemical structure of Redstone looks like, and Minecraft Education Edition, albeit unintentionally, gives us a canon look into what Redstone is made of:

In Minecraft Education Edition, putting a Redstone Block into a Material Reducer shows that it's composed of 31 Carbon, 31 Uranium, and 38 Unobtanium, which we can assume to be measured in grams
Dividing the Redstone Block into Redstone Dust, each Redstone Dust is then composed of approximately 3.4 Carbon, 3.4 Uranium, and 4.2 Unobtanium
Again assuming that's measured in grams, that's 0.17 cm³ of Uranium, 1.496 cm³ of Carbon, and ???³ of Unobtanium per Redstone Dust
So what does this tell us about the chemical structure of Redstone? Basing this on Redstone Dust's composition, we can estimate that each Redstone molecule is composed of 3 Carbon atoms, 3 Uranium atoms, 4 Unobtanium atoms, a little under half of the time it binds to an extra Uranium and/or Carbon, and 20% of the time it binds to an extra Unobtanium
This also has some horrifying implications for how Redstone works:
Redstone would be extremely volatile as the radioactive decay from Unobtanium and Uranium would occasionally release Helium ions through alpha radiation, sometimes breaking apart Carbon into two Beryllium atoms (as it absorbs the extra proton and neutron from the Uranium) or merging into Oxygen
So Redstone should, in theory, be extremely flammable and potentially explosive, which implies that cave static, or the player mining Redstone with an Iron Pickaxe, could lead to a spark that causes an explosive cave-in
As Unobtanium is just a placeholder for unobtainable elements (hence the name), I'm going to estimate Unobtanium in this case as Unbinilium, the placeholder name for element 120
Why?
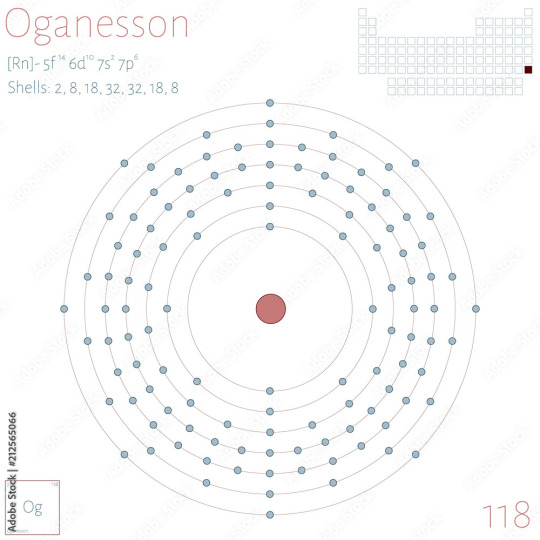
I'm estimating the Unobtanium as Redstone as being larger than the largest man-made element, Oganesson, which holds an impressive 118 protons
Each valence electron shell, from innermost to outermost, can bind with 2, 8, 18, 32, 32, 18, and 8 shells respectively, so I'd like Unobtanium to be an element we haven't discovered yet, and consequently I'd like to jump up to the next shell
While I could estimate with element 119's placeholder, Ununennium, it would have one electron in the next shell, so Unbinilium allows for easier chemical binding
So what does this molecule look like then? Well, horrifyingly...
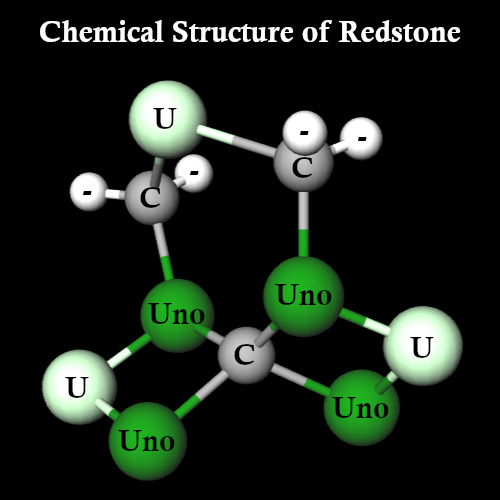
It looks like this. As Redstone forms in crystal lattices, and only two Carbon atoms are free to bind, I can absolutely see why it's so brittle that it breaks into powder.
This makes the structure of Redstone:
C3U3Uno4 (55% of molecules)
C4U3Uno4 (13% of molecules)
C3U4Uno4 (13% of molecules)
C4U4Uno4 (7% of molecules)
C3U3Uno5 (5% of molecules)
C4U3Uno5 (3% of molecules)
C3U4Uno5 (3% of molecules)
C4U4Uno5 (1% of molecules)
An extremely radioactive, flammable, and explosive compound.
#redstone#minecraft#chemistry#physics#education#i would love if someone could submit what a 2D representation of these molecules would look like#i don't actually know how to make them
3K notes
·
View notes
Text
The Victor Ninov situation is one of my favourite cases of scientific fraud because it's rare to see so straightforward an example of someone being brought low by their own hubris.
Like, okay, faking the synthesis of a previously unobserved element: it's one of the few varieties of scientific fraud that actually has a clear gameplan for getting away with it. The physical properties of unobserved elements are, in principle, predictable, and there are only so many ways to go about synthesising them. If you do your homework, it's not outside the realm of possibility that your claimed results will end up being at least mostly consistent with the results of subsequent legitimate efforts to synthesise that element, and any minor discrepancies will end up being dismissed as statistical anomalies and/or the product of sloppy experimental design. It's by no means an easy game to play, but it's a game you can conceivably win.
And Victor Ninov did it. He rolled the dice and he won – twice. His fabricated results for elements 110 and 112 were corroborated by later work, and nobody noticed that his actual data was a crock of shit. He got away with it as cleanly as he could have hoped. It was only the third time he tried it, with element 118, that he biffed it and claimed results which nobody could replicate, and this is the only reason his earlier frauds were discovered. If he'd quit while he was ahead, it's likely the first two incidents never would have come to light.
Like, they say the third time's the charm, and buddy here learned the hard way that sometimes, the opposite also holds true.
2K notes
·
View notes
Text








Happy Valentine's Day!! Have you ever looked at the periodic table and thought, "This would make a great dating game"?? No???
Take a [quiz] to find out which element you are, and see which elements you can bond with! I made a TON of illustrations for this Doodle. Let me know your otp.
#illustration#google doodle#valentines day#chemistry#periodic table#gif#let's go team iodine#id in alt text
2K notes
·
View notes
Text

the chemicals in the water are turning the frogs gay
#rule#196#r/196#shitpost#funny#memes#socialmedia#meme#funny memes#frog#frogs#chemistry#the chemicals the water are turning the frogs gay
2K notes
·
View notes
Text


january this january that
#studyblr#dark academia#math#mathblr#physics#study aesthetic#space#science#astronomy#astrophysics#student#telescope#light academia#physicsblr#chaotic academia#dark acadamia aesthetic#historyblr#literature#chemblr#chemistry#intp#intj#student life#winter#langblr#bookblr#study inspo#study inspiration
911 notes
·
View notes
Text
The Big Three
Geology: I think geology should be one of the big fields of science. It doesn’t really fit neatly as a branch of physics, chemistry, or biology since it draws on all three.
Physics: No. For one, everything is physics so that argument doesn't mean anything.
Biology: UnLeSs iT's StAmP CoLlEcTiNg, right?
Physics: *sighs*
Biology: Also we’re called ‘the big three’, if we added another then it’d be, like, more than three. Some mysterious higher number.
Chemistry: Besides, we’re the big fields because we’re the most important and popular.
Geology: Geology is important! We’re the ones who find all the oil to mine!
Biology: Yeah, could you stop doing that, actually? It’s kinda, y’know, causing the slow apocalypse as we speak. But at least it’s better than the fast nuclear apocalypse, physics.
Physics: Ummm… You know what? I’ve had enough of you stamp collectors with your… uh… collections of things! I'm not letting you join our club unless you can do math.
Biology: *Gasps* not math…
Geology: *hides rock collection behind back* Hey, I do… math. Sometimes.
Physics: Yeah? Like what?
Geology: Oh, y’know, just some geometry and a little geophysical fluid dynamics of the entire mantle, ocean, and atmosphere.
Physics: Oh, shit, I can't even do that. You’re in.
Chemistry: Alright, but don’t let the social sciences know we’re letting new people in or-
Anthropology: HEY GUYS
1K notes
·
View notes
Text
leaving a three hour lab be like:
what time is it? why is it dark outside? where did the sun go?
I'm starving.
I'm never doing that again (literally has the same lab scheduled the following week).
#women in stem#chemistry#organic chemistry#biology#microbiology#stem#college#premed#physics#prevet#science#lab
1K notes
·
View notes
Text








Some of my favorite of the bond illustrations, plus the full version of the completion reward illo, and some little concept sketches. Thanks everyone who played our Chemistry CuPd game! It's up in the [archive] if you ever want to check it out.
#illustration#valentines#google doodle#chemistry#periodic table#id in alt text#I'm so glad everyone enjoyed ArF and Br2
785 notes
·
View notes
