#inorganic chemistry
Text
Plastic waste breaks down over time into nanoplastics (<0.1 μm). Microplastics smaller than 20 μm cannot be removed in currently operating water treatment plants and must be agglomerated to a larger size and then removed. Iron (Fe) or aluminum (Al) based flocculants are used for this purpose, but they are not the ultimate solution as they remain in the water and cause severe toxicity to humans, requiring a separate treatment process.
Dr. Jae-Woo Choi of the Center for Water Cycle Research at the Korea Institute of Science and Technology (KIST) has developed an eco-friendly metal-organic skeleton-based solid flocculant that can effectively aggregate nanoplastics under visible light
irradiation. The research was published in Water Research.
Prussian blue, a metal-organic frameworks-based substance made by adding iron (III) chloride to a potassium ferrocyanide solution, is the first synthetic pigment used to dye jeans a deep blue color and has recently been used to adsorb cesium, a radioactive element, from Japanese nuclear plant wastewater.
Continue Reading.
192 notes
·
View notes
Text


today was my first inorganic chemistry lab of the semester :)
#chemblr#studying#studyspiration#undergraduate#stem major#chemistry#dark academia science#stem dark academia#laboratory#uni student#in the lab#student#studyblr#study motivation#studyspo#inorganic chemistry#inorganic#colourful chemistry
221 notes
·
View notes
Text

Chromyl chloride
#this one is so fucking cool thanks for the submission!!!#chromyl chloride#chemistry#ampoules#wikipedia#wikipedia pictures#inorganic chemistry#chemicals#submission
45 notes
·
View notes
Text
so in round one we figured out the most fuckable block (d), round 2 had the most fuckable group (11) . and now
ROUND THREE
to find out the most fuckable element of the periodic table
once again do reblog. last week didnt have that much engagement so plzz reblog uwu
#poll#polls#most fuckable element of the periodic table#periodic properties#periodic table#periodic table poll#je pense#block chemistry#chemblr#scienceblr#science side of tumblr#desiblr#science stuff#inorganic chemistry
74 notes
·
View notes
Text




More fun colors from lab plus a bonus electrochem hyperlapse.
15 notes
·
View notes
Text


Titration!
7 notes
·
View notes
Text
never did i think i’d be drawing lewis structures my junior year of college but here we are in inorganic chem
23 notes
·
View notes
Note
gosh if i could send an ask through an account you'd /maybe/ recognise i would. alas it is but a mere sideblog so anon will have to do
it's been like forever since i last read breaking bread (life got me in a pickle but fuck it we ball) and i went to check to see how long its been..... 135 days im so sorry rip lmao I'VE MISSED SIX CHAPTWRS T~T
bright side, I will be eating gud and i will try live comment to the best of my ability
I do have a question though! what's your favourite thing you've learned through your chemistry endeavors?
pspspssp love your work so much im beaming digital flowers your way
anon
a n o n
I am so, so fucking glad you're digging my little disaster so much, I love the digital flowers so much they smell like fresh plastic, and I'm so, so fucking glad you asked me this. Bc I *do* have a favorite thing I've learned.
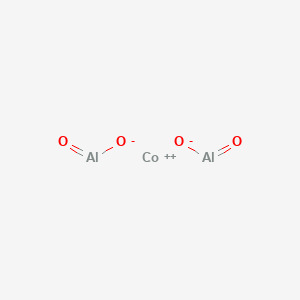
this is CoAl2O4, aka Cobalt (II) aluminate, or Cobalt aluminum oxide. Aka Cobalt Blue. At the macro level, it looks like this.



which is the closest material, physical, can-lay-your-actual-irl-hands-on color equivalent to bayverse Optimus's optics.
Which mean's there a chemical formula for the most beautiful thing ever created by the hands of man or god himself. Those six little bonds, 2 Als, 4 Os, and a Co++ are the first thing I and god-knows-how many others think about when they wake up and the last thing they think about when they fall asleep. Those itty bitty, nondescript little symbols are the minimalist extreme of proof of a loving creator. There is a chemical formula for love in inorganic form.
#I'll stop bleeding my heart w science all over tumblr now#but#goddamn dude#Optimus#Optimus prime#say what you will about Bayverse but Op's design is the closest most people will ever come to seeing an angel#tf#transformers#inorganic chemistry
12 notes
·
View notes
Text
Fluorides
of
transition metals
are
Unstable in low oxidation states.
#d and f block elements#chemistry#inorganic chemistry#pcm#pcb#jee#neet#cbse#cbse 2023#ncert#class 12#desi academia#desi studyblr#queue hai
50 notes
·
View notes
Text
Chemistry Notes (November 2021)
Arrhenius Equation Ex 1
Assigning Formal Charges Ex 2
Avogadro's Law Ex 1
Avogadro's Law Ex 4
Balancing Chemical Equations Ex 10
Calculating Percent by Mass Ex 4
CO2 VSEPR
Density and Crystal Structure Ex 1
Diagrams of Modes of Radioactive Decay
Dissolving NaCl in Water
Enthalpy of Reaction Ex 2
Equations for Alpha Decay Ex 1
H2O Polarity
Ideal Gas Law Ex 5
Mass of Product Ex 6
Moles of Product Ex 10
Niobium Pentafluoride
Parts of the Arrhenius Equation
Percent Yield Ex 1
pH from OH- Ex 4
Relationships of Equilibrium Constants Ex 1
Satisfying Octet Rule Ex 13
Solution Dilution Ex 5
Total Charge of Molecule Ex 3
#chemistry#studyblr#notes#masterlist#master list#studyblr masterlist#studyblr master list#studyblr resources#chem#chem notes#chemistry notes#chemistry masterlist#chem masterlist#inorganic chemistry#general chemistry#mcat#mcat chemistry#mcat resources#mcat masterlist#mcat chem#chemistry problems#chemistry examples
189 notes
·
View notes
Text
Dear p block,
Ever since i laid my eyes on you, i have been overcome with this new feeling of... fucking homicide.
Why the flying fuck are you the way you are p block?????
Sincerely, a hater
#Im back again with my burning hatred for that goddamned chapter#why. Is. It. Like. That.#p block#chemistry#inorganic chemistry#neet exam#neet 2024
2 notes
·
View notes
Text
For the first time, Stanford researchers have found a way to create and stabilize an extremely rare form of gold that has lost two negatively charged electrons, denoted Au2+. The material stabilizing this elusive version of the valued element is a halide perovskite—a class of crystalline materials that holds great promise for various applications including more-efficient solar cells, light sources, and electronics components.
Surprisingly, the Au2+ perovskite is also quick and simple to make using off-the-shelf ingredients at room temperature.
"It was a real surprise that we were able to synthesize a stable material containing Au2+—I didn't even believe it at first," said Hemamala Karunadasa, associate professor of chemistry at the Stanford School of Humanities and Sciences and senior author of the study published Aug. 28 in Nature Chemistry. "Creating this first-of-its-kind Au2+ perovskite is exciting. The gold atoms in the perovskite bear strong similarities to the copper atoms in high-temperature superconductors, and heavy atoms with unpaired electrons, like Au2+, show cool magnetic effects not seen in lighter atoms."
Continue Reading.
147 notes
·
View notes
Text


inorganic lab pics 🧪👩🏻🔬


#chemblr#studying#studyspiration#undergraduate#stem major#chemistry#studyblr#studyspo#laboratory#in the lab#student#uni student#study motivation#inorganic#inorganic chemistry
92 notes
·
View notes
Text
Hiyya!
Today's goals were too:
Go through my python course
Finish my chem homework
I decided to start small since I'm going through burn out right now (I sort of overworked myself but I'm trying to catch up at school haha)
I spent today just trying to catch up with my classes and attendance since I took a break yesterday.

Here's a pic of me asking bard ai to explain my chemistry homework because I got stuck at it
#desi studyblr#chemblr#chemistry#inorganic chemistry#online school#bard ai#a level studyblr#a levels#a level chemistry#burnt out#do your homework#homework#study inspo#studyblr#studying#new studyblr#codeblr#python#self study#codingblr#coding
7 notes
·
View notes
Text
4 notes
·
View notes
Text



Started assisting in an inorganic chemistry lab. There were some beautiful colors produced in the lab by the students. Here's a small sampling.
#science#science side of tumblr#sciblr#chemistry#inorganic chemistry#synthesis#teaching#postdoc life
9 notes
·
View notes