#Biotech and Pharmaceuticals
Text

These words will be very useful for you in writing especially in academic writing.
.
Sentence Starters
.
I help researchers increase engagement with their work. Together we will transform your ideas into unique and intuitive graphics with my reliable process:
1-Pinpoint your goals
2-Sketch and prototype
3-Discuss and iterate
4-Goal accomplished
.
𝐇𝐈𝐑𝐄 𝐌𝐄 (DM Your Design Projects)
.
💎Here to HELP You:
✔️Graphical Abstract Design
✔️Journal Figures Design
✔️Scientific Illustration
✔️Flyer and Poster Design
✔️Infographic Design
✔️Slides Design
#writing tips#writing tools#academic writing#creative writing#writers block#writing#writeblr#biotechnology science#biochemistry#biotech#biology#molecularbiology#biotechnology#bioinformatics#biotech and pharmaceuticals#graphics#science illustration#scientific illustration#illustration#illustragram#commissions#commission open#open commissions#commissions open
117 notes
·
View notes
Text

We’re thrilled to have you in our page!
Take a moment to explore our website, our products, and feel free to drop a comment or message us if you have any questions.
Thank you for being a part of our journey!
2 notes
·
View notes
Text

Apply Now for MSC Biotechnology Admission 2023
Gujarat Biotechnology University (GBU) is a modern university near GIFT City, Gandhinagar. Funded by the Government of Gujarat’s Department of Science and Technology, GBU will create a culture of excellence and innovation with entrepreneurship at its core. GBU offers Masters by Research and PhD biotechnology programmes with a strong translational focus, aiming to deliver biotechnology solutions for society, engaging with the vibrant life science industry in Gujarat, and across India.
Read more: MSc Biotechnology Admission 2023-24
#biotech#biotechnology#biotech and pharmaceuticals#india#education#biotechnology university#Msc Biotechnology University#Apply Now for Msc Biotechnology
2 notes
·
View notes
Text
4 notes
·
View notes
Text
#biotech#pharmaceutical#biopharmaceuticals#t cells#car t cells#biotechnology#biotechtrends#pharmaceutical industry#Pharmarep#Leezettelopatic#pharmaceuticalrep#biotech and pharmaceuticals
2 notes
·
View notes
Text
ONCOCYTE REPORTS Full Year 2023 FINANCIAL RESULTS

Conference Call on Friday, April 12, 2024 at 5:00 a.m. PT / 8:00 a.m. ET
IRVINE, CA, April 12, 2024 - Oncocyte Corporation (Nasdaq: OCX), a precision diagnostics company, today reported financial results for the year ended December 31, 2023.
Recent Highlights
“In 2023, we made significant progress on cost controls and in the development of our transplant monitoring IP,” said Josh Riggs, CEO of Oncocyte. “We achieved reimbursement for VitaGraftTM Kidney, manufactured the first lots of GraftAssure RUO, and ran a competitive partnering process that resulted in the announced Bio-Rad agreement. We look forward to working with them on the commercialization of GraftAssure RUO and the development of VitaGraft Kidney IVD. We continue to benefit from strong support from our core investors and welcome new ones in our recently completed $15.8 million private placement offering. With this partnership and financing we believe that we are well-positioned to meet our critical commercial and regulatory milestones.”
2023 Fourth Quarter and Full-Year Financial Results
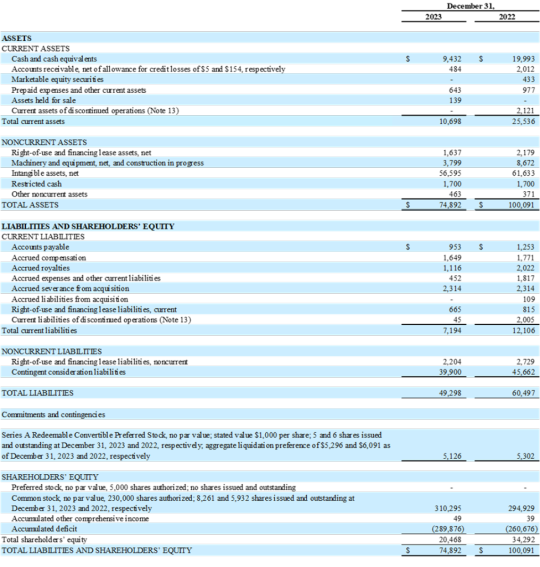
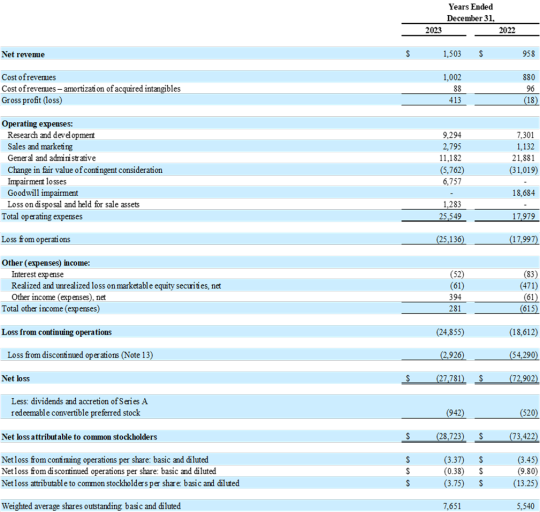
Net revenue for the three months and year ended December 31, 2023, was $314,000 and $1.5 million, respectively, an increase of 15% compared to the fourth quarter 2022 and 57% compared to the full year 2022, due to increased revenue from Pharma Services.
Cost of revenues for the three months ended December 31, 2023 was $431,000, including $409,000 from the cost of diagnostic tests and testing services we performed for our Pharma Services customers, with the remaining cost from noncash amortization expense.
Cost of revenues for the year ended December 31, 2023 was $1.1 million, including $1.0 million from the cost of diagnostic tests and testing services we performed for our Pharma Services customers, with the remaining cost from noncash amortization expense.
Research and development expense for the three months and year ended December 31, 2023, was $2.5 million and $9.3 million, respectively, an increase of 85% compared to the fourth quarter 2022 and 27% compared to the full year 2022. The increases were driven by continued focused investment in developing manufacturable versions of assays including DetermaIOTM, VitaGraft and DetermaCNITM.
Sales and marketing expense for the three months and year ended December 31, 2023, was $582,000 and $2.8 million, respectively, an increase of 74% compared to the fourth quarter 2022 and 147% compared to the full year 2022. The increases were primarily driven by a continued ramp in sales, marketing and commercialization activities related to the recent coverage decision and launch of VitaGraft Kidney.
General and administrative expense for the three months and year ended December 31, 2023, was $1.8 million and $11.2 million, respectively, a decrease of 66% compared to the fourth quarter 2022 and 49% compared to the full year 2022. The decreases were primarily due to decreased stock-based compensation and personnel expenses.
Loss from operations for the three months ended December 31, 2023, was $16.2 million, an increase of 39% compared to fourth quarter 2022. The 2023 loss from operations included a noncash loss of $11.2 million from the change in fair value of contingent consideration, compared to a gain of $13.9 million in 2022.
Loss from operations for the year ended December 31, 2023, was $25.1 million, an increase of 40% compared to the full year 2022. The 2023 loss from operations included a noncash gain of $5.8 million from the change in fair value of contingent consideration, compared to a gain of $31.0 million in 2022.
For Oncocyte’s complete financial results for the year ended December 31, 2023, see the Company’s annual Form 10-K to be filed with the Securities and Exchange Commission on April 15, 2024.
Webcast and Conference Call Information
Oncocyte will host a conference call to discuss fourth quarter and full year 2023 financial results prior to market open on Friday, April 12, 2024 at 5:00 a.m. Pacific Time / 8:00 a.m. Eastern Time. The conference call may be accessed live via telephone by dialing toll free (888) 550-5422 for both domestic and international callers. Once dialed in, ask to be joined to the Oncocyte Corporation call. The live webcast of the call may be accessed by visiting the “Events & Presentation” section of the Company’s website at https://investors.oncocyte.com.
About Oncocyte
Oncocyte is a precision diagnostics company. The Company’s tests are designed to help provide clarity and confidence to physicians and their patients. VitaGraft™ is a clinical blood-based solid organ transplantation monitoring test, GraftAssure™ is a research use only blood-based solid organ transplantation monitoring test, DetermaIO™ is a gene expression test that assesses the tumor microenvironment to predict response to immunotherapies, and the pipeline test DetermaCNI™ is a blood-based monitoring tool for monitoring therapeutic efficacy in cancer patients. For more information, visit https://oncocyte.com/
VitaGraft™, GraftAssure™, DetermaIO™, and DetermaCNI™ are trademarks of Oncocyte Corporation.
Forward-Looking Statements
Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates,” “may,” and similar expressions) are forward-looking statements. These statements include those pertaining to, among other things, the expectation that the Company and Bio-Rad will successfully commercialize GraftAssure RUO and develop VitaGraft Kidney IVD, the belief that the Company is well positioned to meet its critical commercial and regulatory milestones, and other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of diagnostic tests or products, uncertainty in the results of clinical trials or regulatory approvals, the capacity of Oncocyte’s third-party supplied blood sample analytic system to provide consistent and precise analytic results on a commercial scale, potential interruptions to supply chains, the need and ability to obtain future capital, maintenance of intellectual property rights in all applicable jurisdictions, obligations to third parties with respect to licensed or acquired technology and products, the need to obtain third party reimbursement for patients’ use of any diagnostic tests. Oncocyte or its subsidiaries commercialize in applicable jurisdictions, and risks inherent in strategic transactions such as the potential failure to realize anticipated benefits, legal, regulatory or political changes in the applicable jurisdictions, accounting and quality controls, potential greater than estimated allocations of resources to develop and commercialize technologies, or potential failure to maintain any laboratory accreditation or certification. Actual results may differ materially from the results anticipated in these forward-looking statements and accordingly such statements should be evaluated together with the many uncertainties that affect the business of Oncocyte, particularly those mentioned in the “Risk Factors” and other cautionary statements found in Oncocyte’s Securities and Exchange Commission (SEC) filings, which are available from the SEC’s website. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. Oncocyte undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
CONTACT:
Jeff Ramson
PCG Advisory
(646) 863-6893
ONCOCYTE CORPORATION
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share data)
ONCOCYTE CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
Oncocyte Corporation
Reconciliation of Non-GAAP Financial Measure
Consolidated Adjusted Loss from Operations
Note: In addition to financial results determined in accordance with U.S. generally accepted accounting principles (“GAAP”), this press release also includes a non-GAAP financial measure (as defined under SEC Regulation G). We believe the adjusted amounts are more representative of our ongoing performance. The following is a reconciliation of the non-GAAP measure to the most directly comparable GAAP measure:

SOURCE: Oncocyte Corporation
#press release#prism mediawire#stock market#investing#prismdigitalmedia#prismmarketview#nasdaq#healthcare#OCX#oncocyte#biotech and pharmaceuticals
0 notes
Text



#research#college#study motivation#studyblr#books & libraries#studyspo#studygram#capstone#biotechnology#biotech and pharmaceuticals
0 notes
Text
#mechanical seals#manufacturer#mumbai#gujarat#quantech seals#qtseals#pune#tamilnadu#supplier#uae#reverse balanced#karnataka#maharashtra#petrochemical#food and beverages#biotech and pharmaceuticals#biotechnology#oil and gas#industrial equipment#water pump#grundfos pump seal#cartridge
1 note
·
View note
Text
India things!
India aims to increase the Bioeconomy's contribution to GDP (Gross Domestic Product) from 2.6% to 5% by 2030, as outlined in the 'Bioeconomy Report 2022' by the Department of Biotechnology (DBT).
Biotechnology funding in India remains stagnant, with only a 0.0001% allocation of the GDP. Despite a temporary increase during Covid-19, funding levels haven't returned to pre-pandemic standards.
The ‘Guidelines for Genetically Engineered (GE) Insects’, issued by the DBT in April 2023, provide procedural roadmaps for those interested in creating GE insects but have issues.
India's bioeconomy is on a robust growth trajectory, projected to reach USD 150 billion by 2025 and surpass USD 300 billion by 2030.
The sector experienced a remarkable 14.1% increase, reaching USD 80 billion in 2021 compared to USD 70.2 billion in 2020.
In 2021, the sector witnessed the establishment of three biotech startups daily, totaling 1,128 for the year.
With over USD 1 billion invested in research and development, the industry is demonstrating a commitment to innovation and advancement.
Amidst the global pandemic, India administered 4 million Covid-19 vaccine doses and conducted 3 million tests daily, showcasing its resilience and capacity.
Over the past decade, the number of biotech startups has soared from 50 to over 5,300, with expectations of doubling by 2025.
The Biotechnology Industry Research Assistance Council (BIRAC) has played a pivotal role by establishing 74 bio-incubation centers across 21 states/UTs, fostering a supportive environment for bio-entrepreneurs.
Notably, India boasts the second-highest number of USFDA (United States Food and Drug Administration)-approved manufacturing plants outside the US, underscoring its global standing in the biotech industry.
#general knowledge#affairsmastery#india#generalknowledge#current events#current news#ssccgl#upscprelims2024#upsc#upscaspirants#upscexam#upscprelims#upsc2024#dailynews#generalknowledgeindia#biotech and pharmaceuticals#biotechnology#biotech company#biotechinnovation#bioeconomy
0 notes
Text
GABAergic interneuron cell therapy reduces 95% of seizures and improves cognitive function—the interim data from Phase 1/2 clinical trial presented by Neurona Therapeutics.
#GABAergicInterneuronCelltherapyForDrugResistantEpilepsy#GABAergicInterneuronCellTherapyNRTX-1001byNeuronaTherapeutics#DrugResistantUnilateralMesialTemporalLobeEpilepsy(MTLE)#GABAergicInterneuronCellTherapyManufacturing#GABAergicInterneuronCellTherapyPhase1/2ClinicalTrial#GABAergicInterneuronCellTherapyForEpilepsy#NRTX-1001Phase1/2ClinicalTrialforDrugResistantEpilepsy#MedialGanglionicEminence(MGE)Pallial-typeGABAergicInterneurons#medicine#neuroscience#cell therapy#regenerative medicine#positive mental attitude#mentalheathawareness#neuroscientist#Neurona Therapeutics#biotech and pharmaceuticals#biotechnology#health
0 notes
Text
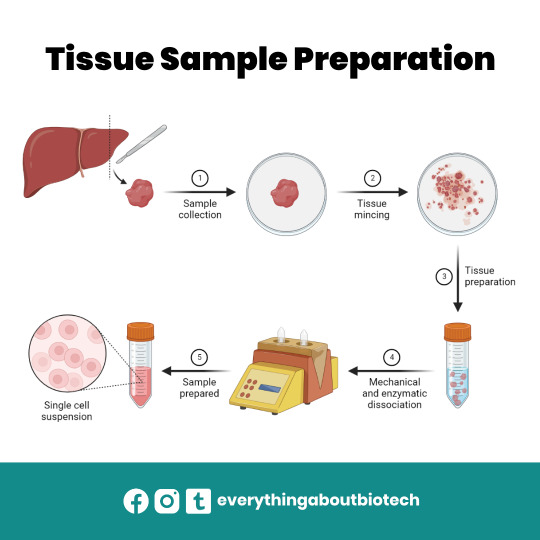
Tissue Sample Preparation
.
I help researchers increase engagement with their work. Together we will transform your ideas into unique and intuitive graphics with my reliable process:
1-Pinpoint your goals
2-Sketch and prototype
3-Discuss and iterate
4-Goal accomplished
.
𝐇𝐈𝐑𝐄 𝐌𝐄 (DM Your Design Projects)
.
💎Here to HELP You:
✔️Graphical Abstract Design
✔️Journal Figures Design
✔️Scientific Illustration
✔️Flyer and Poster Design
✔️Infographic Design
✔️Slides Design
#tissue sample#adipose tissue#tissue#life sciences#biochemistry#biotech#molecularbiology#biology#bioinformatics#biotechnology#biodiversity#science#scientists#biotechnology science#biotech and pharmaceuticals#open commissions#commission#taking commisions#commission art#commisions open
12 notes
·
View notes
Text
20231010 Pharmaceutical & Biotechnology
Architects of Pharmaceutical & Biotechnology will require Computer Driving Licence (ECDL) for core disciplines such as immunology molecular biology and pharmacology.
Biology and related domains are a major field of research and development. AI startups will be regulated in public domain audits.
H.S.H Princess Isabel M Neuchâtel
Principality of Neuchatel
Government of Switzerland
#edcl#pharmacy#biotech and pharmaceuticals#biotechinnovation#neuchatel#swissness#swisskids#ai startups
0 notes
Text
Phosphorylation
Phosphorylation is a key reaction where a phospho group (PO4) is added to protein via enzymatic processes. This allows for protein modification that is central in cell signaling, cellular regulation, cell adhesion, and many more important cellular processes. Phospho protein modification predominantly occurs on amino groups: serine, threonine, and tyrosine. These modified groups on proteins are the focus of post translational modifications. Assay Biotechnology is proud to provide antibodies that are specific for these phosphorylated proteins.
Read more
0 notes
Text

Pulsus conference proudly organizes the three-day event, "11th International Conference on Biotechnology Research," on December 07-09, 2023, in Madrid, Spain”, where competitors from all over entire globe are invited to demonstrate their knowledge, skills, and research expertise in the field of biotechnology.
Biotechnology Conference 2023 welcomes all biotechnologists, biotechnology scientists, academic researchers, industry experts, students, research analysts, industrial professionals, and young scientists involved in biotechnology to take part in the "11th International Conference on Biotechnology Research," which will be hosted in Madrid, Spain, on December 07-09, 2023. The conference's theme is "Emerging Trends and Innovations in Biotechnology." The Biotechnology conference 2023 will be filled with skilled and prominent biotechnology professionals from all over the globe.
The theme and focus of Biotechnology conference 2023 and participants will be capable of presenting their unique research evidence through oral presentations, poster presentations, and video recordings on all aspects with 15+ tracks and scientific sessions.
The Biotechnology conference 2023 therefore provide ideal forum for research scientists, young scholars, industrial representatives, and student communities and individuals to debate and gain knowledge about novel findings in subjects such as genetic variation, cell biology, gene editing, plant regenerative medicine, animal tissue culture, food processing, marine science, molecular genetics, toxicology, environmental engineering, DNA sequencing, plant biotechnology, and so on.
At this event, participants will have the chance to understand about recent breakthroughs in biotechnology, engage in discussion on topics of mutual interest, and invent different professional ties, collaborations, and partnerships. Biotechnology conferences 2023 would empower registrants with the knowledge they need to implement sustainable principles into the development of meaningful products and innovations for society.
We expect that the Biotechnology conference 2023 will make significant contributions to the knowledge base in cutting-edge scientific disciplines of biotechnology.
By establishing an international forum, Biotechnology conference 2023 encourages the interchange of innovative thoughts and creative ideas among scholars, entrepreneurs, academics, and scientific researchers. This platform enables the researchers to honestly express, analyse, and discuss their unique scientific studies through oral and poster demonstrations for the benefit of all biotechnology participants.
Biotechnology conference 2023 is the ideal moment scientists from all around the globe to gather and generate fresh collaborative scientific collaborations, connections, and interactions. With the help of its scientific sessions and tracks, you will be able to assess current technologies and advancements in the research field. The conference will address a wide spectrum of biotechnology-related subjects.
We ensure that this biotechnology conference 2023 will give you an exceptional chance to stay up to date on the latest discoveries in biotechnology while also networking with international professionals and industry experts.
1 note
·
View note
Text
How this UK-based firm is growing a more sustainable cannabis
U.K.-based Glass Pharms claims to be the world’s first firm to grow cannabis in a carbon-neutral way. It actually says it goes one better, and produces in a carbon-negative way — without buying carbon credits that would usually offset emissions.
The business was set up in 2020 and finished building its greenhouse cultivation facility in the south of England in 2023.
CEO James Duckenfield said…
View On WordPress
#Biotech and Pharmaceuticals#business news#carbon neutrality#Environment#Jazz Pharmaceuticals PLC#marijuana#Pharmaceutical industry regulation#Pollution#Science#United Kingdom#United-Guardian Inc
0 notes
Text
Oncocyte and Bio-Rad Partner on Global Launch of Transplant Assay

- Bio-Rad expects to make equity investment in support of deal
- Agreement provides for global exclusivity in transplant monitoring commercialization
- Bio-Rad granted subsequent investment option upon FDA clearance
IRVINE, CA, April 11, 2024 - Oncocyte Corporation (NASDAQ: OCX), a precision diagnostics company, today announced a partnership agreement with Bio-Rad Laboratories (NYSE: BIO), a global leader in life science research and clinical diagnostics products, for the commercialization of its research use only GraftAssure™ assay, powered by Droplet DigitalTM PCR (ddPCRTM)*. The new product is expected to launch in Q2 2024 to a select group of academic transplant centers in the US and EU and more broadly in the second half of the year.
As part of the agreement, Bio-Rad and Oncocyte will co-market the assay inside the US and Germany, with Oncocyte acting as commercial lead. Outside these countries Bio-Rad has been granted exclusive global distribution and commercial rights.
“As we move towards launch, having the support of the Bio-Rad team in the US and Germany gives us the scale we need to meet the market opportunity,” said Josh Riggs, Oncocyte’s CEO. “The QX600 ddPCR platform, along with their expertise in serving the life science market, makes Bio-Rad a natural partner for our transplant technology.”
Going forward, both companies have committed to joint efforts in developing a regulated product designed to facilitate widespread distribution and clinical adoption in the United States and beyond.
Additionally, Bio-Rad has been granted an option for IVD commercial rights at FDA clearance, subject to meeting specific objectives. Exercising the option would come with a second equity investment into Oncocyte. Further details of the agreement can be found in Oncocyte’s filing with the Securities and Exchange Commission.
dd-cfDNA is a proven, non-invasive biomarker with growing demand offering an estimated three million testing opportunities globally and driving a market exceeding $1 billion. Globally, over 157,000 transplants are performed with a 9.1% annual growth rate. GraftAssureTM uses a differentiated technology, Droplet Digital PCR to quantify dd-cfDNA to detect signs of graft damage.
*Droplet Digital, ddPCR and QX600 are trademarks of Bio-Rad Laboratories, Inc.
About Oncocyte
Oncocyte is a precision diagnostics company. The Company’s tests are designed to help provide clarity and confidence to physicians and their patients. VitaGraft™ is a clinical blood- based solid organ transplantation monitoring test, which recently received CMS reimbursement for kidney transplantation. GraftAssure™ is a decentralized research use only blood-based solid organ transplantation monitoring test, DetermaIO™ is a gene expression test that assesses the tumor microenvironment to predict response to immunotherapies, and the pipeline test DetermaCNI™ is blood-based monitoring tool for assessing therapeutic efficacy.
VitaGraft™, GraftAssure™, DetermaIO™, and DetermaCNI™ are trademarks of Oncocyte Corporation.
Forward-Looking Statements
Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates,” “may,” and similar expressions) are forward-looking statements. These statements include those pertaining to, among other things, the expected launch of the new GraftAssure product in 2Q 2024 to a select group of academic transplant centers in the US and EU and more broadly in the second half of the year, the transactions contemplated by the Collaboration Agreement, the expectation of meeting the market opportunity, the anticipated development of a regulated product and system designed to facilitate widespread distribution and clinical adoption of the core technology in the United States and beyond, the expectation for FDA clearance, the possibility that Bio-Rad will exercise its option for IVD commercial rights and with a second equity investment, , and other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of diagnostic tests or products, uncertainty in the results of clinical trials or regulatory approvals, the capacity of Oncocyte’s third-party supplied blood sample analytic system to provide consistent and precise analytic results on a commercial scale, potential interruptions to supply chains, the need and ability to obtain future capital, maintenance of intellectual property rights in all applicable jurisdictions, obligations to third parties with respect to licensed or acquired technology and products, the need to obtain third party reimbursement for patients’ use of any diagnostic tests Oncocyte or its subsidiaries commercialize in applicable jurisdictions, and risks inherent in strategic transactions such as the potential failure to realize anticipated benefits, legal, regulatory or political changes in the applicable jurisdictions, accounting and quality controls, potential greater than estimated allocations of resources to develop and commercialize technologies, or potential failure to maintain any laboratory accreditation or certification. Actual results may differ materially from the results anticipated in these forward-looking statements and accordingly such statements should be evaluated together with the many uncertainties that affect the business of Oncocyte, particularly those mentioned in the “Risk Factors” and other cautionary statements found in Oncocyte’s Securities and Exchange Commission (SEC) filings, which are available from the SEC’s website. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. Oncocyte undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
CONTACT:
Jeff Ramson
PCG Advisory
(646) 863-6893
[email protected]
SOURCE: Oncocyte Corporation
#press release#prism mediawire#stock market#investing#prismdigitalmedia#prismmarketview#nasdaq#healthcare#NYSE#biotech and pharmaceuticals#OCX#BIO#oncocyte
0 notes