#Breyanzi
Text
CAR-T Cell Therapy Market – Transforming lives through personalized CAR-T cell therapy for cancer patients

This latest report researches the industry structure, sales, revenue, price and gross margin. Major producers’ production locations, market shares, industry ranking and profiles are presented. The primary and secondary research is done in order to access up-to-date government regulations, market information and industry data. Data were collected from the CAR-T Cell Therapy manufacturers, distributors, end users, industry associations, governments’ industry bureaus, industry publications, industry experts, third party database, and our in-house databases.
Get a Free Sample PDF of the Report – https://www.growthplusreports.com/inquiry/request-sample/cart-cell-therapy-market/8902
Key Players in the CAR-T Cell Therapy Market: –
Novartis AG
Bristol-Myers Squibb Company
Amgen Inc.
Gilead Sciences Inc. (Kite Pharma)
Johnson & Johnson
Eli Lilly and Company
Sorrento Therapeutics Inc.
ACROBiosystems
Celyad Oncology
Sangamo Therapeutics Inc.
Servier Laboratories
Noile-Immune Biotech Inc.
Miltenyi Biotec
Market Segmentation:
GLOBAL CAR-T CELL THERAPY MARKET – ANALYSIS & FORECAST, BY DRUG TYPE
Kymriah
Yescarta
Abecma
Breyanzi
Tecartus
Carvykti
Others
GLOBAL CAR-T CELL THERAPY MARKET – ANALYSIS & FORECAST, BY INDICATION
Diffuse Large B-Cell Lymphoma
Acute Lymphoblastic Leukemia
Multiple Myeloma
Chronic Lymphocytic Leukemia
Follicular Lymphoma
Mantle Cell Lymphoma
Others
Market segment by Region/Country including: –
-North America (United States, Canada and Mexico)
-Europe (Germany, UK, France, Italy, Russia and Spain etc.)
-Asia-Pacific (China, Japan, Korea, India, Australia and Southeast Asia etc.)
-South America (Brazil, Argentina and Colombia etc.)
-Middle East and Africa (South Africa, UAE and Saudi Arabia etc.)
This report also includes a discussion of the major players across each regional CAR-T Cell Therapy market. Further, it explains the major drivers and regional dynamics of the global CAR-T Cell Therapy market and current trends within the industry.
Request for customization in Report: https://www.growthplusreports.com/inquiry/customization/cart-cell-therapy-market/8902
Key Benefits for Industry Participants and Stakeholders
One can find in-depth research data and industry trends of the CAR-T Cell Therapy Market Research.
The report offers details on potential investment opportunities, including those that are local and sector-specific that may benefit stakeholders and members of the industry.
One can gain a thorough grasp of market dynamics by looking at prices as well as the activities of producers and consumers.
With the use of market research, which will assist in discovering and visualizing new market participants as well as their portfolios, will be better able to make decisions and create an efficient counter strategy to maximize market advantage.
COVID 19 Impact Analysis
The CAR-T Cell Therapy Market Research Reports include a thorough discussion of the coronavirus’s effects in addition to the major market trends. When considering the impact of the COVID-19 on the industry, insights, analysis, projections, and predictions are given in the report study.
Given the breadth of the pandemic’s disruption, it is evident that the current depression is fundamentally different from previous recessions. Due to the sudden drop in demand and growing unemployment, the business climate will alter. In this uncomfortable environment, businesses may carve new roads by embracing novel ideas like ”advance toward localization, cash conservation, supply chain resilience, and innovation.”
CAR-T Cell Therapy Market TOC: https://www.growthplusreports.com/report/toc/cart-cell-therapy-market/8902
the market share and rank (in volume and value), competitor ecosystem, new product development, expansion, and acquisition.
This report stays updated with novel technology integration, features, and the latest developments in the market
This report helps stakeholders to understand the COVID-19 and Russia-Ukraine War Influence on the CAR-T Cell Therapy industry.
This report helps stakeholders to gain insights into which regions to target globally
This report helps stakeholders to gain insights into the end-user perception concerning the adoption of CAR-T Cell Therapy .
This report helps stakeholders to identify some of the key players in the market and understand their valuable contribution.
QUICK BUY: https://www.growthplusreports.com/checkout-8902
Browse Latest Healthcare Reports:
Aptamers Market
Influenza Diagnostics Market
Depression Apps Market
Leukapheresis Market
Mastopexy Market
Catheter Introducer Sheaths Market
Stuttering Therapeutics Market
Fibrous Dysplasia Market
Stoma Care Market
Contact Us:
Manan Sethi
Director, Market Insights
Email: [email protected]
Phone no: +1 888 550 5009
Web: https://www.growthplusreports.com/
About Us
Growth Plus Reports is part of GRG Health, a global healthcare knowledge service company. We are proud members of EPhMRA (European Pharmaceutical Marketing Research Association).
Growth Plus portfolio of services draws on our core capabilities of secondary & primary research, market modelling & forecasting, benchmarking, analysis and strategy formulation to help clients create scalable, ground-breaking solutions that prepare them for future growth and success.
We were awarded by the prestigious CEO Magazine as “Most Innovative Healthcare Market Research Company in 2020.”
0 notes
Text
ABD’de Yaşayan Türk Virolog Tareen’nden Kanser Tedavisinde Müjdeli Haber !

Amerika Birleşik Devletleri’nde yaşayan Virolog Semih Tareen, Biyoteknoloji Şirketinde yıllardır üzerinde çalıştıkları kanser tedavisi için Amerikan Gıda ve İlaç Kurumu FDA’dan faz1 için onay alındığını ve klinik çalışmalara başlanılacağı müjdesini verdi.
Kan kanserlerinin belli türlerini yok edebilecek bilimsel çalışma ile ilgili Bilim Sağlık Haber Ajansı’nın (BSHA) ulaştığı Virolog Semih Tareen, bu başarının bir ekip işi olduğunu, yüzlerce kişinin başarısı olduğunu vurguladı. İnsan sağlığı için sevindirici sonuçlara ulaşıldığına dikkat çeken Tareen şunları söyledi: “Bilimsel çalışmalar bir öncekilerin çalışmaları üzerine eklenerek birikir. Önceki biyoteknoloji şirketimizde benzer bir tedavi FDA tarafından onaylanmıştı ve ruhsat aldı. Breyanzi ismi altında satılan bu tedavide hastanın kendi bağışıklık hücreleri tasarlanıyor. Geliştirdiğimiz tedavi ise allogeneic denen ve sağlıklı kişiden gelen hücreleri kullanacak. Bu yeni yöntemin avantajı hastaya özel bir terapi olmaması ve tek sağlıklı bir donörden pek çok hastaya terapi üretilebilmesidir. Eğer Faz1 çalışmaları başarılı olursa klinik çalışmalarda Faz2’ye geçilecek.”
“Aşı Takviminden Farklı Yürüyebilir”
Hem virüsleri hem de CRISPR teknolojisini kullanarak sağlıklı insandan gelen bağışıklık hücrelerinin laboratuvar ortamında genetik olarak tasarlandığını söyleyen Tareen, “Bu hücreler çoğaltılarak kanser hastasına verilecek ve kanseri tanıyıp yok edebilecek. Deneyler olumlu, sıra Faz1 insan klinik çalışmasındayız. Faz1 belki bir yıl, faz2 belki iki sene sürebilir. Bunlar kanser çalışması olduğu için aşı takviminden farklı yürüyebilir” dedi.
Tüm Kanser Türlerinde Etkili Olacak mı?
Kanser tedavisi alanında yürütülen bilimsel çalışmanın tüm kanser türleri üzerinde etkili olup olmayacağı sorusunu cevaplayan Virolog Tareen, “Hayır tüm kanser türlerinde değil, kan kanserlerinin sadece belli türlerinde denenecek. Henüz insanlarda etkili olup olmayacağını bilemiyoruz. Klinik çalışmalar bunun için var. Fakat preklinik deneylerde etkili. Kanser dediğimiz şey tek bir hastalık değil. Her kanser birbirinden farklı o yüzden tedavisi tamamen farklı. Bugün tedavisi olan bazı kanser çeşitleri var mesela” diye konuştu.
“Uygun Donör Bulma Derdi Olmayacak”
Tek bir bireyin donör olmasıyla çok sayıda insanın sağlığına kavuşmasının öngörüldüğü bilimsel araştırmanın donör uygulaması hakkında bilgi veren Semih Tareen, “Organ naklini düşünün, bizim çalışmamızda organ nakli yerine hücre nakli olacak. Normalde kişiye uygun bir donör bulmak gerekir. Ama bizim yaptığımız genetik tasarımlarla uygun donör derdi olmayacak. Herhangi bir donörden kanseri tedavi edecek hücreler hazırlanabilecek.” (BİLİM SAĞLIK HABER AJANSI)
Read the full article
0 notes
Text
Global Europe CAR-T Cell Therapy Market Size, Trends, and Report
The Europe CAR-T Cell Therapy Market was valued at USD 296.21 Mn in 2020 which expected to reach USD 1,904.40 Mn by 2027 at a CAGR 30.45% from 2020-2027.
The CAR-T Cell Therapy market is a conceptual examination of all commercial activities related to CAR-T Cell Therapy, either directly or indirectly. As a result, a new investor can learn about CAR-T Cell Therapy firms, their important products, their basic strategy, key CAR-T Cell Therapy market trends, and more.
Get a Sample Copy of this Report@ https://qualiketresearch.com/request-sample/Europe-CAR-T-Cell-Therapy-Market/request-sample
Market Drivers
Increasing approvals for CAR-T cell therapy products.
Increase in awareness about the new approach to treat cancer leads to increase in demand for the CAR-T cell therapy products. Thus, the key players in market are engaged in developing new products & thereby drive the growth of Europe CAR-T Cell Market.
For Instance, in June 2020, Kite Pharma, received approval to implement a variation to the Yescarta (axicabtagene ciloleucel) Marketing Authorization from the European Medicine Agency for end-to-end manufacturing. With this approval, Kite’s European manufacturing facility, designed & dedicated to the manufacture of individualized cell therapies, is now fully operational.
Market Restraints
CAR T therapies often come with unique drug development challenges. Some potential challenges associated with CAR T development is likely to hamper the growth of the Europe CAR-T Cell Therapy market. The potential challenges include limited guidance, manufacturing and distribution logistics, products safety etc.
Moreover, high cost involved in research & development for CAR-T cell therapies & lack of expertise as well as inadequate knowledge about CAR-T cell therapies are the major factors among others acting as restraints, and will further challenge the market in the forecast period.
Impact of COVID-19
Many businesses have seen their operations & financial performance suffer as a result of the COVID–19 pandemic and a slowing of global research activity. The negative impact is mostly due to the closure of academic & research institutes, as well as testing laboratories. As a result, the clinical trials conducted for the car T cell therapy has been delayed.
COVID-19 is posing a significant threat to health of vulnerable patients, like immunocompromised patients. CAR-T-cell therapy recipients are at high risk of poor COVID-19 due to their severely immunocompromised state, caused by prior lymphodepleting immunochemotherapy & CAR-T-cell therapy related side effects like B-cell depletion, hypogammaglobulinemia, and cytopenias.
Market Segmentation
The Europe CAR-T Cell Therapy Market is segmented into Type, Application, End-user and Growing System. By Type such as Abecma, Breyanzi , Kymriah, Tecartus, Yescarta. Further, market is segmented into By Application such as Cancer, Lymphoma, Others. By End-user such as Hospitals, Specialty Clinics, Others.
Country Analysis
Europe CAR-T Cell Therapy Market is segmented into fifteen regions such as Germany, France, UK, Turkey, Switzerland, Norway, Sweden, Spain, Denmark, Finland, Iceland, Poland, Luxembourg, Netherlands, and Belgium. Germany dominated the market and accounted for the largest revenue share of 15.10% in 2020. The region is expected to continue its dominance over the forecast period. UK is expected to grow at significant growth rate, as end-user such as Hospitals and Specialty Clinics segment are growing in the country. Due to rapid advances in the healthcare infrastructure and disposable income, the market
Get Discount on this Report@ https://qualiketresearch.com/request-sample/Europe-CAR-T-Cell-Therapy-Market/ask-for-discount
Key Players
Various key players are listed in this report such as Mustang Bio Inc, Calgene Corporation, Bluebird Bio Inc., Kite Pharma, Inc, CARsgen Therapeutics, Ltd., Legend Biotech, Immune Therapeutics, Pfizer Inc, Bellicum Pharmaceuticals, Inc, Sorrento Therapeutics, Inc, Novartis.
Market Taxonomy
By Type
Abecma
Breyanzi
Kymriah
Tecartus
Yescarta
By Application
Cancer
Lymphoma
Others
By End-user
Hospitals
Specialty Clinics
Others
By Country
Germany
France
UK
Turkey
Switzerland
Norway
Sweden
Spain
Denmark
Finland
Iceland
Poland
Luxembourg
Netherlands
Belgium
Key Questions Addressed by the Report
What are the Key Opportunities in Europe CAR-T Cell Therapy Market?
What will be the growth rate from 2020 to 2027?
Which segment/region will have highest growth?
What are the factors that will impact/drive the Market?
What is the competitive Landscape in the Industry?
What is the role of key players in the value chain?
Browse Full Report https://qualiketresearch.com/reports-details/Europe-CAR-T-Cell-Therapy-Market
0 notes
Text
Oh, he’ll then foam is a deadly disease I’m posting this because this may be an investment vehicle although it’s still experimental. I do have an extensive science background and I was I am teacher in gifted education for chemistry atusf.edu.
Oh, he’ll then foam is a deadly disease I’m posting this because this may be an investment vehicle although it’s still experimental. I do have an extensive science background and I was I am teacher in gifted education for chemistry atusf.edu.
Bristol Myers Squibb – CAR T Cell Therapy Breyanzi® Approved as Relapsed or Refractory Large B-cell Lymphoma Second-Line Therapy in Japan
View On WordPress
0 notes
Text
The CAR-T therapies market is characterized by a healthy pipeline of promising therapies, and is projected to be worth around USD 14 Billion in 2030
Given the success of approved CAR-T cell therapies, such as KYMRIAH®, YESCARTA®, TECARTUS® and BREYANZI®, this upcoming class of biologics are anticipated to carve out a significant share of the multi-billion dollar cancer immunotherapy market
Roots Analysis has announced the addition of “CAR-T Cell Therapy Market (3rd Edition) by Target Indications (NHL, Multiple Myeloma, Chronic Lymphocytic Leukemia, Acute Lymphoblastic Leukemia, Follicular Lymphoma, Mantle Cell Lymphoma, Hepatocellular Carcinoma and Others), Target Antigens (CD19, BCMA, CD19 / CD22, GPC3 and EGFR), Key Players and Key Geographies (North America, Europe, Asia Pacific, Latin America, Middle East and North Africa, and Rest of the World) – Industry Trends and Global Forecasts, 2021-2030” report to its list of offerings.
Given their ability to selectively direct a cell mediated immune response against cancer cells and, thereby, offer prolonged periods of disease remission, several CAR-T cell therapies provide a promising therapeutic strategy for advanced stage cancers and are expected to achieve blockbuster status. With four approved products and many candidate therapies under evaluation for the treatment of multiple disease indications, the CAR-T cell therapy market is characterized by a healthy and growing pipeline. Further, with lucrative financial support and notable increase in partnerships, the CAR-T-cell therapies market is abuzz with activity.
To order this 795+ page report, which features 165+ figures and 270+ tables, please visit https://www.rootsanalysis.com/reports/view_document/car-t-therapies-market/269.html
Key Market Insights
Over 755 CAR-T cell therapies are currently approved / under development
Close to 40% of the aforementioned candidates are in preclinical and discovery stages, while more than 25% are being evaluated in clinical stages (phase I/II and above). Examples of late-stage clinical candidates include bb2121, CD123/CLL1 CAR-T CD19 CAR-T and LCAR-B38M CAR-T / JNJ-68284528.
Currently, the focus is on developing product candidates to treat various types of cancers
Over 95% of the products in the development pipeline are being evaluated for the treatment of hematological malignancies, including (in decreasing order of number of pipeline products) acute lymphoblastic leukemia, non-Hodgkin's lymphoma, multiple myeloma, and acute myeloid leukemia. Only 2% of current pipeline candidates are being developed for the treatment of non-oncological indications.
Extensive efforts are underway to improve CAR constructs
Majority of the product candidates in the clinical pipeline, including the four approved drug products, are based on second generation CARs. Further, a number of novel therapies armed with fourth generation CAR constructs, CAR-based products containing humanized scFv and bispecific CARs (CD19+CD20 or CD19+CD22 or CD19+CD30) are being evaluated worldwide.
China is leading the product development efforts related to CAR-T cell therapies, in terms of number of active trials and supporting hospitals
In the last 10 years, over half of the 410 clinical trials evaluating various types of CAR-T cell therapies, were registered in China. In addition, owning to a favorable clinical research environment, China is presently considered to be among the leading regions in the CAR-T cell therapy space, with close to 40 industry players and more than 100 non-industry players, including hospitals and universities, contributing to this field.
Partnership activity within this domain has grown at a CAGR of 26%, between 2011 and 2020
More than 220 agreements were inked related to CAR-T cell therapies, with the maximum activity being reported in 2018. Majority of partnership deals signed within this domain were R&D agreements (21%), technology licensing (20%) and product development and commercialization agreements (11%).
Over USD 13 billion has been invested by both private and public investors, across more than 205 instances
It is important to mention that, between 2013 and 2020, majority of the funding was acquired through venture capital rounds (37%), other equity financing elements (24%), grants (12%) and secondary offerings (12%).
The market is anticipated to grow at a CAGR of over 28%, during the period 2021-2030
Growth in this domain is anticipated to be primarily driven by encouraging clinical trial results and the recent success of the four approved CAR-T cell therapies. North America (primarily the US) and Europe are expected to capture over 75% of the market share by 2030, in terms of the sales-based revenues.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/269/request-sample.html
Key Questions Answered
§ Who are the leading industry and non-industry players in this market?
§ What are the prevalent R&D trends in CAR-T cell therapies domain?
§ What are the key therapeutic areas for which CAR-T cell therapies are being / have been developed?
§ What are the challenges faced by stakeholders in this industry?
§ Which are the key geographies where extensive research on CAR-T cell therapies is being conducted?
§ Who are the key investors in this domain?
§ Who are the key opinion leaders / experts that can help in driving product development efforts in this field?
§ What kind of partnership models are commonly adopted by industry stakeholders?
§ What kind of contract manufacturing support is available for CAR-T cell therapies?
§ What kind of promotional strategies are likely to be adopted for CAR-T cell therapies that are approved / commercialized in future?
§ What are the factors that are likely to influence the evolution of this upcoming market?
§ How is the current and future market opportunity likely to be distributed across key market segments?
The USD 14 billion (by 2030) financial opportunity within the CAR-T cell therapy market has been analyzed across the following segments:
§ Disease indication
§ Non-Hodgkin lymphoma
§ Multiple myeloma
§ Chronic lymphocytic leukemia
§ Acute lymphoblastic leukemia
§ Follicular lymphoma
§ Mantle cell lymphoma
§ Hepatocellular carcinoma
§ Colorectal cancer
§ Target antigens
§ CD19
§ BCMA
§ CD19, CD22
§ GPC3
§ EGFR
§ Key Geographical Regions
North America
Europe
Asia Pacific
Latin America
Middle East and North Africa
Rest of the World
The report features inputs from eminent industry stakeholders, according to whom CAR-T cell therapies are soon likely to witness increased adoption given their broad scope of applications in various advanced stage oncological disorders. The report includes detailed transcripts of discussions held with the following experts:
§ Tim Oldham (Chief Executive Officer, Cell Therapies)
§ Troels Jordansen (Chief Executive Officer, Glycostem Therapeutics)
§ Wei (William) Cao (Co-Founder, Chairman and Chief Executive Officer, Gracell Biotechnologies)
§ Miguel Forte (Chief Operating Officer, TxCell)
§ Adrian Bot (Vice President, Scientific Affairs, Kite Pharma)
§ Vincent Brichard (Vice President, Immuno-Oncology, Celyad)
§ Brian Dattilo (Manager of Business Development, Waisman Biomanufacturing)
§ Aino Kalervo (Competitive Intelligence Manager, Strategy & Business Development, Theravectys)
§ Xian-Bao Zhan (Professor of Medicine and Director, Department of Oncology, Changhai Hospital)
§ Enkhtsetseg Purev (Assistant Professor of Medicine, University of Colorado)
The research includes brief profiles, featuring an overview of the company, its financial information (if available), and a description of its product(s), highlighting type of therapy and current development status. Each company profile includes technology portfolio (if available), recent developments related to T-cell immunotherapies and manufacturing capabilities of the companies.
§ Autolus
§ bluebird bio
§ CARsgen Therapeutics
§ Celgene (A Bristol Myers Squibb Company)
§ Cellectis
§ Cellular Biomedicine Group
§ Innovative Cellular Therapeutics
§ Iovance Biotherapeutics
§ Kite Pharma (A Gilead Sciences Company)
§ Kuur Therapeutics
§ Noile-Immune Biotech
§ Novartis
§ Shanghai Genechem
§ Sinobioway Cell Therapy
§ Takara Bio
§ Ziopharm Oncology
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/car-t-therapies-market/269.html or email [email protected]
You may also be interested in the following titles:
1. Global T-Cell Therapies Market (5th Edition), 2021 – 2030
2. mRNA Therapeutics and Vaccines Market, 2020-2030
3. Gene Therapies Market (4th Edition): Industry Trends and Global Forecasts, 2020-2030
4. Oncolytic Virus Therapy Market: Pipeline Review, Developer Landscape and Competitive Insights, 2020-2030
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at [email protected]
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
0 notes
Text
Bristol Myers must face $6.4 bln lawsuit over delayed cancer drug

A U.S. judge on Friday refused to dismiss a $6.4 billion lawsuit accusing Bristol Myers Squibb Co of delaying its Breyanzi cancer drug to avoid payments to shareholders of the former Celgene Corp, which the drugmaker bought for $80.3 billion in 2019.
U.S. District Judge Jesse Furman in Manhattan rejected Bristol Myers' claim that it was never properly notified about its alleged default on its merger obligations by UMB Bank NA, the trustee representing the former Celgene shareholders.
Read the full article
0 notes
Text
Bristol Myers must face $6.4 billion lawsuit over delayed cancer drug
Bristol Myers must face $6.4 billion lawsuit over delayed cancer drug
By Jonathan Stempel
NEW YORK (Reuters) – A U.S. judge on Friday refused to dismiss a $6.4 billion lawsuit accusing Bristol Myers Squibb Co of delaying its Breyanzi cancer drug to avoid payments to shareholders of the former Celgene Corp, which the drugmaker bought for $80.3 billion in 2019.
U.S. District Judge Jesse Furman in Manhattan rejected Bristol Myers’ claim that it was never properly…

View On WordPress
0 notes
Text
Sanofi’s ADC is targeting CEACAM5 in patients with NSCLC. CEACAM5 is expressed in 20% of patients with NSCLC Adenocarcinoma

Phase I/II study of tusamitamab ravtansine (Sanofi) in patients with non-sq having locally advanced or metastatic NSCLC for which no standard alternative therapy is available.
Tusamitamab ravtansine (SAR408701) is an ADC composed of a humanized monoclonal antibody that binds carcinoembryonic antigen-related cell adhesion molecule-5 (CEACAM5) and a cytotoxic maytansinoid that selectively targets CEACAM5-expressing tumor cells.
A total of 24 patients were treated for ≥ 6 months, 15 patients for ≥ 9 months, 11 patients for ≥ 12 months, 6 patients for ≥ 24 months, and 2 patients for ≥ 42 months. At the data cutoff, 5 patients remained on treatment, 1 for > 3.5 y. For patients treated ≥ 12 months, the median treatment duration was 26.6 months.
Of 15 patients with PR in the prior analysis, as of Dec 2021, PR was still observed in 10 patients (67%) treated for ≥ 6 months, 8 patients (53%) for ≥ 9 months, and 7 patients (47%) for ≥ 12 months. For the 11 patients treated ≥ 12 months, 7 had PR, and 4 had SD. Of the 11 patients treated ≥ 12 months, 9 had high CEACAM5 expression, and 2 had moderate CEACAM5 expression; most had prior treatment with an anti-PD1/PD-L1.
Patients treated for ≥ 12 months had better ECOG performance status and fewer prior treatments than the overall group. Of patients treated for ≥ 12 months, PR occurred irrespective of CEACAM5 expression level. Only 1 patient treated for ≥ 12 months discontinued due to a treatment-emergent adverse event (TEAE). Corneal events (keratitis/keratopathy) were the most frequent TEAEs.
KOL insights
“The observed long-term clinical benefit and safety profile of Tusa support its further clinical development; a Phase III study is ongoing to evaluate Tusa monotherapy in previously treated pts with high CEACAM5 expressing NSQ NSCLC”–Expert Opinion.
Conclusion
CEACAM5 is expressed in about 20% of patients with NSCLC adenocarcinoma but is not found in normal lung tissue. This makes CEACAM5 a potentially attractive therapeutic target; therefore, it is currently being explored as a potential biomarker. Understanding CEACAM5 may potentially give us more insights to target this malignancy with a poor prognosis.
Until now, Tusamitamab ravtansine clinical trial results have been most promising in NSQ-NSCLC; in fact, it is the most advanced novel agent in clinical testing targeting CEACAM5, specifically NSLC patients. The ADC has already entered phase III development for NSCLC; moreover, it is also being evaluated in Phase II trials for Metastatic Breast and Pancreatic Cancer.
To Get a Detailed analysis of ASCO Conference 2022 Abstracts, Visit: ASCO 2022 Detailed Coverage | ASCO 2022 Conference | ASCO Conference 2022 | ASCO Abstract 2022
Some of the Latest ASCO Abstract 2022 Launched:-
Can Breyanzi be a hit CAR-T in second-line treatment after failing in the first-line setting for the patients with R/R Large B-cell lymphoma (LBCL)?
Teclistamab, a soon-to-be-approved bispecific antibody, demonstrates its promise in Multiple Myeloma during ASCO 2022.
Merus’ Zenocutuzumab, a HER2-HER3 Bispecific Antibody, Successfully Targets NRG1 Fusions in Lung and Pancreatic Cancer
Latest Pharmaceutical Market Research Reports 2022 by Delveinsight
Hepatic Cirrhosis Market
Shigellosis Market
Tuberculosis Market
B-Cell Maturation Antigen Targeted Therapies Market
Oncolytic Virus Cancer Therapy Pipeline
Coronary Microvascular Dysfunction CMD Market
Medical Marijuana Market
Facial Lines Market
Adult T-Cell Leukemia Market
Shingles Market
Kaposi's Sarcoma Market
Surgical Mask & Respirator Market
Stem Cell Market
0 notes
Text
Cell Therapy Bioprocessing Market 2022: Size, Share, Growth, Trends and 2028 Forecast
The cell therapy bioprocessing is expected to reach US$ 30,052.61 million in 2028 from US$ 11,192.50 million in 2020. The market is estimated to grow with a CAGR of 13.5% from 2021-2028.
The cell therapy bioprocessing market has been analyzed on the basis of technology, cell type, end user, and region. The market based on region is segmented into North America, Europe, Asia Pacific, the Middle East and Africa, and South and Central America. The report emphasizes on parameters such as market trends, technological advancements, market dynamics, and leading company’s competitive landscape analysis to offers insights and in-depth analysis of the cell therapy bioprocessing market. It also includes the analysis of COVID-19 pandemic across the market in all the key regions.
Growing Approvals for Cell Therapies
Cell therapies have shown positive results in treating various chronic diseases including rare genetic disorders by offering regenerative medicines and personalized medicines. Increasing need of treating chronic diseases have pushed the research and development activities resulting in growing cell therapy production and product approvals. Few instances are listed below for the cell therapies approvals that have contributed to the growth of the cell therapy bioprocessing market.
In May 2019, the Food and Drug Administration (FDA) approved Zolgensma, manufactured by AveXis, Inc. A subsidiary of Novartis AG. Zolgensa is designed to treat spinal muscular atrophy in children below two years and is given by infusing one-time into the vein.
In July 2020, Kite Pharma, Inc., a Subsidiary of Gilead Company, received FDA approval for its Tecartus. Tecartus is (CAR) T cell therapy designed to treat refractory mantle cell lymphoma (MCL) in adults. According to toe Gilead Company, Tecartus is the first approved (CAR) T cell therapy for mantle cell lymphoma, which is expected to be a new frontier in the treatment of mantle cell lymphoma.
In February 2021, Juno Therapeutics, Inc., a subsidiary of Bristol-Myers Squibb Company, received approval for its Breyanzi. Breyanzi is a cell-based gene therapy intended to treat certain types of large B-cell lymphoma in adults. The treatment is given after patient have not responded to minimum two other types of systemic treatment. However, Breyanzi in 2019, faced regulatory setback since its first filing; it is currently under the European Medicines Agency’s review and was validated in July 2020.
Thus, growing product developments have resulted in various product approvals that reflects the increase in cell therapies. Therefore, it is expected that the growing approvals for cell therapies are enormously increasing the cell therapy bioprocessing, which, in turn is likely to drive the market’s growth over the coming years
…
For More Info Read our Press Release: https://www.theinsightpartners.com/pr/cell-therapy-bioprocessing-market
0 notes
Photo

2021 drug approvals: In a year dominated by COVID, biopharma managed to deliver 55 new drugs #fdaapproval #MSL #FSTP #BoardcertifiedMSL #BCMSLcert “Each year, many scientists and other experts working in the biopharma industry see years of their work culminate in FDA approvals. With the COVID-19 crisis stretching into its third year, the 2021 batch of new drug approvals clearly demonstrates that the sector has learned how to operate in a pandemic. All told, the industry nabbed 55 FDA approvals in 2021. They include the controversial accelerated approval for Biogen's Alzheimer's drug Aduhelm, Pfizer's record-shattering COVID-19 vaccine, Comirnaty, and many others. Not all 2021 approvals commanded as many headlines as Aduhelm and Comirnaty. Aside from those notable companies, also represented in 2021's crop of new approvals were Amgen, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Merck and Regeneron. And among the dozens of new drugs green-lit last year, companies such as BridgeBio, ChemoCentryx and Apellis won their first-ever FDA approvals, setting them up to make their commercial debuts. RELATED: FDA missing more approval target dates as COVID slows inspections Yet, some applications did face pandemic challenges. The FDA struggled to keep up with its inspection duties during the pandemic, leading to regulatory delays for certain drugmakers. In November, the agency reported that 55 new drug applications were facing inspection delays. Avadel Pharmaceuticals and UCB were among those to experience inspection-related delays in 2021. “ 2021 drug approvals: In a year dominated by COVID, biopharma managed to deliver 55 new drugs 1. #Verquvo 2. #Cabenuva 3. #Lupkynis 4. #Tepmetko 5. #Ukoniq 6. #Breyanzi 7. #Evkeeza 8. #Cosela 9. Amondys 45 10. #Pepaxto 11. #Nulibry 12. #Azstarys 13. #Fotivda 14. #Ponvory 15.#Zegalogue 16. #Abecma 17. #Qelbree 18. #Nextstellis 19. #Jemperli 20. #Zynlonta 21. #Empaveli 22. #Rybrevant 23. #Lybalvi 24. #Truseltiq 25. #Lumakras … https://lnkd.in/gwV7JXXe https://www.instagram.com/p/CYVaOqVpos3/?utm_medium=tumblr
#fdaapproval#msl#fstp#boardcertifiedmsl#bcmslcert#verquvo#cabenuva#lupkynis#tepmetko#ukoniq#breyanzi#evkeeza#cosela#pepaxto#nulibry#azstarys#fotivda#ponvory#zegalogue#abecma#qelbree#nextstellis#jemperli#zynlonta#empaveli#rybrevant#lybalvi#truseltiq#lumakras
0 notes
Text
Lisocabtagene maraleucel
Lisocabtagene maraleucel (liso-cel; JCAR017; Anti-CD19 CAR T-Cells) is an investigational chimeric antigen receptor (CAR) T-cell therapy designed to target CD19, [1][2] which is a surface glycoprotein expressed during normal B-cell development and maintained following malignant transformation of B cells. [3][4][5] Liso-cel CAR T-cells aim to target and CD-19 expressing cells through a CAR…
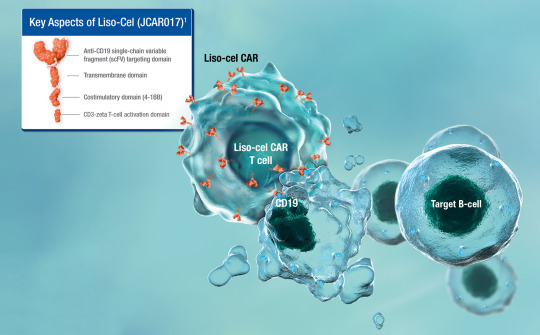
View On WordPress
0 notes
Text
Breyanzi approved for certain types of large B-cell lymphoma
Breyanzi approved for certain types of large B-cell lymphoma
Cell-based gene therapy Breyanzi (lisocabtagene maraleucel) has been approved to treat adults with certain types of large B-cell lymphoma who have not responded to other systemic treatment, the U.S. Food and Drug Administration announced Friday.
The chimeric antigen receptor T cell therapy was approved for relapsed or refractory large B-cell lymphoma, including diffuse large B-cell lymphoma,…

View On WordPress
0 notes
Link
0 notes
Text
BMS gets EU panel backing for CAR-T therapy Breyanzi $BMY
BMS gets EU panel backing for CAR-T therapy Breyanzi $BMY
BMS gets EU panel backing for CAR-T therapy Breyanzi $BMYfirstwordpharma.com/story/5489111
– FirstWord Pharma (@fwpharma)09:30 – Jan 28, 2022
from @fwpharma Tweets https://twitter.com/fwpharma/status/1487115966999842826
via IFTTT
View On WordPress
0 notes
Text
Impact of COVID-19 on CAR T-cell Therapy Market
CAR T-cell Therapy Market: Introduction
According to the report, the global CAR T-cell therapy market was valued at US$ 1.1 Bn in 2020 and is projected to expand at a CAGR of 30.6% from 2021 to 2031. CAR T-cell therapy is a recently approved treatment option for various types of cancer. CAR T cell therapy is provided by removing or harvesting T cells from a patient with cancer, transfecting the cells with CAR genes that are directed against the patient’s tumor type, expanding the modified T cell population, and reinfusing the cells back into the patient. Currently, Kymriah, Yescarta, Tecartus, Breyanzi, and ABECMA are the CAR T-cell therapy approved products for acute lymphocytic leukemia, diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, multiple myeloma, and others.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=83242
The global CAR T-cell therapy market is driven by increase in incidence of cancer and strong product pipeline. North America dominated the global CAR T-cell therapy market in 2020 and the trend is anticipated to continue during the forecast period. Highly structured healthcare industry, early adoption of new products, high prevalence rate of cancer, and presence of major players are expected to propel the CAR T-cell therapy market in North America. Asia Pacific is likely to be a highly lucrative market for CAR T-cell therapy during the forecast period. The market in the region is projected to expand at a high CAGR from 2021 to 2031.
Request for Analysis of COVID19 Impact on CAR T-cell Therapy Market- https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=83242
Increase in Incidence of Cancer and Strong Product Pipeline to Drive Global Market
Increase in incidence of cancer and strong product pipeline are anticipated to boost the growth of the global CAR T-cell therapy market. According to the WHO, one in five people develop cancer during their lifetime, and one in eight men and one in 11 women die from the disease. These new estimates suggest that more than 50 million people are living within five years of a past cancer diagnosis. According to the WHO, in 2020, around 544,352 new cases of non-Hodgkin lymphoma were recorded globally.
Strong product pipeline is a major factor driving the demand for CAR T-cell therapy products. For instance, Autolus Therapeutics plc has one innovative product, AUTO1, in phase I trial for treatment of adult acute lymphoblastic leukemia. AUTO1 contains obecabtagene autoleucel, which is a CD19 CAR T cell investigational therapy designed to overcome the limitations in clinical activity and safety compared to current CD19 CAR T cell therapies.
Request for Custom Research - https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=83242
Axicabtagene Ciloleucel to Dominate CAR T-cell Therapy Market
In terms of product type, the global CAR T-cell therapy market has been classified into axicabtagene ciloleucel, tisagenlecleucel, brexucabtagene autoleucel, lisocabtagene maraleucel, idecabtagene vicleucel, and others. The axicabtagene ciloleucel segment dominated the global CAR T-cell therapy market in 2020 and the trend is expected to continue during the forecast period. Yescarta is a product, which contains axicabtagene ciloleucel. Increase in demand for Yescarta in the treatment of diffuse large B-cell lymphoma and follicular lymphoma is likely to boost the growth of the segment during the forecast period. In October 2017, Kite Pharma, Inc. received the U.S. FDA approval for Yescarta for adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.
Tisagenlecleucel was the second largest segment in terms of market share in 2020. Increase in demand for Kymriah for treatment of acute lymphoblastic lymphoma and product approval in different countries are likely to drive the segment.
Enquiry before Buying CAR T-cell Therapy Market Report - https://www.transparencymarketresearch.com/sample/sample.php?flag=EB&rep_id=83242
Diffuse Large B-cell Lymphoma to be Highly Lucrative
Based on indication, the global CAR T-cell therapy market has been categorized into acute lymphocytic leukemia, diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, multiple myeloma, and others. The diffuse large B-cell lymphoma segment dominated the CAR T-cell therapy market due to increase in incidence of diffuse large B-cell lymphoma. Diffuse large B-cell lymphoma is the most common type of non-Hodgkin lymphoma. Increase in number of cases of non-Hodgkin lymphoma is likely to propel the segment during the forecast period. According to WHO, globally, around 544,352 new non-Hodgkin lymphoma cases were recorded in 2020.
Hospitals to Emerge as Significant End-user
In terms of end-user, the global CAR T-cell therapy market has been divided into hospitals and cancer treatment centers. The hospitals segment led the global CAR T-cell therapy market in terms of revenue in 2020 and the trend is projected to continue during the forecast period. The hospitalization for cancer treatment is anticipated to boost the growth of the hospitals segment. Moreover, increase in use of CAR T-Cell therapy in the treatment of cancers contributes to the segment's large market share. The segment is driven by surge in number of cancer patients who visit hospital for treatment of cancer.
Buy now CAR T-cell Therapy Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=83242<ype=S
North America to Dominate CAR T-cell Therapy Market
In terms of region, the global CAR T-cell therapy market has been segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America dominated the global CAR T-cell therapy market in 2020, followed by Europe. North America’s large market share can be ascribed to technologically advanced research and treatment platforms, increase in number of cancer patients, new product launches, and presence of major players. The CAR T-cell therapy market in Asia Pacific is anticipated to expand at a high CAGR from 2021 to 2031. This can be ascribed to the presence of developing countries with commercial hubs, expansion of business organizations, rise in awareness about CAR T-cell therapy, improvement in healthcare infrastructure, and increase in investments by companies.
Competition Landscape
The global CAR T-cell therapy market is consolidated due to limited approved products. Key players operating in the global market include Pfizer, Inc., Novartis AG, Bristol-Myers Squibb, Amgen, Inc., Sorrento Therapeutics, Inc., Johnson & Johnson Services, Inc., Gilead Sciences, Inc., Merck & Co., Inc., and bluebird bio, Inc.
More Trending Reports by Transparency Market Research:
D-dimer Testing Market D-dimer tests are used for diagnosing conditions related broadly to deep vein thrombosis (DVT), pulmonary embolism, and disseminated intravascular coagulation (DIC). Among all the applications, the demand for the test for diagnosing DVT is prominent.
Pyrogen Testing Market North America dominated the global pyrogen testing market in 2018and the trend is anticipated to continue during the forecast period. New drug approvals by the Food and Drug Administration (FDA) and increase in demand for pyrogen testing for medical devices are expected to drive the pyrogen testing market in North America.
Conserving Devices Market While nasal cannulae for supplementary supply of oxygen is reliable and effective, it is very wasteful. To address this, oxygen conserving methodologies have emerged to improve the efficiency of oxygen delivery for patients requiring oxygen therapy. This is because oxygen conserving devices enable a high degree of portability, improve the efficiency of oxygen delivery, and lighten the load for these patients.
About Us Section:
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants, use proprietary data sources and various tools and techniques to gather, and analyse information. Now avail flexible Research Subscriptions, and access Research multi-format through downloadable databooks, infographics, charts, interactive playbook for data visualization and full reports through MarketNgage, the unified market intelligence engine. Sign Up for a 7 day free trial!
Contact Us
Mr. Rohit Bhisey
Transparency Market Research,
90 State Street, Suite 700,
Albany, NY 12207
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Email: [email protected]
Website: https://www.transparencymarketresearch.com/
0 notes