#Drug Discovery Services Market Growth
Text
Drug Discovery Services Market Growth 2024 Along with Business Insights and Forecast to 2030

****Everything You Need to Know About Drug Discovery Services everything is Here....!
The Comprehensive study on Drug Discovery Services Market includes historical data as well as share, size, and projection information for the major players, geographies, applications, and product categories for the years 2024 to 2030. The Market study includes comprehensive insights on the competitive environment, description, broad product portfolio of key players, SWOT analysis, and significant business strategy implemented by rivals, revenue, Porters Five Forces Analysis, and sales projections. The report also features an impact analysis of the market dynamics, highlighting the factors currently driving and limiting market growth, and the impact they could have on the short, medium, and long-term outlook. The main goal of the paper is to further illustrate how the latest scenario, the economic slowdown, and war events affect the market for Drug Discovery Services.
Drug Discovery Services Market is growing at a +14.9% CAGR during the forecast period 2024-2030. The increasing interest of the individuals in this industry is that the major reason for the expansion of this market.
The Top Key Players profiled in the report:
Eurofins Scientific SE, Evotec SE, WuXi AppTec, Syngene International Limited, Curia Global Inc., Pharmaron Beijing Co., Ltd., Piramal Enterprises Limited, Thermo Fisher Scientific Inc., Jubilant Pharmova Limited, GenScript Biotech Corporation, Oncodesign Services, and Selvita S.A.
Click the link to get a free sample copy of the report :
(*If you have any special requirements, please let us know and we will provide you with the report as you wish.)
Drug Discovery Services Market Segmentation:
Drug Discovery Services Market by Process, 2020-2029, (USD Billion)
Target Selection
Hit-to-lead
Drug Discovery Services Market by Drug Type, 2022-2029, (USD Billion)
Small Molecule
Biologics
Drug Discovery Services Market by Therapeutic Area, 2022-2029, (USD Billion)
Oncology
Neurology
Infectious
Based on geography, the global market for Drug Discovery Services and Disruptions has been segmented as follows:
North America (United States, Canada, Mexico)
South America (Brazil, Argentina, Ecuador, Chile)
Asia Pacific (China, Japan, India, Korea)
Europe (Germany, UK, France, Italy)
Middle East Africa (Egypt, Turkey, Saudi Arabia, Iran) and more.
Strategic Points Covered in Drug Discovery Services Market Directory:
To study and analyze the global market size (value & volume) by company, key regions/countries, products and application, history data, and forecast to 2030.
To understand the structure of market by identifying its various sub segments.
To share detailed information about the key factors influencing the growth of the market (growth potential, opportunities, drivers, industry-specific challenges and risks).
Focuses on the key global manufacturers, to define, describe and analyze the sales volume, value, market share, market competition landscape, SWOT analysis and development plans in next few years.
To analyze the growth trends, future prospects, and their contribution to the total market.
To project the value and volume of submarkets, with respect to key regions (along with their respective key countries).
To analyze competitive developments such as expansions, agreements, new product launches, and acquisitions in the market.
To strategically profile the key players and comprehensively analyze their growth strategies.
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the Drug Discovery Services
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the Drug Discovery Services market strategies, geographic and business segments of the leading players in the market.
Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the Drug Discovery Services
Take a look at the full report with detailed TOC here:
Some of the key questions scrutinized in the study are:
Which companies are expanding litanies of products with the aim to diversify product portfolio?
Which companies have drifted away from their core competencies and how have those impacted the strategic landscape of the Drug Discovery Services market?
Which companies have expanded their horizons by engaging in long-term societal considerations?
Which firms have bucked the pandemic trend and what frameworks they adopted to stay resilient?
What are the marketing programs for some of the recent product launches?
We offer customization on the Drug Discovery Services market report based on specific client requirements:
20% free customization.
Five Countries can be added as per your choice.
Five Companies can add as per your choice.
Free customization for up to 40 hours.
After-sales support for 1 year from the date of delivery.
Get More: https://exactitudeconsultancy.com/primary-research/
Thank you for your interest in the Drug Discovery Services Market research publications; you can also get individual chapters or regional/country report versions such as Germany, France, China, Latin America, GCC, North America, Europe or Asia……
Other Reports:
Patient Lifting Equipment market
Electronic Drug Delivery Systems market
Medical Aesthetics market
Vivo Toxicology market
Long-term Care Software market
About Us:
Exactitude Consultancy is a Market research & consulting services firm which helps its client to address their most pressing strategic and business challenges. Our professional team works hard to fetch the most authentic research reports backed with impeccable data figures which guarantee outstanding results every time for you. So, whether it is the latest report from the researchers or a custom requirement, our team is here to help you in the best possible way.
Contact: Exactitude Consultancy
PHONE NUMBER +1(704) 266-3234
EMAIL ADDRESS: [email protected]
#Drug Discovery Services Market#Drug Discovery Services Market growth#Drug Discovery Services Market size#Drug Discovery Services Market analysis 2024#Drug Discovery Services Market CAGR#Drug Discovery Services Growth#Drug Discovery Services Market 2024#Drug Discovery Services Market analysis by types#Drug Discovery Services Market data#Drug Discovery Services Market Outlook#Drug Discovery Services Market Size and Share#Drug Discovery Services Strategic Industry Latest News#Top Companies Analysis By Drug Discovery Services Market#Drug Discovery Services Market Trends#Drug Discovery Services Market Forecast#Drug Discovery Services Industry
0 notes
Link
Drug Discovery Services Market Trends and Insights By Drug Type (Small Molecule Drug and Biologics Drug), By Types Of Services (Drug Metabolism and...
#market research future#drug discovery services market#drug discovery services#drug discovery industry trends#drug discovery market growth
0 notes
Text
An Overview of the Age Related Macular Degeneration Market: Trends and Insights
The age-related macular degeneration (AMD) market is influenced by various trends and insights that shape its dynamics and growth trajectory.
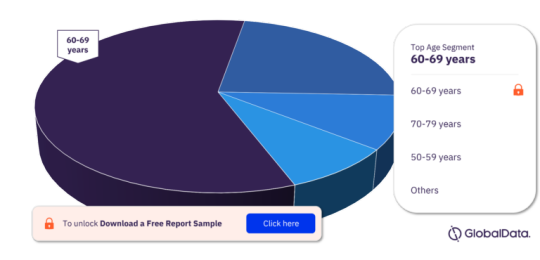
For more age segment insights, download a free report sample
Here's an overview of some key trends and insights in the AMD market:
Prevalence and Demographics: AMD primarily affects older adults, particularly those aged 50 and above. With global aging populations, the prevalence of AMD is increasing steadily. This demographic trend drives demand for AMD treatments and diagnostic solutions.
Advancements in Diagnostic Technologies: Innovations in diagnostic technologies, such as optical coherence tomography (OCT) and fundus autofluorescence imaging, have revolutionized the early detection and monitoring of AMD. Early diagnosis allows for timely intervention and management, potentially slowing disease progression and preserving vision.
Treatment Landscape Evolution: The treatment landscape for AMD has evolved significantly over the years. The introduction of anti-vascular endothelial growth factor (anti-VEGF) therapies, such as ranibizumab and aflibercept, has revolutionized the management of neovascular (wet) AMD, leading to improved visual outcomes for patients.
Emerging Therapeutic Approaches: Alongside anti-VEGF therapies, several emerging therapeutic approaches are being explored for AMD treatment. These include novel drug delivery systems, gene therapies, complement inhibitors, and regenerative medicine approaches aimed at addressing various aspects of AMD pathophysiology.
Personalized Medicine: There is a growing emphasis on personalized medicine in AMD treatment, driven by the recognition of inter-individual variability in treatment response and disease progression. Biomarkers, genetic testing, and imaging biomarkers are being investigated to tailor treatment strategies based on individual patient characteristics.
Healthcare Policy and Reimbursement Landscape: Reimbursement policies and healthcare regulations influence access to AMD treatments and diagnostic services. Variations in reimbursement policies across regions can impact market access and adoption rates for innovative therapies.
Patient-Centric Care Models: Patient-centric care models are gaining prominence in AMD management, focusing on holistic patient care, education, and support services. Multidisciplinary care teams comprising ophthalmologists, optometrists, retinal specialists, and allied healthcare professionals play a crucial role in delivering comprehensive care to AMD patients.
Research and Development Investments: Pharmaceutical companies, biotechnology firms, and academic institutions continue to invest heavily in AMD research and development (R&D). Clinical trials are underway to explore novel therapeutic targets, combination therapies, and innovative treatment modalities to address unmet needs in AMD management.
Global Market Expansion: The AMD market is expanding globally, with significant growth opportunities in emerging markets such as Asia-Pacific and Latin America. Rising healthcare expenditure, improving healthcare infrastructure, and increasing awareness of AMD contribute to market growth in these regions.
Collaborations and Partnerships: Collaboration between industry players, research organizations, and healthcare providers is essential for driving innovation and accelerating the development of new AMD therapies and diagnostic solutions. Collaborative efforts facilitate knowledge sharing, resource pooling, and the translation of scientific discoveries into clinical practice.
In summary, the AMD market is characterized by ongoing advancements in diagnostics and therapeutics, a shift towards personalized medicine, and a focus on patient-centric care models. Continued investments in research, collaborations, and global market expansion efforts are expected to shape the future landscape of AMD management.
0 notes
Text
Pharmaceutical API Manufacturing Market Size, Volume, Demand, Outlook | BIS Research

Pharmaceutical Active Pharmaceutical Ingredient (API) manufacturing is a critical aspect of the pharmaceutical industry, serving as the foundation for the development and production of various drugs. API manufacturing involves the synthesis, extraction, and purification of chemical compounds that serve as the active components in pharmaceutical formulations.
The global highly potent API market is projected to reach $84.20 billion by 2033 from $27.44 billion in 2023, growing at a CAGR of 11.86% during the forecast period 2023-2033.
Pharmaceutical API Manufacturing Overview
Pharmaceutical Active Pharmaceutical Ingredient (API) manufacturing is the process of synthesizing, extracting, and purifying chemical compounds that serve as the active components in pharmaceutical drugs. APIs are essential for the efficacy and therapeutic effects of medicines.
Applications of Pharmaceutical API Manufacturing Market
Drug Development
Generic Drug Production
Contract Manufacturing
Nutraceuticals and Dietary Supplements
Emerging Markets
Veterinary Medicine
Download our sample page click here !
Pharmaceutical API Manufacturing Market Segmentation
Segmentation 1: by Type
Segmentation 2: by Therapeutic Area
Segmentation 3: by Type of Manufacturing
Segmentation 4: by Type of Synthesis
Segmentation 5: by End User
Segmentation 6: by Region
China dominated the Asia-Pacific highly potent API market in 2022. The country has a growing population with cancer, and hormonal disorder. Highly potent API offers effective and accessible results for these conditions.
Key Companies are as follows
Almac Group
Asymchem Inc.
BASF Pharma Solutions
CARBOGEN AMCIS
CordenPharma International
Market Drivers and Trends
Drivers
Increasing demand for pharmaceuticals
Patent Expirations of Blockbuster Drugs
Increasing Focus on Personalized Medicine
Emerging market growth
Trends
Speciality API
Continuous Manufacturing
Biosimilars and Complex Generics
These market drivers and trends shape the evolving landscape of the Pharmaceutical API Manufacturing Market, driving innovation, efficiency, and competitiveness in the industry.
Recent Developments in the Highly potent API Market
In December 2022, Almac concluded the initial phase of its good manufacturing practice (GMP) active pharmaceutical ingredient (API) facility expansion as part of a multi-million-pound investment program.
In October 2022, Asymchem Inc., a prominent global provider of contract development and manufacturing services, and AUM Biosciences (AUM), a global biotech company in the clinical stage, with a focus on the discovery, acquisition, and development of next-generation targeted oncology therapeutics, jointly declared the successful conclusion of their inaugural GMP production campaign for AUM601.
Click here to have a look at Life Sciences & Biopharma page !
Key Question Answers
Q What is the estimated global market size for the highly potent API market?
Q What are the different types of highly potent API market available in the market?
Q How has the COVID-19 outbreak affected the future trajectory of the highly potent API market?
Q What are the key trends influencing the global highly potent API market, and what is their potential for impacting the market?
Q What does the patent landscape of the global highly potent API market look like? Which year and country witnessed the maximum patent filing between January 2020 and December 2023?
Conclusion
In conclusion, the Pharmaceutical API Manufacturing Market stands as a cornerstone of the pharmaceutical industry, providing the essential active ingredients necessary for the development and production of life-saving medications, growing demand for pharmaceuticals driven by factors such as the prevalence of chronic diseases and an aging population, the market continues to expand.
0 notes
Text
Artificial Intelligence in Healthcare Market to Witness an Outstanding Growth by 2033
Market Definition
Artificial intelligence (AI) in healthcare is a broad term that covers a wide range of applications and technologies. AI technologies can be used to help doctors and other healthcare professionals diagnose and treat diseases, make predictions about patient health, and improve the efficiency of care delivery. AI is also being used to develop new drugs and personalized treatments, and to create digital assistants that can help patients manage their health.
Market Outlook
The global Artificial Intelligence in Healthcare market was valued at USD 12.4 Billion in 2022 and it is anticipated to grow up to USD 45.6 Billion by 2032, at a CAGR of 13.9% during the forecast period.
The market is driven by several factors, including the need to manage data more effectively and optimize healthcare costs, the growth of public-private partnerships, and the increased regional spending on healthcare. Additionally, the market is anticipated to grow as opportunities in geriatric population care with AI technology, imaging, and diagnostics to generate data for research development arise. Moreover, the growing shortage of healthcare workforce drove the adoption of AI/ML technologies. Therefore, AI algorithms can be trained to analyze patient health information which further supports care providers in quickly diagnosing the condition and devising an accurate treatment regime.
Small-molecule drug discovery benefits from AI in four ways; access to new biology, improved or unique chemistry, higher success rates, and speedier and less expensive discovery procedures. For instance, FDA’s Center for Drug Evaluation and Research (CDER) approved 50 brand-new pharmaceutical and biological products in 2021. About 33 of the 50 novel medications and biological products approved for use had tiny molecules, while 17 were monoclonal antibodies and other large molecules. Such aforementioned factors boosting the growth of the market and accelerating the adoption rate of AI in healthcare.
To Know More@ https://www.globalinsightservices.com/reports/artificial-intelligence-in-healthcare-market
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample@ https://www.globalinsightservices.com/request-sample/GIS20011/
Global Artificial Intelligence in Healthcare Market Segmentation
By Component
Software Solutions
Hardware
Services
By Application
Robot-Assisted Surgery
Clinical Trials
Hospital Workflow
Therapy Planning
Wearables
Virtual Assistants
Medical Imaging & Diagnosis
Others
Request Customization@ https://www.globalinsightservices.com/request-customization/GIS20011/
Major Players in the Global Artificial Intelligence in Healthcare Market
The overall competitive rivalry remains moderately high in the market studied. The growing presence of big players in the industry is expected to intensify competitive rivalry during the forecast period. The global Bioactive Ingredients Market report includes players such as AiCure, APIXIO, Inc., Atomwise, Inc, Butterfly Network, Inc., Cyrcadia Health Inc, Enlitic, Inc, IBM Corporation, iCarbonX, Insilico Medicine Inc, NVIDIA Corporation, others.
Request Discounted Pricing@ https://www.globalinsightservices.com/request-special-pricing/GIS20011/
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis.
Buy your copy here@ https://www.globalinsightservices.com/checkout/single_user/GIS20011/
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1-833-761-1700
Website: https://www.globalinsightservices.com/
About Global Insight Services:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
0 notes
Text
Pharmacovigilance Market 2024 Key Factors and Emerging Opportunities with Current Trends Analysis 2031
According to recent market analysis, the pharmacovigilance market was valued at USD 7.05 billion in 2023 and is projected to reach USD 12.03 billion by 2031, growing at a compound annual growth rate (CAGR) of 6.9% during the forecast period of 2024-2031. This substantial growth underscores the importance of pharmacovigilance in ensuring drug safety and public health.
Emerging Trends and Opportunities
Technological Advancements: The integration of advanced technologies such as artificial intelligence (AI), machine learning (ML), and big data analytics is revolutionizing pharmacovigilance processes. These technologies enable more efficient adverse event detection, signal detection, and risk management, thereby enhancing patient safety.
Expansion of Outsourcing Services: Pharmaceutical companies are increasingly outsourcing pharmacovigilance activities to specialized service providers. This trend is driven by the need for cost-effective solutions, expertise in regulatory compliance, and access to advanced technology platforms.
Focus on Real-World Evidence (RWE): There is a growing emphasis on leveraging real-world data to complement traditional clinical trial data in pharmacovigilance. RWE provides valuable insights into drug safety and effectiveness in real-world patient populations, contributing to more informed decision-making by regulatory authorities and healthcare stakeholders.
Globalization of Clinical Trials: The globalization of clinical trials has resulted in diverse patient populations and increased regulatory complexities. Pharmacovigilance plays a crucial role in monitoring drug safety across different geographical regions, ensuring compliance with regulatory requirements, and addressing unique healthcare challenges in various markets.
Download Free Sample Copy of Report: https://www.snsinsider.com/sample-request/3095
Key Drivers Propelling Growth
Rising Incidence of Adverse Drug Reactions (ADRs): The increasing use of pharmaceuticals and biopharmaceuticals has led to a higher prevalence of adverse drug reactions, driving the demand for pharmacovigilance services to monitor and mitigate these risks.
Stringent Regulatory Requirements: Regulatory agencies worldwide are implementing stricter pharmacovigilance regulations to enhance drug safety and public health. Compliance with these regulations necessitates robust pharmacovigilance systems and processes, driving market growth.
Growing Pharmaceutical Industry: The pharmaceutical industry's continued expansion, fueled by advancements in drug discovery and development, is contributing to the growth of the pharmacovigilance market. Pharmaceutical companies are investing in pharmacovigilance to ensure the safety and efficacy of their products throughout their lifecycle.
Challenges and Considerations
Data Quality and Integration: The quality and integration of pharmacovigilance data from disparate sources remain significant challenges. Ensuring data accuracy, completeness, and standardization is essential for effective signal detection and risk management.
Resource Constraints: Pharmacovigilance activities require skilled personnel, sophisticated technology infrastructure, and substantial financial resources. As the volume and complexity of pharmacovigilance tasks increase, companies may face challenges in resource allocation and capacity building.
Regulatory Compliance: Keeping pace with evolving regulatory requirements poses challenges for pharmaceutical companies and pharmacovigilance service providers. Compliance with diverse regulations across multiple jurisdictions requires continuous monitoring, adaptation, and investment in regulatory intelligence.
Data Privacy and Security: With the digitization of pharmacovigilance processes and the increasing use of electronic health records, ensuring data privacy and security is paramount. Regulatory compliance, data encryption, and cybersecurity measures are critical considerations in safeguarding sensitive pharmacovigilance data.
Key Takeaways from the Market
The pharmacovigilance market is poised for substantial growth and evolution in the coming years, driven by technological advancements, regulatory dynamics, and the expanding pharmaceutical industry. Key takeaways include:
Focus on Innovation: Innovation in technology and methodologies will continue to drive efficiency and effectiveness in pharmacovigilance processes.
Collaboration and Partnerships: Collaboration between pharmaceutical companies, regulatory agencies, and pharmacovigilance service providers is essential for advancing drug safety initiatives and addressing emerging challenges.
Investment in Talent and Infrastructure: Investment in talent development and technology infrastructure is crucial for building robust pharmacovigilance capabilities and ensuring compliance with evolving regulatory requirements.
Adaptation to Regulatory Changes: Proactive monitoring of regulatory changes and swift adaptation of pharmacovigilance practices are imperative for maintaining compliance and sustaining business growth in a dynamic regulatory landscape.
In conclusion, the pharmacovigilance market's growth trajectory reflects its critical role in safeguarding patient safety and ensuring the efficacy of pharmaceutical products. Embracing emerging trends, overcoming challenges, and capitalizing on opportunities will be key to unlocking the full potential of pharmacovigilance in the global healthcare landscape.
0 notes
Link
0 notes
Text
Understanding the Dynamics of the Psoriasis Market: Drivers, Barriers, and Future Outlook
Psoriasis is a chronic autoimmune skin condition characterized by patches of red, inflamed skin covered with silvery scales. It occurs when the immune system mistakenly attacks healthy skin cells, leading to rapid turnover of skin cells. Normally, skin cells are replaced every 3 to 4 weeks, but in psoriasis, this process happens every few days, resulting in a buildup of cells on the skin's surface.
Psoriasis Market Drivers
The psoriasis market is influenced by several key drivers that shape the development of treatments, therapies, and overall market dynamics. Some of the significant drivers in the psoriasis market include:
Increasing Prevalence: Psoriasis is a common chronic skin condition, affecting millions of people worldwide. As the prevalence of psoriasis continues to rise, particularly in developed countries, there is a growing demand for effective treatments and therapies.
Advancements in Research and Development: Ongoing research and development efforts in the field of dermatology and immunology have led to the discovery of novel therapeutic targets and treatment modalities for psoriasis. This includes the development of biologic drugs, small molecule inhibitors, and targeted therapies that offer improved efficacy and safety profiles compared to traditional treatments.
Expanding Treatment Options: The psoriasis market has witnessed the introduction of a wide range of treatment options, including topical medications, phototherapy, systemic medications, and biologic agents. The availability of diverse treatment modalities allows healthcare providers to tailor treatment plans to individual patient needs, improving overall management and outcomes.
Focus on Personalized Medicine: There is a growing emphasis on personalized medicine in the treatment of psoriasis, driven by advances in genomics, biomarker research, and precision medicine approaches. By identifying specific genetic, immunologic, and molecular factors associated with psoriasis, healthcare providers can develop targeted therapies that are more effective and well-tolerated by patients.
Patient Awareness and Advocacy: Increased awareness about psoriasis among patients, healthcare providers, and the general public has led to greater recognition of the impact of the condition on quality of life and overall health. Patient advocacy organizations play a crucial role in raising awareness, promoting research, and advocating for improved access to treatment and healthcare services for individuals with psoriasis.
Growing Healthcare Expenditure: Rising healthcare expenditure, particularly in developed countries, has facilitated greater investment in the development and commercialization of new psoriasis treatments and therapies. Pharmaceutical companies are incentivized to innovate and bring new products to market to address the unmet needs of patients with psoriasis.
Shift towards Biologic Therapies: Biologic drugs have revolutionized the treatment of moderate to severe psoriasis, offering higher efficacy and lower risk of adverse effects compared to traditional systemic medications. The increasing adoption of biologic therapies, coupled with the introduction of biosimilars, has contributed to the growth of the psoriasis market.
Emerging Markets: Emerging markets, particularly in Asia-Pacific and Latin America, present significant growth opportunities for the psoriasis market. Factors such as improving healthcare infrastructure, rising disposable incomes, and increasing awareness about psoriasis are driving demand for advanced treatment options in these regions.
Psoriasis Market Barriers
Limited Understanding of Disease Pathogenesis: Despite significant progress in understanding the underlying mechanisms of psoriasis, there are still gaps in knowledge regarding its precise etiology and pathogenesis. This limited understanding can impede the development of targeted therapies and personalized treatment approaches.
Complexity of Psoriasis Treatment: Psoriasis is a complex and heterogeneous disease, with varying clinical manifestations and treatment responses among patients. Developing effective treatments that address the diverse needs of patients with different disease phenotypes and comorbidities can be challenging.
High Development Costs: The development of new psoriasis treatments, particularly biologic drugs and targeted therapies, involves substantial research and development costs, as well as lengthy and expensive clinical trials. High development costs can deter pharmaceutical companies from investing in psoriasis drug development, especially for niche patient populations or less profitable markets.
Regulatory Hurdles: Regulatory approval processes for new psoriasis treatments can be rigorous and time-consuming, requiring extensive preclinical and clinical data to demonstrate safety, efficacy, and quality. Delays in regulatory approval can prolong the time to market and increase development costs for pharmaceutical companies.
Market Saturation and Competition: The psoriasis market is becoming increasingly crowded, with multiple treatment options available, including generics, biosimilars, and novel therapies. Market saturation and intense competition can limit the commercial success of new psoriasis treatments, particularly if they offer marginal improvements over existing therapies.
Access and Affordability Issues: Access to psoriasis treatments, especially advanced biologic drugs, may be limited in certain regions or healthcare systems due to factors such as high costs, reimbursement policies, and healthcare disparities. Affordability issues can prevent some patients from accessing the treatments they need, leading to disparities in healthcare outcomes.
Safety Concerns and Adverse Effects: While biologic drugs have revolutionized the treatment of psoriasis, they are associated with certain safety concerns, including an increased risk of infections, malignancies, and immune-mediated adverse events. Safety concerns and adverse effects may influence treatment decisions and patient adherence, particularly in the long term.
Stigma and Psychosocial Impact: Psoriasis is often associated with stigma and negative psychosocial effects, including low self-esteem, depression, and social isolation. The psychosocial impact of psoriasis can affect patient quality of life and treatment outcomes, highlighting the need for holistic approaches to psoriasis care.
Future Psoriasis Market Analysis
Analyzing the future of the psoriasis market involves considering various factors that could influence its trajectory, including emerging trends, technological advancements, regulatory developments, and evolving patient needs. Here's a prospective analysis of the future psoriasis market:
Biologic Dominance: Biologic drugs have transformed the treatment landscape for moderate to severe psoriasis. In the future, biologics are likely to maintain their dominance, with continued development of novel agents targeting specific pathways involved in psoriasis pathogenesis. This includes next-generation biologics with improved efficacy, safety profiles, and dosing regimens.
Biosimilars Expansion: The market for biosimilar versions of biologic drugs used in psoriasis treatment is expected to expand further. Biosimilars offer cost savings and increased access to treatment, driving their adoption in both developed and emerging markets. However, regulatory pathways and market acceptance will continue to shape the pace of biosimilar uptake.
Personalized Medicine: Advances in genomics, biomarker research, and precision medicine will facilitate the development of personalized treatment approaches for psoriasis. Biomarker-based diagnostics and targeted therapies tailored to individual patient characteristics will improve treatment outcomes and minimize adverse effects. Pharmacogenomic testing may also play a role in optimizing treatment selection and dosing.
Innovative Therapies: Beyond biologics, there is growing interest in innovative therapies for psoriasis, including small molecule inhibitors, gene therapies, and cell-based therapies. These approaches offer potential advantages such as oral administration, enhanced specificity, and long-term remission. Early-stage research in these areas shows promise, but further clinical validation and commercialization are needed.
Digital Health Solutions: Digital health technologies, including telemedicine, mobile apps, wearables, and remote monitoring devices, will increasingly integrate into psoriasis management. These tools enable remote patient monitoring, medication adherence tracking, lifestyle management support, and access to virtual consultations with healthcare providers. Digital health solutions enhance patient engagement, improve treatment adherence, and facilitate personalized care delivery.
Patient-Centric Care: There is a growing emphasis on patient-centered care in psoriasis management, recognizing the importance of addressing patients' holistic needs and preferences. Multidisciplinary care models that integrate dermatologists, rheumatologists, psychologists, and other healthcare professionals will become more prevalent. Patient support programs, educational resources, and peer-to-peer networks will also play a crucial role in empowering individuals living with psoriasis.
Health Equity and Access: Efforts to improve health equity and access to psoriasis care will remain a priority. This includes addressing disparities in healthcare access, affordability, and treatment outcomes across different demographic groups and geographic regions. Public health initiatives, advocacy campaigns, and policy interventions can help reduce barriers to care and promote equitable access to effective treatments for all individuals with psoriasis.
Research and Collaboration: Continued investment in psoriasis research, clinical trials, and collaborative initiatives will drive innovation and evidence-based practice in the field. Academic-industry partnerships, collaborative research networks, and patient registries will facilitate data sharing, knowledge exchange, and the development of consensus guidelines for psoriasis management.
Evolving Psoriasis Treatment Outlook
The outlook for psoriasis treatment is evolving rapidly, driven by advances in scientific research, technological innovation, and a growing understanding of the disease's underlying mechanisms. Here's an overview of the evolving treatment outlook for psoriasis:
Targeted Therapies: There is a shift towards targeted therapies that specifically inhibit key molecules and pathways involved in psoriasis pathogenesis. These therapies offer enhanced efficacy and safety compared to traditional systemic treatments. Examples include biologic agents targeting interleukin (IL)-17, IL-23, and Janus kinase (JAK) pathways, as well as small molecule inhibitors of phosphodiesterase 4 (PDE4) and tyrosine kinases.
Personalized Medicine: Personalized medicine approaches are increasingly being explored in psoriasis treatment. Biomarker research, genetic profiling, and pharmacogenomics enable healthcare providers to tailor treatment strategies to individual patient characteristics, optimizing efficacy and minimizing adverse effects. Personalized medicine may involve selecting the most appropriate therapy based on disease phenotype, genetic profile, biomarker expression, and treatment response predictors.
Topical Therapies: While systemic therapies and biologics dominate the landscape for moderate to severe psoriasis, there are ongoing efforts to improve topical treatment options. Novel topical formulations, including combination therapies, nanotechnologies, and sustained-release formulations, aim to enhance drug delivery, increase efficacy, and minimize side effects. Topical treatments remain an important component of psoriasis management, especially for mild to moderate cases and localized disease.
Alternative Delivery Methods: Innovations in drug delivery methods are expanding treatment options for psoriasis. These include oral formulations, injectables, patches, implants, and microneedle arrays. Alternative delivery methods offer advantages such as improved convenience, reduced dosing frequency, enhanced patient adherence, and targeted drug delivery to affected skin areas. Novel delivery systems also enable the administration of biologic agents via routes other than subcutaneous or intravenous injection.
Combination Therapies: Combination therapy approaches are being explored to maximize treatment efficacy and address different aspects of psoriasis pathophysiology. Combinations of biologic agents with conventional systemic therapies, topical treatments, phototherapy, or other biologics may offer synergistic effects and improved outcomes compared to monotherapy. Rational drug combinations targeting multiple pathways involved in psoriasis pathogenesis hold promise for achieving better control of disease activity and reducing treatment resistance.
Long-term Management Strategies: The focus is shifting towards long-term management strategies that aim to achieve sustained remission, prevent disease progression, and minimize relapses. Treat-to-target approaches, maintenance therapy regimens, and proactive management strategies are being increasingly emphasized. Early intervention, regular monitoring, and individualized treatment adjustments based on disease activity and patient preferences are key principles of long-term management.
Digital Health Integration: Digital health technologies are revolutionizing psoriasis care delivery and patient engagement. Telemedicine platforms, mobile apps, wearable devices, and electronic health records enable remote monitoring, self-management, and virtual consultations. Digital health tools support treatment adherence, lifestyle modifications, and patient education, empowering individuals with psoriasis to actively participate in their care and improve treatment outcomes.
Patient-Centered Care Models: Patient-centered care models prioritize the holistic needs and preferences of individuals living with psoriasis. Multidisciplinary care teams, shared decision-making, and patient support programs play a central role in delivering comprehensive, patient-centric care. Psychosocial support, educational resources, and peer-to-peer networks help address the emotional and social impact of psoriasis, enhancing overall well-being and quality of life.
Role of Companies in the Psoriasis Market
In the Psoriasis market, companies such as Hangzhou Highlightll Pharmaceutical Co., Ltd, Biohaven Pharmaceuticals, Inc., SFA Therapeutics, Bristol-Myers Squibb, Ventyx Biosciences, Inc, Amgen, AbbVie, Alumis Inc, DICE Therapeutics, Inc., UCB Pharma, Janssen Research & Development, LLC, Arcutis Biotherapeutics, Inc., AnaptysBio, Inc., Sun Pharmaceutical Industries Limited, Novartis, Pfizer, Boehringer Ingelheim, KoBioLabs, Abcentra, Aclaris Therapeutics, and others play a pivotal role in driving innovation, research, development, and the provision of treatments and therapies for individuals suffering from this chronic inflammatory skin condition. These companies encompass pharmaceutical giants, biotechnology firms, medical device manufacturers, and healthcare service providers, each contributing uniquely to the advancement of Psoriasis management. Pharmaceutical companies lead the charge in developing novel drugs, ranging from topical corticosteroids to biologics targeting specific immune pathways implicated in Psoriasis pathogenesis.
Psoriasis Market Outlook - Key Conclusion and Analysis
The Psoriasis market is undergoing a transformative period, driven by advances in research, innovation in therapeutic approaches, and shifting treatment paradigms. While significant progress has been made in improving outcomes for patients with Psoriasis, several barriers continue to challenge the market's expansion, including high treatment costs, safety concerns, and regulatory hurdles. Looking ahead, personalized medicine, novel therapeutic targets, and digital health solutions are poised to shape the future of Psoriasis management, offering new hope for patients and caregivers alike. Efforts to address these challenges and capitalize on emerging opportunities will be critical in advancing the field and ultimately improving the lives of individuals living with Psoriasis.
Get a more detailed overview, at: Psoriasis Market Outlook and Forecast
0 notes
Text
Multiplex Assay Market: Growth Assessment 2024-2032
The global multiplex assay market is set to advance at a CAGR of 8.56% during the forecast period of 2024 to 2032. Get more insights into our latest reports
As per Triton Market Research, the Global Multiplex Assay Market report is segmented by Product and Service (Instruments and Accessories, Reagents and Consumables, Software and Services), Application (Clinical Diagnostics, Research and Development, Companion Diagnostics), Industry Vertical (Research Institutes, Pharmaceutical & Biotechnology Companies, Clinical Laboratories, Hospitals), Type (Nucleic Acid-Based Multiplex Assay, Protein-Based Multiplex Assay, Other Multiplex Assays), Technology (Multiplex PCR, Multiplex Protein Microarray, Other Technologies), and Regional Outlook (Middle East and Africa, North America, Europe, Asia-Pacific, Latin America).
The report highlights the Market Summary, Industry Outlook, Impact Analysis, Porter’s Five Forces Analysis, Key Buying Impact Analysis, Market Attractiveness Index, Key Market Strategies, Market Drivers, Challenges, Opportunities, Analyst Perspective, Competitive Landscape, Research Methodology and scope, Global Market Size, Forecasts & Analysis (2024-2032).
According to Triton’s market research report, the global multiplex assay market is set to advance at a CAGR of 8.56% during the forecast period 2024-2032.

A multiplex assay is a technique for simultaneously detecting and quantifying various analytes, including proteins, biomolecules, growth factors, and cytokines. It enhances the efficiency of analysis by amplifying multiple targets in a polymerase chain reaction (PCR).
Factors such as technological advancements, rapid growth of companion diagnostics, and growth in drug discovery initiatives create lucrative opportunities for the multiplex assay market. Multiplex assays encompass various research technologies, ranging from basic Petri dishes to automated robotics tailored for High Content Screening (HCS). Embracing target-based drug discovery models with a focus on cell and systems biology promises to mitigate failures and reduce costs in the initial stages of drug development.
However, issues related to cross-reactivity and assay interference, challenges in achieving adequate assay range, and lack of skilled professionals hamper the expansion of the studied market.
The Asia-Pacific is projected to witness the fastest growth over the forecast period. The market experiences growth due to a surge in demand for healthcare infrastructure, the expansion of hospitals in emerging nations, and the development of the R&D sector. Additionally, healthcare reforms and technological progress in the field contribute to this growth.
The well-known companies in the multiplex assay market consist of Abcam Limited, Agilent Technologies Inc, Becton, Bio-Rad Laboratories Inc, Seegene Inc, Dickinson and Company, Bio-Techne Corporation, F Hoffmann-la Roche Ltd, Merck Millipore, Olink Proteomics AB, Illumina Inc, Qiagen NV, Randox Laboratories Ltd, Siemens Healthineers AG, and Thermo Fisher Scientific Inc.
The forecast suggests that the multiplex assay market will witness a transition from a moderate to a significant level of competition due to potential new entrants. Moreover, the rising incidence of various diseases, including neurological disorders, cancer, and infectious ailments, is set to open avenues for emerging enterprises to conduct research and innovate in the field of pharmaceuticals, technologies, and therapeutic interventions for these health conditions.
#multiplex assay#Lifesciences#Diagnostic & Biotechnology#triton market research#market research reports
0 notes
Link
#market research future#drug discovery services market#drug discovery services#drug discovery industry trends#drug discovery market growth
0 notes
Text
Drug Discovery Outsourcing Market Size & Demand by 2033

Between 2023 and 2033, the Global Drug Discovery Outsourcing Industry is projected to grow at a consistent compound annual growth rate of 7.2%. The market, which is projected to be valued at US$ 3.75 billion in 2023, is expected to surpass a market share of US$ 7.52 billion by 2033.Expanding the pharmaceutical industry and the higher penetration of digital technology and AI integration are transforming the market.
Furthermore, the research and development procedure and the expansion of drug discovery vendors in emerging economies like China and India fuel the market growth.A massive portion of the research around small and large drug molecules (80%) is outsourced through pharma giants. This is due to the cheaper costs, easy workflow, increased workforce, and enhanced quality.
The transformed drug industry with synergistic drugs and advanced anti-infection drugs is also shaping the market dynamics. At the same time, virus outbreaks such as Ebola and Coronavirus are increasing the market’s consumer base.Government policies integrate their medical policies, allowing pharma giants to collaborate and build the advanced drug discovery space.
Thus, the demand for outsourced drug delivery services is in high demand.The surge in respiratory patients with issues like tuberculosis, bronchitis, etc., also consumes a big chunk of the market. These conditions have increased lately due to the post-corona impacts.
Request a Sample PDF of this Reporthttps://www.futuremarketinsights.com/reports/sample/rep-gb-16937
0 notes
Text
Epigenetic Market Report Is Booming Worldwide | Bis Research Report
DNA methylation detection technology stands out as a crucial tool for understanding gene regulation, epigenetics, and disease mechanisms. With advancements in genomics and epigenetics, the global DNA methylation detection technology market has been witnessing remarkable growth, driven by the increasing demand for personalized medicine, precision oncology, and epigenetic research.
The global DNA methylation detection technology market is projected to experience substantial growth over the forecast period 2023-2033. Moreover, the market value for 2023 was $2.80 billion, which is expected to reach $12.32 billion by 2023, growing at a CAGR of 15.96%
DNA Methylation Detection Technology Overview
DNA methylation is an epigenetic mechanism that involves the addition of a methyl group to the DNA molecule, typically at cytosine bases within CpG dinucleotides. This modification plays a pivotal role in regulating gene expression, genomic stability, and cellular differentiation.
Market Segmentation
Segmentation 1: by Product
Segmentation 2: by Technology
Segmentation 3: by Application
Segmentation 4: by End User
Grab a look at our free sample page click here !
Epigenetic Market
The field of epigenetics, which focuses on changes in gene expression that do not involve alterations to the DNA sequence itself, has gained significant attention in recent years for its potential in understanding and treating various diseases.
Various factors involved are market growth, technological advancements, therapeutic potential
The Epigenetic Market Report forecasts continued expansion of the epigenetic market, fueled by ongoing research advancements, increasing investment in biotechnology and pharmaceutical sectors, and growing adoption of epigenetic-based diagnostics and therapeutics.
Applications of Epigenetics
Disease Biomarker Discovery
Cancer Diagnostics and Prognostics
Drug Discovery and Development
Epigenetic Editing
Agricultural Biotechnology
Epigenetic market focuses on understanding how environmental factors influence gene expression and cellular phenotype without altering the DNA sequence.
Epigenetic market is characterized by rapid innovation driven by advances in technology and increasing recognition of the importance of epigenetics in health and disease.
Market Drivers for Epigenetic Market Report
Factors drive the growth and development of the epigenetic market are as follows
Increasing Research Activities
Rising Incidence of Chronic Diseases
Advancements in Genomic Technologies
Growing Awareness and Expansion
Regulatory Support and Initiatives
The market drivers collectively contribute to the growth and expansion of the epigenetic market, fueling advancements in research, diagnostics, therapeutics, and agricultural biotechnology.
Role of Epigenetics in DNA Methylation Detection Technology
Some key aspects of the epigenetic market's role in DNA methylation detection include:
Research Tools and Kits
High Throughput Technologies
Epigenetic Analysis Services
Therapeutic Target Identification
Development of Biomarkers
The epigenetic market plays a multifaceted role in DNA methylation detection, providing essential tools, services, and expertise that drive research advancements and clinical applications in the field of epigenetics
Recent Developments in the Global DNA Methylation Detection Technology Market
Pacific Biosciences of California, Inc., and GeneDx initiated a research collaboration with the University of Washington. This partnership focuses on studying the effectiveness of long-read whole genome sequencing in improving diagnostic accuracy in neonatal care.
Ultima Genomics, Inc. announced a partnership with New England Biolabs to integrate NEB's NEBNext reagents and library preparation kits into Ultima's sequencing platforms. This collaboration would develop and optimize next-generation sequencing (NGS) processes for DNA, RNA, and methylation sequencing applications.
Key Players
Agilent Technologies, Inc.
Abcam plc.
Bio-Rad Laboratories, Inc.
Illumina, Inc.
QIAGEN N.V.
Thermo Fisher Scientific, Inc.
And many others
Visit our Precision medicine page for more understanding!
Key Questions Answered
▪ What is the estimated global market size for the global DNA methylation detection technology market?
▪ Who are the key players in the global DNA methylation detection technology market?
▪ What are the different types of products available in the global DNA methylation detection technology market?
▪ Which region holds the largest market share in the global DNA methylation detection technology market?
▪ How has the COVID-19 outbreak affected the future trajectory of the global DNA methylation detection technology market?
Conclusion:
The global DNA methylation detection technology market holds immense potential for driving advancements in basic research, clinical diagnostics, and personalized medicine. With ongoing technological innovations, expanding applications, and growing investments in epigenetics research, this market is poised for continued growth and innovation, contributing to our understanding of human health and disease at the molecular level.
0 notes
Text
Artificial Intelligence in Healthcare Market Size and Forecast to 2030
The size of the global artificial intelligence in healthcare market reached USD 14.1 billion in 2023 and is projected to exceed USD 34.2 billion by 2030, demonstrating a growth rate of more than 13.5% from 2024 to 2030.
Market Definition:
Artificial Intelligence (AI) in healthcare refers to the utilization of advanced computational algorithms and data analytics techniques to interpret complex medical data, enhance decision-making processes, and improve patient outcomes within the healthcare sector. AI systems in healthcare encompass a wide range of applications, including medical image analysis, predictive analytics, personalized treatment planning, virtual health assistants, and drug discovery. By leveraging vast amounts of patient data, AI algorithms can identify patterns, detect anomalies, and provide valuable insights to healthcare professionals, leading to more accurate diagnoses, tailored treatment plans, and proactive disease management. Ultimately, the integration of AI technologies into healthcare workflows has the potential to revolutionize the delivery of medical services, optimizing resource utilization, reducing healthcare costs, and enhancing overall quality of care for patients worldwide.
Artificial Intelligence in Healthcare Market Drivers and Trends:
Rising Demand for Personalized Medicine
The rising demand for personalized medicine is a significant driving factor of the artificial intelligence in healthcare market. Personalized medicine aims to tailor medical treatment to the individual characteristics of each patient, considering factors such as genetic makeup, lifestyle, and environmental influences. This approach offers the potential for more effective and targeted interventions, improving patient outcomes and reducing adverse reactions to treatments.
AI plays a crucial role in realizing the promise of personalized medicine by analyzing vast amounts of patient data to identify patterns, correlations, and predictive markers. Machine learning algorithms can sift through complex genetic data, medical imaging scans, and clinical records to uncover insights that enable healthcare providers to develop personalized treatment plans tailored to each patient’s unique needs.
Furthermore, AI-driven predictive analytics empower healthcare professionals to anticipate disease progression, identify individuals at higher risk of developing certain conditions, and intervene pre-emptively to prevent or mitigate adverse health outcomes. By harnessing AI technologies, healthcare providers can deliver more proactive, preventive, and precise care, ultimately leading to better patient outcomes and reduced healthcare costs.
The growing adoption of personalized medicine coupled with advancements in AI algorithms is driving increased investment and innovation in AI-driven healthcare solutions. As the healthcare industry continues to embrace personalized approaches to patient care, the demand for AI technologies to support these initiatives is expected to grow, further fueling the expansion of the artificial intelligence in healthcare market.
Growing Awareness of AI’s Potential Benefits
Growing awareness of AI’s potential benefits is a significant driving factor propelling the artificial intelligence in healthcare market forward. As stakeholders across the healthcare ecosystem become more informed about the capabilities of AI, they increasingly recognize its potential to transform healthcare delivery and improve patient outcomes. Healthcare providers are acknowledging AI’s capacity to enhance diagnostic accuracy, streamline administrative tasks, and optimize treatment planning, leading to more efficient and effective patient care.
Moreover, as patients become more aware of AI’s potential benefits, they are more willing to embrace AI-driven technologies to monitor their health, receive personalized treatment recommendations, and actively participate in their healthcare decision-making process. This heightened awareness among patients contributes to the growing demand for AI-enabled healthcare solutions.
Furthermore, regulatory agencies are also recognizing the importance of AI in healthcare and are actively working to establish guidelines and standards to ensure the safe and ethical use of AI technologies. This regulatory support provides assurance to healthcare providers and encourages them to integrate AI solutions into their practices.
Additionally, as the broader public discourse around AI in healthcare expands, investors and industry stakeholders are increasingly allocating resources towards AI research and development initiatives. This influx of investment further accelerates the advancement and adoption of AI technologies in the healthcare sector.
Therefore, the growing awareness of AI’s potential benefits among healthcare stakeholders, including providers, patients, regulators, and investors, is driving the rapid expansion of the artificial intelligence in healthcare market, fueling innovation and paving the way for transformative changes in healthcare delivery.
Artificial intelligence in healthcare Market Restraints and Challenges:
Data Privacy and Security Concerns
While data privacy and security concerns are often viewed as barriers to the adoption of artificial intelligence (AI) in healthcare, they paradoxically also act as driving factors for the market. In an era of increasingly sophisticated cyber threats and stringent regulations like the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in the European Union, healthcare organizations are compelled to invest in AI-driven solutions that enhance data protection measures. This heightened focus on safeguarding patient data fosters the development and adoption of AI technologies that offer advanced encryption, anonymization, and access control features to mitigate privacy and security risks.
Furthermore, as the volume and complexity of healthcare data continue to grow exponentially, traditional methods of data security become inadequate to address emerging threats. AI-based cybersecurity solutions leverage machine learning algorithms to detect anomalies, identify potential vulnerabilities, and proactively respond to security breaches in real-time. This proactive approach to data security not only protects sensitive patient information but also instills confidence among healthcare stakeholders regarding the reliability and integrity of AI-driven healthcare systems.
Moreover, healthcare organizations are increasingly recognizing the strategic imperative of prioritizing data privacy and security as a competitive differentiator in the marketplace. By investing in AI solutions that prioritize data protection and comply with regulatory requirements, healthcare providers can enhance patient trust, mitigate legal and reputational risks, and gain a competitive edge in an increasingly digital and interconnected healthcare landscape. Thus, data privacy and security concerns serve as catalysts for innovation and investment in AI-driven cybersecurity solutions, driving the growth of the artificial intelligence in healthcare market.
Integration Complexity
Integration complexity serves as both a driving factor and a challenging aspect of the Artificial Intelligence (AI) in Healthcare Market. As healthcare organizations strive to adopt AI technologies to improve patient care and operational efficiency, the need to integrate these AI systems with existing healthcare IT infrastructure becomes increasingly critical. Integration complexity drives innovation and market growth by fostering demand for interoperability solutions and specialized integration services that facilitate seamless incorporation of AI technologies into clinical workflows.
Moreover, the complexity of integrating AI systems with diverse data sources, such as electronic health records (EHRs), medical imaging archives, and wearable devices, drives the development of innovative data interoperability standards and middleware solutions tailored to the healthcare industry. This creates opportunities for technology vendors and solution providers to offer specialized integration tools and services, driving market expansion and fostering a robust ecosystem of AI-enabled healthcare solutions.
Additionally, integration complexity acts as a catalyst for collaboration and partnerships between AI technology developers, healthcare IT vendors, and healthcare providers. These collaborations aim to address interoperability challenges, streamline integration processes, and develop standardized interfaces that facilitate the seamless exchange of data between AI systems and existing healthcare IT systems. By fostering collaboration and knowledge-sharing across industry stakeholders, integration complexity drives innovation and accelerates the adoption of AI technologies in healthcare.
Furthermore, the growing demand for AI-driven healthcare solutions underscores the importance of addressing integration complexity to ensure successful implementation and maximize the benefits of AI technologies. Healthcare organizations are increasingly investing in interoperability solutions and integration services to overcome integration challenges and unlock the full potential of AI in improving patient outcomes, enhancing operational efficiency, and driving innovation in healthcare delivery. Thus, integration complexity serves as a driving force behind the growth and advancement of the artificial intelligence in healthcare market, stimulating innovation, fostering collaboration, and accelerating the adoption of AI technologies across the healthcare industry.
Segmental Overview
The artificial intelligence in healthcare market is classifiedintocomponent, application, and region.
Major Players in the Artificial Intelligence in Healthcare Market
The market players in the global artificial intelligence in healthcare market are APIXIO, Inc, Atomwise, Inc, AiCure, Butterfly Network, Inc, iCarbonX, Enlitic, Inc, Cyrcadia Health Inc, Insilico Medicine Inc, IBM Corporation, and NVIDIA Corporation.
Know More- https://nexbindinsight.com/pharmaceutical-and-healthcare/artificial-intelligence-in-healthcare-market-size
0 notes
Text
Liquid Scintillation Analyzer Market Growth and Restrain Factors Analysis 2023 – 2033
A Liquid Scintillation Analyzer (LSA) is an instrument used to measure the activity of radioactive isotopes in a liquid sample. The LSA works by measuring the amount of light emitted from a sample as a result of radioactive decay. This light is detected by a photomultiplier tube and converted into an electrical signal. The electrical signal is then analyzed by the LSA to determine the activity of the sample.
Market Dynamics
Liquid scintillation analyzer technology is a type of analytical instrument used for measuring radioactivity in liquids. It is used for a variety of applications, such as environmental monitoring, medical research, and industrial processes. As technology continues to advance, liquid scintillation analyzers are becoming increasingly sophisticated and capable of providing more accurate and reliable results.
One of the key trends in liquid scintillation analyzer technology is the development of more sensitive detectors. These detectors are capable of detecting even the smallest amounts of radioactivity. This allows for more precise measurements and improved accuracy. This is especially important for applications such as environmental monitoring, where small amounts of radioactivity can be hazardous. Additionally, this increased sensitivity allows for better detection of low-level radioactive materials, which can be difficult to detect with traditional methods.
Another key trend is the development of more automated systems. Automation makes the process of measuring radioactivity much faster and more efficient. Automated systems can also be programmed to perform multiple measurements in a single run, allowing for greater accuracy and more reliable results. Automation also reduces the amount of time and resources required to perform measurements.
To Know More: https://www.globalinsightservices.com/reports/liquid-scintillation-analyzer-market/?utm_id=1014
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers, and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample: https://www.globalinsightservices.com/request-sample/GIS26665/?utm_id=1014
Market Segments
The Liquid Scintillation Analyzer market can be segmented by product type, application, end-user, and region. By Product Type, the market can be divided into Counter, Analyzer, Consumable and Accessories, Software, and Services. By Application, the market can be divided into Drug Discovery and development, Immunoassays, Protein Analysis, Cell Biology, and Others. By End User, the market can be divided into Biopharmaceutical Companies, Academic and Research Institutes, Hospitals, and Others. By region, the market is divided into North America, Europe, Asia-Pacific, and the Rest of the World.
Request Customization: https://www.globalinsightservices.com/request-customization/GIS26665/?utm_id=1014
Key Player
The Liquid Scintillation Analyzer market includes players such as Thermo Fisher Scientific(US), PerkinElmer(US), Hidex Oy(FI), Berthold Technologies(DE), Packard(US), Beckman Coulter(US), Rotem Industries(IL), LabLogic Systems(UK), Agilent Technologies(US), and LabLogic Systems(UK), among others.
Request Discounted Pricing: https://www.globalinsightservices.com/request-special-pricing/GIS26665/?utm_id=1014
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porter 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis
Forecast Period – 2024-2033
Base Year – 2023
Buy your copy here: https://www.globalinsightservices.com/checkout/single_user/GIS26665/?utm_id=1014
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with the lead analyst of the report
Infographic Excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after-sales service
About Global Insight Services:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with the highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1-833-761-1700
Website: https://www.globalinsightservices.com/
0 notes
Text
Distribution Channels: Reaching Your Target Audience in the Leukemia Therapeutics Market

The Leukemia Therapeutics Market, valued at USD 8275.45 million in 2022, is poised for remarkable growth, projected to reach USD 13491.42 million by 2030, with a steady CAGR of 6.3% over the forecast period of 2023-2030. This growth trajectory underscores the evolution of treatment options, driven by emerging trends, key drivers, and a myriad of opportunities. Nevertheless, challenges persist, necessitating careful consideration and strategic planning.
Emerging Trends and Opportunities:
Immunotherapy Dominance: The emergence of immunotherapy, particularly chimeric antigen receptor (CAR) T-cell therapy, has revolutionized leukemia treatment. This personalized approach harnesses the body's immune system to target and destroy cancer cells, offering promising outcomes for patients.
Precision Medicine: Advances in genomics and molecular diagnostics have paved the way for precision medicine in leukemia treatment. Tailoring therapies based on individual genetic profiles enhances efficacy while minimizing adverse effects, heralding a new era of personalized care.
Targeted Therapies: Targeted therapies, including tyrosine kinase inhibitors and monoclonal antibodies, have demonstrated efficacy in specific leukemia subtypes. With ongoing research and development, the repertoire of targeted agents continues to expand, offering novel treatment options and improved patient outcomes.
Adoption of Novel Drug Delivery Systems: Innovations in drug delivery systems, such as nanoparticles and liposomal formulations, enhance drug stability, bioavailability, and targeted delivery to leukemia cells. These advancements mitigate systemic toxicity and optimize therapeutic efficacy, driving market growth.
Get Free PDF Sample Copy of Report: https://www.snsinsider.com/sample-request/3135
Key Drivers Propelling Growth:
Rising Disease Burden: The increasing incidence and prevalence of leukemia globally propel the demand for effective therapeutics. Factors such as aging populations, environmental exposures, and genetic predispositions contribute to the escalating disease burden, driving market growth.
Technological Advancements: Continuous advancements in biotechnology, genomics, and drug discovery expedite the development of innovative leukemia therapeutics. Collaborations between academia, pharmaceutical companies, and research institutions fuel R&D efforts, driving therapeutic innovation and market expansion.
Regulatory Support: Supportive regulatory frameworks and expedited approval pathways streamline the development and commercialization of leukemia therapeutics. Regulatory agencies prioritize accelerated approval for breakthrough therapies, fostering a conducive environment for market growth.
Increasing Healthcare Expenditure: The growing healthcare expenditure, coupled with improved access to healthcare services, augments the adoption of advanced leukemia therapeutics. Government initiatives, private investments, and healthcare reforms further bolster market growth, ensuring broader patient access to innovative treatments.
Challenges and Considerations:
High Development Costs: The high cost and complexity associated with drug development pose significant challenges for market players. Research-intensive processes, stringent regulatory requirements, and clinical trial expenses escalate development costs, impacting pricing strategies and market accessibility.
Limited Access to Advanced Therapies: Disparities in healthcare infrastructure and access impede the widespread adoption of advanced leukemia therapeutics, particularly in low- and middle-income countries. Addressing barriers to access, including affordability, infrastructure, and healthcare literacy, is imperative for equitable distribution of innovative treatments.
Resistance and Relapse: Despite advancements in leukemia therapeutics, treatment resistance and disease relapse remain formidable challenges. Tumor heterogeneity, clonal evolution, and microenvironmental factors contribute to therapeutic resistance, necessitating ongoing research to overcome resistance mechanisms and improve treatment outcomes.
Key Takeaways from the Market:
Immunotherapy and precision medicine are poised to dominate the leukemia therapeutics landscape, offering personalized and targeted treatment approaches.
Collaborative efforts between stakeholders, including pharmaceutical companies, research institutions, and regulatory agencies, are crucial for driving therapeutic innovation and market growth.
Addressing challenges related to access, affordability, and therapeutic resistance is essential for optimizing patient outcomes and ensuring equitable distribution of advanced leukemia therapeutics.
In conclusion, the Leukemia Therapeutics Market is undergoing a transformative phase, propelled by technological advancements, rising disease burden, and supportive regulatory frameworks. While challenges persist, opportunities abound for stakeholders to leverage emerging trends and drive therapeutic innovation, ultimately improving patient outcomes and advancing the fight against leukemia.
Buy This Exclusive Report: https://www.snsinsider.com/checkout/3135
0 notes