#ZyCovD
Text
#Battle Against COVID-19 : Zydus Cadila Confirms Commencing Supplies Of ZyCoV-D Vaccine To Centre
0 notes
Text
সতর্কতামূলক ডোজ হিসাবে কোন টিকা দেওয়া হবে? সরকার এই উত্তর দিয়েছে
সতর্কতামূলক ডোজ হিসাবে কোন টিকা দেওয়া হবে? সরকার এই উত্তর দিয়েছে
করোনা টিকা:স্বাস্থ্যসেবা এবং ফ্রন্টলাইন কর্মীরা যারা ভারতে 10 জানুয়ারি থেকে করোনা ভ্যাকসিনের দুটি ডোজ নিয়েছেন এবং 60 বছরের বেশি বয়সী ব্যক্তিদের আরেকটি ডোজ দেওয়া হবে, যাকে সতর্কতা ডোজ বলা হচ্ছে। হয়। যারা এখন পর্যন্ত ভ্যাকসিন নিয়েছেন তাদের একই ভ্যাকসিনের সতর্কতামূলক ডোজ দেওয়া হবে, কিন্তু আগামী সময়ে কি আরেকটি টিকার ডোজ দেওয়া হবে? ICMR-এর মহাপরিচালক, অধ্যাপক বলরাম ভার্গবের মতে, টিকাদান…
View On WordPress
#ZyCoVD#করোনা টিকা#করোনা ভ্যাকসিন#করোনা ভ্যাকসিনেশন#করোনাভাইরাস#কোভাসিন#কোভিশিল্ড#কোভ্যাক্সিন#প্রতিরোধমূলক ভ্যাকসিন#ভারতে বুস্টার ডোজ#ভারতে বোস্টার ডোজ#সতর্কতা ভ্যাকসিন#সতর্কতামূলক ডোজ#স্পুটনিকভি
0 notes
Text
Work on developing dengue vaccines under way: ICMR chief
Balram Bhargava, director general, Indian Council for Medical Research. (File photo: PTI)
With a rise in dengue cases across the country, work has started on on developing dengue vaccines. Balram Bhargava, director general, Indian Council for Medical Research, said on Thursday at the health ministry briefing that companies have started on trials for the Dengue vaccine.
“Dengue vaccine is a very…

View On WordPress
#Balram Bhargava#covid vaccination programme#dengue vaccine#dengue vaccines#icmr chief#Rajesh Bhushan#zycovd#zydus cadila covid 19 vaccine
0 notes
Photo

Swipe left to read more. Follow @the_unopinion for more updates. #coronavirus #covid #12 #covid19 #covid_19 #discover #covidvaccine #corona #pandemic #stayhome #quarantine #lockdown #india #news #publichealth #pfizer #zycovd #cadila #the_unopinion https://www.instagram.com/p/CS12vgDFqZq/?utm_medium=tumblr
#coronavirus#covid#12#covid19#covid_19#discover#covidvaccine#corona#pandemic#stayhome#quarantine#lockdown#india#news#publichealth#pfizer#zycovd#cadila#the_unopinion
0 notes
Text
0 notes
Text
Emergency approval for Zydus Cadila COVID-19 vaccine to take few more days, say sources
Emergency approval for Zydus Cadila COVID-19 vaccine to take few more days, say sources
द्वारा एएनआई
NEW DELHI: भारत का ड्रग रेगुलेटर ड्रग्स कंट्रोलर जनरल ऑफ इंडिया (DCGI) कुछ और दिनों में Zydus Cadila को अपने COVID-19 वैक्सीन ZyCoV-D के लिए आपातकालीन उपयोग प्राधिकरण (EUA) देने पर विचार करेगा, सूत्रों ने ANI को बताया।
सूत्रों ने कहा, “विषय विशेषज्ञ समिति (एसईसी) की इस सप्ताह बैठक होने की उम्मीद है और वह डेटा की समीक्षा करेगी। हालांकि, यूरोपीय संघ के लिए अंतिम मंजूरी कुछ बैठकों के…

View On WordPress
0 notes
Text
COVID 19 Vaccine India LIve: Coronavirus Narendra Modi Speech Today | Oxford Covidshield, Covaxin, ZyCovD Vaccine Review by PM Modi
COVID 19 Vaccine India LIve: Coronavirus Narendra Modi Speech Today | Oxford Covidshield, Covaxin, ZyCovD Vaccine Review by PM Modi
[ad_1]


PM Modi to visit three cities to review vaccine progress (Representational Image)
Covid 19 Vaccine progress in India Live Updates:Prime Minister Narendra Modi is on a three-city tour today to review the progress of Covid-19 vaccine development in India. PM Modi is visiting Ahmedabad, Pune and Hyderabad – three cities where research and development of Indian vaccine candidates are being…
View On WordPress
#Coronavirus#Covaxin#Covid#Covidshield#India#Live#Modi#Narendra#Oxford#Review#speech#Today#vaccine#ZyCovD
0 notes
Text
Clinical Trial of Covid-19 Vaccine: ZyCoV-D
Clinical Trial of Covid-19 Vaccine: ZyCoV-D
Clinical Trial of Covid-19 Vaccine: ZyCoV-D
Recently, India has started phase I/II clinical trials of Covid-19 vaccine – ZyCoV-D, designed and developed by Zydus (a pharmaceutical company) with support from the Department of Biotechnology (DBT).
The adaptive phase I/II clinical trials will assess the safety,…
View On WordPress
0 notes
Photo
ZyCoV-D: Zydus Cadila begins human clinical trials of coronavirus vaccine candidate Image Source : AP ZyCoV-D: Zydus Cadila begins human clinical trials of coronavirus vaccine candidate Drug firm Zydus Cadila on Wednesday announced that it has started human clinical trials of its coronavirus vaccine candidate ZyCoV-D.
#begins#Cadila#Candidate#Clinical#Coronavirus#coronavirus medicine COVAXIN#coronavirus vaccine#coronavirus vaccine zycov-d#Human#Trials#Vaccine#zycovd#Zydus#Zydus Cadila coronavirus vaccine#zydus cadila human trials
0 notes
Link
The Indian drug regulator's subject expert committee on Friday recommended approving ZyCoV-D, Zydus Cadila's three-dose Covid vaccine, for emergency use.
Zydus application claims efficacy of 66.6%. SEC is of a view that Zydus needs to submit additional data for 2-dose vaccine. ZyCoV-D COVID vaccine is for all above 12 years of age and the vaccine has to be administered in three doses – the first dose, and the remaining doses after 28 and 56 days.
1 note
·
View note
Text
Coronavirus India vaccine: Covaxin, ZyCOV-D move to phase II clinical trials
Coronavirus India vaccine: Covaxin, ZyCOV-D move to phase II clinical trials
Coronavirus India vaccine: Covaxin, ZyCOV-D move to phase II clinical trials While Covaxin was the first Indian vaccine to be issued the go-ahead for the advent of clinical trials in the first week of July, ZyCOV-D was the first vaccine to head to the trials.
Source link
View On WordPress
0 notes
Photo
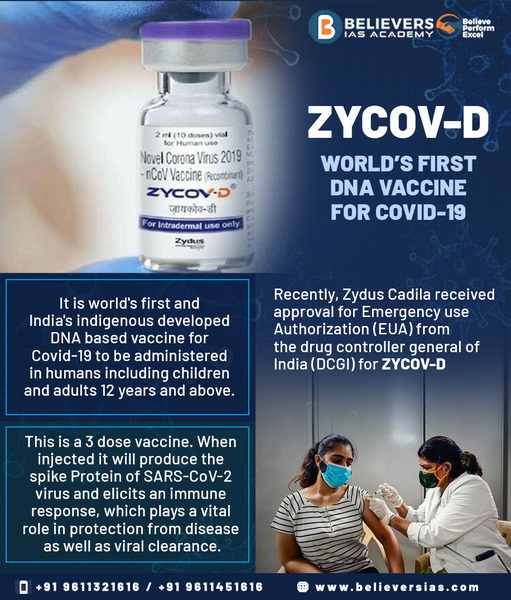
ZYCOV- D vaccine is an innovative leap for India, which has been the first to approve a DNA platform shot for Covid-19 in the world. Zydus claims that its vaccine is 66.6 per cent effective against symptomatic coronavirus cases and 100 per cent for moderate Covid-19.
Visit: https://believersias.com/ for details.
Email: [email protected] / [email protected]
Subscribe our YouTube channel for latest updates on important topics
https://www.youtube.com/channel/UCZcg7G1YDRa4zKN77c3YphA
Call @ +91 9611321616 / +91 9611451616
#BelieversIAS #ZycovD #zycovdvaccine #covidvacccine2021 #news #IASacademy #UPSC #onlinecourse #onlineclasses #civilservices #coaching #BestIASCoaching #IASPreparation #upscaspirant #online #library #upsccoaching #iasexam #upscprelims #currentaffairs #ips #upscstudymaterial #Exams #career #Training #Knowledge #Bengaluru
0 notes
Link
#ZyCov-D vaccine#Prime Minister#CDSCO India#COVID-19#innovative zeal of India’s scientists#India is fighting COVID-19
0 notes
Text
How made-in-India ZyCoV-D will be different from other vaccines. Read.
#ZycovD #ZydusCadila #covidvacccine #Covid19India #coronavirusindia #DNA #madeinindia #AtmanirbharBharat #storypitch
0 notes