#and cyclo-hexane
Photo



Hydro carbons and bromine.
#gif#science#hydrocarbons#alkanes#alkenes#cycloalkanes#if I remember right it's was#hexane#hexene#and cyclo-hexane#chemistry
4 notes
·
View notes
Text
Nomenclature - Basics
Nomenclature is the method for naming chemical compounds, both inorganic and organic. It is a systematic method for naming compounds so that it’s structure can drawn according to its name, or named based off its structure. Usually, IUPAC rules are followed, and is used in most organic chemistry courses. Before learning the nomenclature for more complex structures, the parent names and some basic rules should be noted.
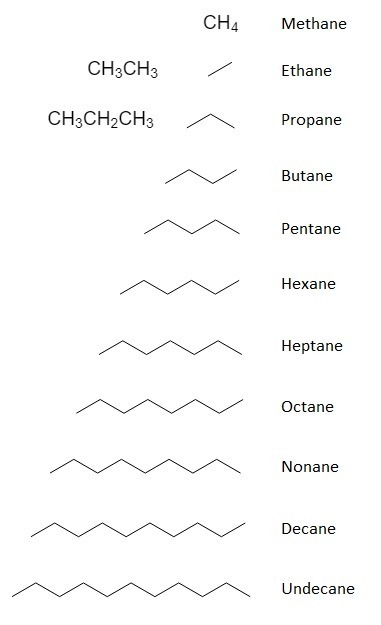
This is a list containing the parent names of organic compounds that contain up to 11 carbons. There are parent names for compounds containing more than 11 carbons, but most professors usually only require you to know the first 11 or so. You will need to memorize these.

If a chain of carbons is in a ring, ‘cyclo’ is placed before the parent name. So, a chain of 6 carbons in a ring will be cyclohexane.

The shape of the compound doesn’t change it’s parent name; molecules are always vibrating and rotating, so its conformation doesn’t influence its IUPAC name. The three drawings of hexane are conformational isomers, and represent the same compound.
To determine the parent chain of a compound, number it starting from the end. If its a ring, the numbering doesn’t matter unless there is a substituent or side chain hanging off of it, in which case, begin numbering where the side chain or substituent is attached to the ring.
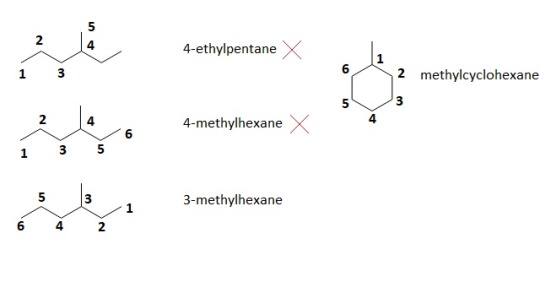
In carbon chains, always number it so that you get the longest chain possible. Also, like numbering rings, number the compound so that any substituent or side chain has the lowest number. Thus, the correct way to name the compound on the left is 3-methylhexane, since a methyl group, a CH3, is attached to the 3rd carbon when correctly numbering the chain.
219 notes
·
View notes
Text
The Thirium Theor...ium
Oi Raakx, what’s this about thirium? Well let me tell you that I spent a pretty decent amount of time trying to analyze what Thirium 310 may contain.
So feel free to read below if you like science rambles! This is also a really brief summary since I dind’t want to tie all the connections out, because they’re...obvious. If you’d like to correct me on anything, please feel free!
And if you don’t believe me, I’ve spoken with my organic chemistry professor, my lab assistant, and have done a little of my own research while trying to understand.
So, let’s get into the t h e o r y.
Thirium contains Benzene and a possible transition metal (most likely Copper or Cobalt to create the blue color)
P.S. if you want to know what it smells like, think almonds + metal. That’s just my theory.
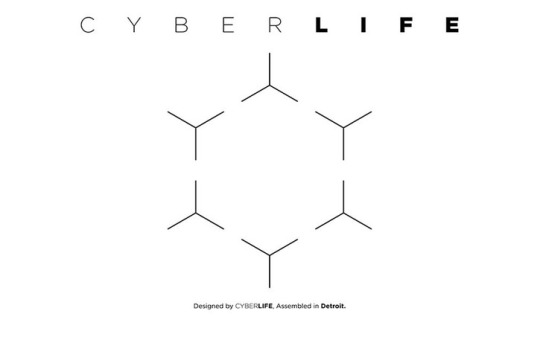
The CyberLife logo is what is known to the world of science as a “cyclohexane” - cyclo being a circle and hexane being 6 carbons and is very similar to that of a benzene ring.

Common properties of Thirium 310 as shown in the game:
Used in the drug “Red Ice” -messes up hormones
Is blue
Transfers a “life energy” through androids + electronic signals to their biocomponents
Evaporates and leaves traces only possible to be found by other androids or special tools
Made of Thirium + chemicals found in the arctic
I believe benzene plays a role in thirium, along with its blue color marker cobalt (yes, I chose to lean towards cobalt, and I’ll explain why).
Benzene is generally a colorless liquid with a sweet odor and is highly flammable. It also evaporates quickly into the air and is heavier than air particles, so it may sink into lower level areas. It’s formed from natural processes + human activities, such as volcanoes and forest fires, crude oil, gasoline, and cigarette smoke. It is also one of the top 20 chemicals for production volume. Some industries use benzene for plastics, synthetic fibers, dyes, drugs, and pesticides.
Benzene in effect to humans will cause bone marrow to not produce enough red blood cells, leading to anemia, and can damage the immune system by changing the levels of antibodies and the loss of white blood cells.
Exposure and Consumption of benzene will lead to:
Drowsiness
Dizziness
Rapid/irregular heartbeat
Tremors
Confusion
Unconsciousness
Vomiting
Stomach irritation
Death
To name a few…
Now, about Cobalt (a quick summary):
Slightly toxic by skin contact and moderately toxic by ingestion
Chemically active metal
Used in aircraft engine parts in allows with corrosion and wear resistant uses
Widely used in batteries and electroplating (covering an object with a thin layer of metal)
Makes the blues and greens in glass
Essential to many living creatures as and is a part of Vitamin B12
Magnets
Here’s a picture of it in liquid form

So, what’s the point of all this?
Benzene shares many similar properties to Thirium 310. It evaporates into the air and becomes unseeable except through special tools (I suppose a spectrometer is responsible for this). It sinks to lower level areas (thus why I believe you can still find T310 traces because it stays there instead of floating away into the air). Also benzene is flammable.. SPOILER...think about when Markus went ablaze. END OF SPOILER
Many chemicals (ie. gasoline/oil...such as that benzene is used in) are getting spilled into the Arctic (and many chemicals needed for T310 aside Thirium are mined in the arctic).
It can cause a lot of toxicity in the bloodstream of the body which may affect it if ingested (perhaps through the Red Ice drug?).
The Cobalt makes for the blue color and transfers electric signals, and is also used as a metal for strength + support.
Mix Benzene and Cobalt together and you may just have yourself some actual Thirium 310!!
Well, almost. All you’re missing now is a chemical tag that allows you to identify the android, just as we can use our blood to identify who we are. So...we almost have Thirium 310.
BUT IT’S JUST A THEORIUM.
A GAME THEORIUM.
THANKS FOR WATCHINGIUM.
#mun speaks#thirium#thirium 310#dbh#detroit become human#does anyone care about this#or am I the only one that's fascinated
92 notes
·
View notes
Text
WHAT IS THE COMPOSITION OF GASOLINE?
We can see better the correct response to the inquiry "At what temperature does fuel solidify?" by distinguishing the components that form gas.
For one thing, gas is certifiably not a plain substance. It isn't unadulterated in any way. Rather, it is a blend of different hydrocarbons and added substances – hundreds in amount. Regular ones are ethanol, toluene, octane, hexane, and heptane. Then again, discretionary ones are butane and pentane relying upon a specific region's winter atmosphere. Most synthetic substances specified can have a few auxiliary structures called isomers. That prompts the way that all things shaping fuel have their own liquefying point. Clearly, they likewise have distinctive solidifying focuses.
AT WHAT TEMPERATURE DOES GASOLINE FREEZE?
You may have contemplated the appropriate response officially in light of the way that fuel is a blend. At what temperature does fuel solidify once more? None precisely. Gas does not have the alleged the point of solidification. A similar thought goes for flame wax and diesel. Or maybe, fuel has a solidifying range.
While the temperature is slipping, gas is evolving step by step. The initial segment is little bits of dregs and gums leaving the fluid. Next, when it gets greatly chilly, the heaviest hydrocarbon atoms like cyclo-heptane and iso-nonane gradually set. They would look waxy. Presently, when the temperature gets increasingly horrendous, fuel turns out to be significantly waxier and slushier until the point that the staying fluid components are the lightest atoms.
In the mean time, a few silt frame because of contaminations in the gas tank. Cool temperature just makes them significantly more strong. Luckily, the channel situated in the fuel line can really get these silt. Things deteriorate just if gas turns out to be greatly waxy. In this stage, we are talking - 40 to - 200 degrees Fahrenheit. The range is wide since gas has different kinds of blends and mixes. Another factor is the wellspring of fuel's raw petroleum.
Since refineries can really change a specific blend of gas, they can give unique mixes to zones with an amazingly cool atmosphere. They can influence fuel to have a lower solidifying range. An illustration is ethanol gas.
WHAT ARE THE SYMPTOMS OF A FROZEN FUEL LINE?
It is difficult to discuss the side effects of solidified gas. Some time before gas ever solidifies inside the auto, the primary components to get solidified are water and water vapor. In this way, we should discuss the indications of a solidified fuel line. All things considered, the purpose of asking "At what temperature does gas solidify?" is to counteract outrageous winter issues auto proprietors may confront.
At the point when both water and water vapor solidifies inside the car, they square gas in the fuel line from entering the motor. To see whether gas is now hindered by solidified water, here are side effects to keep an eye out for:
Steady STOPPING OR SPUTTERING DURING MOVEMENT
A few people discover the need to drive their auto regardless of whether the outside temperature gets insufferably chilly key covers. The terrible news is that regardless of how the auto produces warm while you are driving, the gas tank can at present stop. You would know whether the tank is as of now breaking down because of chilly. The auto would begin to slow down or sputter. In reality, regardless of whether the tank is still alright, a solidified gas line can even now give this indication.
Inability TO START
You ought not stress excessively if the motor does not begin. For whatever length of time that it can at present turn over, simply hold up until the point when the temperature gets sufficiently warm car key covers. With persistence, your auto can work regularly regardless of whether it is cold outside. As should be obvious, you should not hold up until the point that gas solidifies inside the auto. Essentially deal with your auto often amid winter.
Inability TO TURN OVER
While the fuel line is as yet solidifying – not completely solidified yet – the motor can in any case turn over despite the fact that it can't begin any longer. You would know whether the fuel line is as of now best case scenario if the motor totally neglects to turn over. Try not to stress; there are courses on the most proficient method to settle a solidified fuel line.
HOW Might WE FIX A FROZEN FUEL LINE?
At the point when it becomes absolutely necessary, here are useful arrangements in defrosting a solidified fuel line:
PUSH YOUR CAR INTO AN ENCLOSED AREA.
In any case, when you hear winter is coming, ensure that your vehicle is in a sheltered place. It is simply a terrible plan to leave your auto in the open amid winter. In a most dire outcome imaginable, for example, leaving your auto in the front yard medium-term until the point that snow falls, the underlying advance you have to do is to push your auto towards the carport. In any case, ensure that you arranged the zone key cover. The carport must be dry and warm to effectively defrost the solidified fuel line. Holding up will take hours, however the entire thought is compelling. To accelerate holding time, horses on lamp fuel or electric warmers inside the carport, particularly confronting the auto. This influences the defrosting to process quicker to one hour as it were. Be watchful however; autos are extremely combustible so put a major space between the warmth source and the car.
On the off chance that you would encounter a troublesome time getting your auto out of the snow, look at how to make the main endeavor and continue to design B if vital.
PUT ANTIFREEZE ADDITIVES IN THE TANK.
Regardless of what season it is, go to a car parts store and buy liquid catalyst added substances. These medications are exceptionally valuable amid winter, so recollect forget to have one in your carport. It is prescribed to put a few containers of liquid catalyst medicines into the tank. Regardless you need to sit tight for a couple of hours to let the radiator fluid totally blend with the fuel, yet it truly works. You can likewise make the holding up time speedier. Simply shake the auto forward and backward to influence the radiator fluid to mix with the fuel rapidly.
HOW Might WE PREVENT A FROZEN FUEL LINE?
To stay away from all the problem of defrosting a solidified fuel line, counteractive action is critical. Here are a few hints on the best way to keep the fuel line from solidifying beside keeping the auto inside your carport:
KEEP THE TANK FULL.
This diminishes dampness in the framework. Water vapor is the principle guilty party for solidifying fuel lines all things considered. Buildup produces water, and we as a whole realize that water solidifies in chilly temperature.
Include ANTIFREEZE TREATMENT.
Liquid catalyst is both an answer and an avoidance factor with regards to solidified fuel lines. Simply make a point to take after the item's guidelines.
Rundown
For the last time, at what temperature does fuel solidify? The correct answer is none. Fuel is made out of different components with various solidifying focuses. Along these lines, essentially, it doesn't have a the point of solidification. Or maybe, it has a scope of temperatures that step by step change the fuel's unique fluid frame. In view of different kinds of gas blends and mixes, the typical range is - 40 to - 200 degrees Fahrenheit. Gas is as of now viewed as solidified on the off chance that it transforms into a thick ooze or wax. To thoroughly maintain a strategic distance from solidified gas inside the auto, center around keeping the fuel line from solidifying amid winter. When you take great care of the fuel line, aversion of solidified gas takes after.
For different concerns including gas, realize why a few autos possess a scent like gas when stopped. On a positive note, discover fundamental propensities that assistance you spare gas.
1 note
·
View note
Text
Chemical technology revolution in oil plants
Chemical, petrochemical, and pharmaceutical businesses all employ chemical technologists. Manufacturing, research and development (R & D), consultancy, quality control, and a range of other activities are all possible in these businesses. Additionally, chem techs employed by these organizations may be used to perform quality control and other routine tests, as well as aid in chemical and biochemical research, such as analysis, industrial chemistry, environmental protection, and chemical engineering. Chemical technology has allowed people to develop more types of usage and products.
Skills are needed.
Chemical technologists, on the whole, are more likely than chemical technicians to be given more autonomy and more difficult responsibilities. R & D is the most prevalent job for chemical technologists. They are frequently employed in a laboratory setting under the guidance of a chemist or chemical engineer.
They may help set up and conduct chemical tests, as well as operate laboratory equipment under supervision. They must adhere to quality control criteria that have been established. They may also compile data for analytical research and participate in the writing of study results.In some nations, national certification for chemical technologists and technicians is necessary.
The insight
Chemicals aren't enough for onshore producers. They want knowledgeable, dependable, and responsive partners. We aim to make your operations safer and more economical in both the conventional and unconventional sectors, from first oil through aging assets. Our onshore chemical production solutions are underpinned by a worldwide network of data insights and supplied with problem-solving and support from the ground up. We have facilities near all major oil and gas plays, and our full-cycle water management skills complement our decades of production experience.
A huge turn after chemical technology improved
Commercial gasoline is made up of a diverse range of hydrocarbons. Gasoline can be made in a variety of compositions to fulfill a variety of engine performance requirements. As a result, the chemical composition of gasoline is unknown. In order to start a cold engine, the performance criteria change with the season, with more volatile mixes in the winter. The composition of the final product at the refinery varies depending on the crude oil used, the type of processing units present, how those units are run, and which hydrocarbon streams the refinery chooses to employ when mixing the final product.
Other organic molecules, such as organic ethers (deliberately added), as well as minor amounts of pollutants, particularly organosulfur compounds, can be found in gasoline (which are usually removed at the refinery).
Benzene, in particular, and olefin (alkene) concentrations are currently regulated in many nations. Because alkane isomers, such as isomerate or alkylate, have a higher octane rating than n-alkanes, these regulations have led to an increased preference for them. The benzene limit for all grades of motor gasoline in the European Union is set at one percent by volume. This is commonly accomplished by not feeding C6, namely cyclo-hexane, into the reformer unit, where it would be transformed into benzene. As a result, the reformer unit only receives (desulphurized) heavy virgin naphtha (HVN).
Different refinery streams are combined to generate gasoline, and each has its own properties.
0 notes
Text
Got a quiz in ochem and i’m a damn idiot holy shit. I drew the structure for 3-methylcylohexane instead of 3-methylhexane :/// Realized it didn’t say “cyclo” right when I had to turn it in.
2 notes
·
View notes
Text
Global and Chinese Cyclo Hexane Di Methanol (CAS 105-08-8) Industry, 2017 Market Research Report By Radiant Insights
The 'Global and Chinese Cyclo Hexane Di Methanol Industry, 2012-2022 Market Research Report' is a professional and in-depth study on the current state of the global Cyclo Hexane Di Methanol industry with a focus on the Chinese market. The report provides key statistics on the market status of the Cyclo Hexane Di Methanol manufacturers and is a valuable source of guidance and direction for companies and individuals interested in the industry.
Download Full Research Report @: http://www.radiantinsights.com/research/global-and-chinese-cyclo-hexane-di-methanol-cas-105-08-8-industry-2017
Firstly, the report provides a basic overview of the industry including its definition, applications and manufacturing technology. Then, the report explores the international and Chinese major industry players in detail. In this part, the report presents the company profile, product specifications, capacity, production value, and 2012-2017 market shares for each company. Through the statistical analysis, the report depicts the global and Chinese total market of Cyclo Hexane Di Methanol industry including capacity, production, production value, cost/profit, supply/demand and Chinese import/export.
The total market is further divided by company, by country, and by application/type for the competitive landscape analysis. The report then estimates 2017-2022 market development trends of Cyclo Hexane Di Methanol industry. Analysis of upstream raw materials, downstream demand, and current market dynamics is also carried out. In the end, the report makes some important proposals for a new project of Cyclo Hexane Di Methanol Industry before evaluating its feasibility. Overall, the report provides an in-depth insight of 2012-2022 global and Chinese Cyclo Hexane Di Methanol industry covering all important parameters.
Request a Free Sample Copy of this Report: http://www.radiantinsights.com/research/global-and-chinese-cyclo-hexane-di-methanol-cas-105-08-8-industry-2017/request-sample
Table of Contents
Chapter One Introduction of Cyclo Hexane Di Methanol Industry
1.1 Brief Introduction of Cyclo Hexane Di Methanol
1.2 Development of Cyclo Hexane Di Methanol Industry
1.3 Status of Cyclo Hexane Di Methanol Industry
Chapter Two Manufacturing Technology of Cyclo Hexane Di Methanol
2.1 Development of Cyclo Hexane Di Methanol Manufacturing Technology
2.2 Analysis of Cyclo Hexane Di Methanol Manufacturing Technology
2.3 Trends of Cyclo Hexane Di Methanol Manufacturing Technology
Chapter Three Analysis of Global Key Manufacturers
3.1 Company A
3.1.1 Company Profile
3.1.2 Product Information
3.1.3 2012-2017 Production Information
3.1.4 Contact Information
3.2 Company B
3.2.1 Company Profile
3.2.2 Product Information
3.2.3 2012-2017 Production Information
3.2.4 Contact Information
See More Reports of This Category by Radiant Insights:
http://www.radiantinsights.com/catalog/chemicals
About Radiant Insights
Radiant Insights is a platform for companies looking to meet their market research and business intelligence requirements. It assist and facilitate organizations and individuals procure market research reports, helping them in the decision making process. The Organization has a comprehensive collection of reports, covering over 40 key industries and a host of micro markets. In addition to over extensive database of reports, experienced research coordinators also offer a host of ancillary services such as, research partnerships/ tie-ups and customized research solutions.
Contact Details:
Michelle Thoras
Corporate Sales Specialist, USA
Radiant Insights, Inc
28 2nd Street, Suite 3036, San Francisco, CA 94105, United States
Phone: 1-415-349-0054
Toll Free: 1-888-202-9519
Email: [email protected]
Web: http://www.radiantinsights.com/
#Cyclo Hexane Di Methanol (CAS 105-08-8) Industry#Cyclo Hexane Di Methanol (CAS 105-08-8) Market#Market Research
0 notes
Text
Multi Component Green Synthesis of Bis-Pyrrol Indoline-2-Ones Triazoles Catalysed By Cu NPS on Activated Carbon in Water-JuniperPublishers
Journal of Chemistry-JuniperPublishers
Abstract
A variety of potentially biological active multi component green synthesis of Bis-pyrrol indoline-2-ones triazoles possessing N- alkyl furan/ N- alkyl thiophene /N- alkyl pyrrol by catalysed Cu-NPs on activated carbon in water involving green synthetic technology have been developed. It involves in three steps. The first step involves the synthesis of 1-prop-2-ynyl indoline-2,3-dione(3) from indoline -2,3-dione and 3-chloropropanone(2). The intermediate1-prop-2-ynyl indoline-2,3-dione(3) was converted into 1-((1-furan/thiophene/pyrrol/phenyl/4- methyl phenyl/4-fluoro phenyl/4- trifluro methyl phenyl/4-nitro phenyl)-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)indiline-2,3-dione (5a-h) by 1,3- dipolar cyclo addition with 2-bromo methyl-1H-furan/ 2-bromo methyl-1H-thiophene/ 2-bromo methyl-1H-pyrrol benzyl bromide/4-methyl benzyl bromide/4-fluoro benzyl bromide/4-tri fluoro benzyl bromide/4- nitro benzyl bromide (4a-h) catalysed by Cu-NPs on activated carbon catalysed click reaction in neat water depicted in step-2. The step three involves green synthesis of 1-((1-furan/thiophene/pyrrol/phenyl/4- methyl phenyl/4-fluoro phenyl/4- trifluro methyl phenyl/4-nitro phenyl)-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl) -3,3-di(1H-pyrrol-2-yl) indoline-2-ones(7a-h) from 1-((1-1-((1-furan/ thiophene/pyrrol/phenyl/4-methyl phenyl/4-fluoro phenyl/4- trifluro methyl phenyl/4-nitro phenyl)-2-yl)-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)indiline-2,3-dione(5a-h) and pyrrol (6) in presence of selected ionic liquid. The newly synthesized triazoles (7a-h) were characterized by spectral analysis. The synthetic route was shown in scheme-1. Antimicrobial activity of 7a-h was studied in presence of Ag NPs by disk diffusion method.
Keywords: Click Reaction; (Bmim) Oh (1-Butyl-3-Methylimidazolinium Hydroxide) Bis-Pyrrol Indoline Triazoles; Cu-Nps; Eco-Friendly Synthesis; Ag-Nps.
Introduction
Heterocycles play an important role in biochemical processes and, therefore, they are very frequently found as substructures in numerous pharmaceutical products [1]. Among them, 1,2,3- and 1,2,4-triazoles are known to possess remarkable biological properties as antitumor, antiviral, anti-inflamMicrobial Experimentationory, analgesic, antifungal or antibacterial agents [2]. Nowadays, click chemistry[3] represents a pivotal tool in the discovery of new therapeutic compounds and in medicinal chemistry, since it allows molecular diversity in a direct, precise and selective manner.[4] In particular, the Huisgen 1,3-dipolar cycloaddition of azides and alkynes [5] have become a synthetic cornerstone, the paramount discovery by the groups of Meldal [6] and Sharpless [7] of its copper(I)-catalysed version (CuAAC). [8] This powerful methodology, which is considered the paradigm of a click reaction, gives a straight access into the 1,2,3-triazole moiety with high reliability and selectivity. This particular nucleus is used as a robust linker of complexes On the other hand, owing to our dedication to study and understand the reactivity of active metals and nanoparticles, [9] molecular architectures of significance in different fields, and especially in synthetic routes to bioactive molecules [10]. Copper nanoparticles (CuNPs) were formed either from CuCl22H2O or anhydrous CuCl2 under the aforementioned conditions. These CuNPs (10 mol%) effectively catalysed the 1,3-dipolar cycloaddition of organic azides and terminal alkynes in remarkably short reaction times (10-120 min) [11], the CuNPs underwent dissolution under the reaction conditions (Et3N, THF, 65 °C) the same could not be reused. In order to overcome this inconvenience, supported catalysts based on CuNPs were developed for better recyclability and stability than the unsupported counterparts. [12] In this sense, a catalyst consisting of oxidized copper nanoparticles on activated carbon, readily prepared under mild conditions, which manifested a high versatility in the multicomponent Huisgen 1,3-dipolar cycloaddition in water. [13] The organic halides, azides as aryldiazonium salts, anilines, epoxides and alkene, were proven to be appropriate substrates in this process. In order to expand the applicability of this methodology, we have reported here in our results on the multicomponent synthesis of an array of 1,2,3-triazoles, derived from some natural products and a synthetic one, with potential biological activity.
Bis Pyrrol-2-yl-indoline-2 ones derivatives have been drawing the attention of synthetic organic chemists due to their wide spectrum of biological properties [14]. 3,3-Diaryloxindoles known to exhibit a wide range of biological activities such as antibacterial [15], antiprotozoal [16], anti-inflamMicrobial Experimentationory [17] and anticancer activity [18]. A reaction which involves ionic liquids as catalysts [19] and /or media [20] in reactions have been widely used in organic Trans forMicrobial Experimentationions due to their advantages such as good solvating ability, negligible vapor pressure, high polarity and ease of work-up. [Bmim]OH (1-butyl-3-methylimidazolinium hydroxide) is one such task-specified ionic liquid which acts as reaction medium as well as a basic catalyst and has got varied applications [21] in the field of synthetic methodology development. In view of our continued interest in indoles [22] and the development of green procedures for the synthesis of diverse heterocyclic compounds of biological significance, we now report a simple and efficient method for the synthesis and use of Cu NPs of Bis-Pyrrol indoline triazoles 7a-h using [Bmim] OH as a task-specific ionic liquid.
Experimental Section
Melting points were obtained with a Reichert Thermovar apparatus and are un corrected. Infrared analysis was performed with a Jasco 4100LE (Pike MIRacle ATR) spectrophotometer; wavenumbers are given in cm-1. NMR spectra were recorded on Bruker Avance 400 spectrometers (400 MHz for 1H NMR; 100 MHz for 13C NMR); chemical shifts are given in (5) parts per million and coupling constants (J) in Hertz. Activated charcoal (Norit CA1, Aldrich), and sodium azide (Across) were commercially available. All the starting Microbial Experimentationerials and other reagents were commercially available of the best grade (Aldrich) and were used without further purification. THF was dried in a Sharlab PS-400-3MD solvent purification system using an alumina column. Propargylation of the substrates was done following a literature procedure [23].
General Procedure for the synthesis of (3):
To a stirred suspension of sodium hydroxide (1.0 g, 42 m mol, and 1:2 eq.) in THF (25 ml) at room temperature, Indole-2,3- dione(1) was added at room temperature and the reaction mixture was stirred at same temperature for 30 min, 3-chloro -1-propyne (7.01 g, 42.0 mmol, 1:2 eq) was added drop wise to the stirring solution and the reaction mixture was stirred for an additional 3 hr. The progress of the reaction was monitored by TLC with hexane and ethyl acetate (7:3) as mobile phase, after completion of the reaction, the reaction mixture was poured on to cooled water, then extracted with ethyl acetate twice (2x20M). The combined organic layer was washed with water, brine, dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure to get crude compound of (3), then the crude compound was purified by silica gel column chroMicrobial Experimentationography, eluted with 5% EtOAc and 95% pet ether to afford pure 1-prop-2-ynyl indoline-2,3-dione(3). The yield of the compound was 70% with melting point 139-1410C. The structure of compound was characterized by spectral data (IR and 1H-NMR,) and elemental analysis.
Spectral data of Compound (3):
The IR(KBr) spectra of 1-Prop-2-ynyl indoline-2,3-dione(3) was recorded in the range of 4000-400 cm'1 in KBr pellet reflect the molecular structure and showed the characteristics bands 3250 cm-1 (C =C-H str.),2861 and 2841 cm-1(Aliphatic CH str.),2150 cm-1 (C = C str.),1743 cm-1 and 1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline2.3- dione). 1H-NMR (400MHz, DMSO-d6) spectrum of 1-Prop- 2-ynyl indoline-2,3-dione(3) was recorded in DMSO-d6 showed the signals at 8 PPm 7.19-7.83 (m,4H,Ar-H protons), 3.70 (s, 2H,CH2 attached to (nitrogen of indoline-2,3-dione) and 2.25(s, H, acetylinic-CH proton) Anal. Caled.(%) for C11H7NO2: C:71.35,H:3.78,N:7.56, found C: 69.25,H:2.95,N:6.75.
General procedure for the synthesis of (5a-h):
A mixture of NaN3 (72 mg. 1.1mmol) 2- bromo methyl furan(4a)/ 2-bromo methyl thiophene(4b)/ 2-bromo methyl pyrrol (4c) (1m mol) benzyl bromide/4-methyl benzyl bromide/4-fluoro benzyl bromide/4-tri fluoro benzyl bromide/4- nitro benzyl bromide and1- prop-2-ynyl indoline-2,3-dione(3) (1 m mol) were added to a suspension of Cu NPs/C (20mg. 0.5 mol% Cu) in H2O (2mL) . The reaction mixture was warmed to 700C and monitored by TLC and /or GLC until total conversion of the starting Microbial Experimentationerials. Water (30 mL) was added to the resulting mixture, followed by extraction with EtoAc (3x10 mL). The collected organic phases were dried with anhydrousMgSO4 and the solvent was removed in vacuum to give the corresponding (5a-h) which were purified by recrystellisation in EtoAc.
Spectral data for the Compound-5a:
1-((1-furan-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione(5a),Yield 70%, M.P-154-1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743-1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2.3- dione), 1500,1250,1000,850 cm-1 (Characteristics of furan signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-furan-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5a) was recorded in DMSO-d6 showed the signals at 8 PPm 6.18-7.83(m,7H,C6H4and C4H3 rings),2.35(s,2H,CH2-flankedbetween indolone nucleus and 1.2.3- triazole system), 4.84 (s, 2H, CH2-flanked between1,2,3- triazole system and furan ring.7.17(s,H,1,2,3-triazole ring),7.21(unequal doublet, H,CH attached to oxygen atom of furan ring). Anal. Caled.(%) for C18H19N4O3: C:63.52,H:5.92,N:16.46, found C:61.31,H:4.82,N:14.23.
Spectral data for the Compound-5b:
1-((1-thiophene-2-yl)methyl)-1H-1,2,3-triazole-4- yl) methyl) indoline-2,3-dione(5b), Yield70% M.P-154- 1560C,IR-(KBr) 2861 and 2841cm'1(Aliphatic CH str.),1743- 1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm'1 (C-N str, of indoline 2,3-dione), 3100,1400,1100,850,700 cm-1 (Characteristics of thiophene signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-thiophene-2-yl] methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5b) was recorded in DMSO-d6 showed the signals at 8 PPm 6.18-7.83(m,7H,C6H4and C4H3 rings),2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.84 (s, 2H, CH2-flanked between1,2,3-triazole system and thiophene ring), 7.17(s,H,1,2,3-triazole ring),6.91(unequal doublet ,H,CH attached to sulphur atom of thiophene ring). Anal. Caled.(%) for C18H19N4O2 S: C:60.65,H:5.66,N:15.72, found C:58.34,H:3.33,N:13.41.
Spectral data for the Compound-5c:
1-((1-pyrrol-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione(5c), Yield70% M.P-154-1560C,IR-(KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2.3- dione), 1600, 1400, 3500, 720 cm-1 (Characteristics of pyrrol signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-pyrrol-2-yl) methyl)-1H-1,2,3-triazole-4' yl) methyl) indoline-2,3-dione, (5c) was recorded in DMSO-d6 showed the signals at 8 PPm 6.18-7.83(m,7H,C6H4and C4H3 rings),2.35(s,2H,CH2-flanked between indolone nucleus and 1.2.3- triazole system), 5.03 (s, 2H, CH2-flanked between1,2,3- triazole system and pyrrol ring), 7.17(s,H,1,2,3-triazole ring),6.91(unequal doublet ,H,CH attached to NH atom of pyrrol ring). Anal. Caled.(%) for C18H20N5O2: C:63.89,H:5.96,N:20.76, found C:58.44,H:3.53,N:18.43
Spectral data for the Compound-5d:
1-((1-phenyl-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione(5d), Yield 70%,M.P-154-1560C,IR-(KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2.3- dione), 3000,2000-1600,1500,1000 cm-1 (Characteristics of phenyl signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-phenyl-2-yl) methyl)-1H-1,2,3-triazole-4' yl) methyl) indoline-2,3-dione, (5d) was recorded in DMSO-d6 showed the signals at 8 PPm 6.18-7.83(m,7H,C6H4and C4H3 rings),2.35(s,2H,CH2-flanked between indolone nucleus and 1.2.3- triazole system), 5.03 (s, 2H, CH2-flanked between1,2,3- triazole system and phenyl ring), 7.17(s,H,1,2,3-triazole ring),6.36(unequal doublet ,H,CH attached to NH atom of phenyl ring). Anal. Caled.(%) for C20H20N4O2:C:68.95,H:5.79,N:16.08,found C:57.43,H:4.56,N:14.06.
Spectral data for the Compound-5e:
1-((1-(4-methylphenyl-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione(5e), Yield70% M.P-154-1560C,IR- (KBr) 2861 and 2841cm-1 (Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2.3- dione), 3200, 2950, 1600-1500, 1100 cm-1(Characteristics of 4-methyl phenyl signals), 3030, 1500, 1100, 810, 720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-4-methyl phenyl -2-yl) methyl)- 1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5e) was recorded in DMSO-d6 showed the signals at 8 PPm 7.0-7.5(m,9H,C6H4and C6H5 rings),2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3-triazole system and benzene ring), 7.30(s,H,1,2,3-triazole ring), Anal. Caled.(%) for C21H22NO2: C:69.59,H:6.12,N:15.46, found C:66.48,H:5.10,N:14.23.
Spectral data for the Compound-5f:
1-((1-(4-fluorophenyl-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione (5f),Yield70% M.P-154-1560C,IR- (KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2.3- dione), 3000,1600,1500, 1210, 900,750cm-1(Characteristics of 4-fluorophenyl signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-4-fluorophenyl -2-yl) methyl)- 1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5f) was recorded in DMSO-d6 showed the signals at 8 PPm 7.0- 7.5(m,9H,C6H4and C6H5 rings), 2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3-triazole system and benzene ring), 7.320(s,H,1,2,3-triazole ring), Anal. Caled.(%) for C20H22N4O2: C:65.56,H:5.2 3,N:15.29, found C:63.33,H:4.12,N:13.15.
Spectral data for the Compound-5g:
1-((1-(4-trifluorophenyl-2-yl)methyl)-1H-1,2,3-triazole- 4-yl) methyl) indoline-2,3-dione (5g),Yield70% M.P-154- 1560C,IR-(KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743- 1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2,3-dione), 1350-1150,1000,1550,850,750 (Characteristics of 4-trifluorophenyl signals),3030, 1500, 1100, 810,720cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-4-tri fluorophenyl -2- yl) methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5g) was recorded in DMSO-d6 showed the signals at 8 PPm 7.0- 7.5(m,9H,C6H4and C6H5 rings), 2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3-triazole system and benzene ring), 7.30(s,H,1,2,3-triazole ring), Anal. Caled.(%) for C21H19N4O2: C:60.57,H:4.60,N:13.46, found C:58.46,H:2.30,N:11.23.
Spectral data for the Compound-5h:
1-((1-(4-nitrophenyl-2-yl)methyl)-1H-1,2,3-triazole-4-yl] methyl) indoline-2,3-dione (5h), Yield70% M.P-154-1560C,lR- (KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2,3-dione), 3100, 2900, 1620, 1420,1000,850,800cm- 1 (Characteristics of 4-nirophenyl signals),3030, 1500, 1100, 810,720cm-1 (Characteris tics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-4-tri fluorophenyl -2- yl) methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5g) was recorded in DMSO-d6 showed the signals at 8 PPm7.0- 7.5(m,9H,C6H4and C6H5 rings), 2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3-triazole system and benzene ring), 7.30(s,H,1,2,3-triazole ring), Anal. Caled.(%) for C20H19N5O4: C:61.06,H:4.87,N:17.80, found C:57.03,H:2.43,N:14.12.
General procedure for the synthesis of (7a-h):
A mixture of 1-((1- furan/thiophene/pyrrol/phenyl/4- methyl phenyl/4-fluoro phenyl/4- trifluro methyl phenyl/4-nitro phenyl)-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)indiline-2.3- dione/1-((1-thiophene-2-yl)methyl)-1H-1,2,3-triazol-4-yl] methyl)indoline-2,3-diones(5a-h) (1.0 mmol) and pyrrol (6, 2.00 mmol) and [Bmim]OH (10 ml) was heated at 1000 C until the completion of reaction as checked by TLC. To the resulting oily reaction mixture was added ethanol (10 ml) to force out the crude product from the polar ionic liquid reaction medium. The separated solid mass was collected by filtration and dried in oven to obtain crude (7a-h). The latter, were recrystallized from ethanol to get the pure (7a-h). The filtrate consisting of the ionic liquid and ethanol were evaporated to remove ethanol and the recovered ionic liquid was reused for subsequent reactions. To compensate for the loss of some ionic liquid during the work up procedure, an amount (5 ml) of fresh [Bmim] OH was added after the 4 runs of the reactions.
Spectral data for the Compound-7a:
1-((1-(1-furan-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl) indoline -2-one(7a), Yield 70%, M.P-154- 1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743- 1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2,3-dione), 1500,1250,1000,850 cm-1 (Characteristics of furan signals),3030,1500,1100,810,720 cm-1 (Characteristics of1.2.3- triazole system),3 500,1600,1400,72 0cm-1(Characteristics of pyrrol signals) 1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-(1-furan-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl) indoline -2-one(7a), was recorded in DMSO-d6 showed the signals at δ PPm 7.0-7.50(m,7H,C6H4and C4H3 rings),5.90-6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2- flankedbetween indolone nucleus and 1,2,3-triazole system), 4.84 (s, 2H, CH2-flanked between1,2,3-triazole system and furan ring.7.17(s,H,1,2,3-triazole ring),7.21(unequal doublet, H,CH attached to oxygen atom of furan ring). Anal. Caled.(%) for C11H7NO2: C:71.35,H:3.78,N:7.56, found C: 69.25,H:2.95,N:6.75.
Spectral data for the Compound-7b:
1-((1-(1-thiophene-2-yl)methyl)-1H-1,2,3-triazol-4-yl) methyl)-3,3-di(1H-pyrrol-2-yl) indoline -2-one(7b), Yield 70%, M.P-154-1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743-1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2,3-dione), 3100,1400,1100,850,700 cm-1 (Characteristics of thiophene signals), 3500,1600,1400,720cm- Characteristics of pyrrol signals)3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-(1-thiophene-2-yl)methyl)-1H-1.2.3- triazol-4-yl)methyl)-3,3-di(1H-pyrrol-2-yl) indoline -2-one (7b), was recorded in DMSO-d6 showed the signals at δ PPm 7.0- 7.50(m,7H,C6H4and C4H3 rings),5.90-6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2-flankedbetween indolone nucleus and 1.2.3- triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3- triazole system and thiophene ring.7.17(s,H,1,2,3-triazole ring),6.91(unequal doublet, H,CH attached to sulphur atom of thiophene ring).
Spectral data for the Compound-7c:
1-((1-(1-pyrrol-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl) indoline -2-one(7c), Yield 70%, M.P-154- 1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743- 1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2,3-dione), 1600,1400,3500,720 cm-1 (Characteristics of pyrrol signals),3030,1500,1100,810,720 cm-1(Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-(1-pyrrol-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl) indoline -2-one (7c), was recorded in DMSO-d6 showed the signals at 8 PPm 7.0-7.50(m,7H,C6H4and C4H3 rings),5.90-6.95(m,9H,tri-pyrrol ring),2.35(s,2H,CH2- flankedbetween indolone nucleus and 1,2,3-triazole system), 5.03 (s, 2H, CH2-flanked between1,2,3-triazole system and pyrrol ring. 6.36(s,H,1,2,3-triazole ring),6.91(unequal doublet, H,CH attached to NH atom of pyrrol ring).
Spectral data for the Compound-7d:
1-((1--phenyl-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl)indoline -2-one(7d), Yield 70%, M.P-154- 1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743- 1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2,3-dione), 3000,2000-1600,1500,1000 cm-1 (Characteristics of phenyl signals),3500,1600,1400,720cm-1 (Characteristics of pyrrol signals), 3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-(1-phenyl-2-yl)methyl)-1H-1,2,3- triazol-4-yl)methyl)-3,3-di(1H-pyrrol-2-yl) indoline -2-one (7d), was recorded in DMSO-d6 showed the signals at 8 PPm7.0- 7.50(m,9H,C6H4and C6H5 rings),5.90-6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2-flankedbetween indolone nucleus and1.2.3- triazole system), 2.35 (s, 2H, CH2-flanked between1,2,3- triazole system and phenyl ring. 7.42(s,H,1,2,3-triazole ring).Anal. Caled.(%] for C30H34N6O: C:72.85,H:6.93,N:16.99, found C:70.43,H:5.52,N:14.54.
Spectral data for the Compound-7e:
1-((1-(4-methylphenyl-2-yl)methyl)-1H-1,2,3-triazol-4-yl] methyl]-3,3-di(1H-pyrrol-2-yl] indoline-2-one(7e], Yield 70%, M.P-154-1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.],1743-1691 cm-1(C=O of indoline 2,3-dione] and 1294 cm-1 (C-N str. of indoline 2,3-dione], 3200,2900,1600,1500,1100- 1000cm'1 (Characteristics of 4-methyl phenyl signals], 3500,1600,1400,720cm-1(Characteristics of pyrrol signals],3030,1500,1100,810,720 cm-1 (Characteristics of1.2.3- triazole system],1H-NMR (400MHz, DMSO-d6) spectrum of(( 1-(1-(4-methyl phenyl-2-yl)methyl)-1H-1,2,3-triazol' 4-yl]methyl]-3,3-di(1H-pyrrol-2-yl] indoline -2-one (7e], was recorded in DMSO-d6 showed the signals at δ PPm 7.0- 7.50(m,9H,C6H4and C6H5 rings],5.90-6.95(m,6H,bis-pyrrol ring],2.35(s,2H,CH2-flankedbetween indolone nucleus and1.2.3- triazole system], 2.35 (s, 2H, CH2-flanked between 1,2,3- triazole system and4-methyl phenyl ring]. 7.42(s,H,1,2,3-triazole ring]. Anal. Caled.(%] for C31H36N6O: C:73.20, H:7.13,N:16.52, found C:71.12,H:5.11,N:14.31.
Spectral data for the Compound-7f:
1-((1-(4-fluoro phenyl-2-yl)methyl)-1H-1,2,3-triazol-4-yl]methyl]-3,3-di(1H-pyrrol-2-yl] indoline-2-one(7f],Yield 70%, M.P-154-1560C,lR-(KBr] 2861 and 2841 cm- 1(Aliphatic CH str.],1743-1691 cm-1(C=O of indoline 2.3- dione] and 1294 cm-1 (C-N str. of indoline 2,3-dione], 3000,1600,1500,1210,900,750cm-1 (Characteristics of 4-fluoro phenyl signals], 3500, 1600, 1400, 720cm-1(Characteristics of pyrrol signals], 3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system],1H-NMR (400MHz, DMSO-d6] spectrum of(( 1-(1-(4-fluoro phenyl-2-yl)methyl)-1H-1,2,3- triazol-4-yl]methyl]-3,3-di(1H-pyrrol-2-yl] indoline -2-one (7f], was recorded in DMSO-d6 showed the signals at PPm 7.0- 7.50(m,9H,C6H4and C6H5 rings],5.90-6.95(m,6H,bis-pyrrol ring],2.35(s,2H,CH2-flankedbetween indolone nucleus and 1.2.3- triazole system], 2.35 (s, 2H, CH2-flanked between1,2,3- triazole system and4-fluoro phenyl ring). 7.42(s,H,1,2,3-triazole ring]. Anal. Caled.(%] for C30H33N6O: C:70.29, H:6.49, N:16.39, found C:68.15,H:4.25,N:14.24.
Spectral data for the Compound-7g:
1-((1-(4-tri fluoro phenyl-2-yl)methyl)-1H-1,2,3- triazol-4-yl]methyl]-3,3-di(1H-pyrrol-2-yl] indoline-2-one(7g], Yield 70%, M.P-154-1560C,lR-(KBr] 2861 and 2841 cm-1(Aliphatic CH str.],1743-1691 cm-1(C=O of indoline2.3-dione] and 1294 cm-1 (C-N str. of indoline 2,3-dione], 1350-1150,1000,1550,850,750cm-1(Characteristics of 4-trifluoro phenyl signals), 3500, 1600, 1400,720cm-1 (Characteristics of pyrrol signals],3030,1500,1100,810,720 cm- 1(Characteristics of 1,2,3-triazole system],1H-NMR (400MHz, DMSO-d6) spectrum of((1-(1-(4-tri fluoro phenyl-2-yl) methyl)-1H-1,2,3-triazol-4-yl)methyl)-3,3-di(1H-pyrrol-2-yl] indo line -2-one(7g), was recorded in DMSO-d6 showed the signals at 8 PPm 7.0-7.50(m,9H,C6H4and C6H5 rings),5.90- 6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 2.35 (s, 2H, CH2- flanked between1,2,3-triazole system and 4-tri fluoro phenyl ring). 7.42(s,H,1,2,3-triazole ring). Anal. Caled.(%) for C31H33N6O: C:66.18,H:5.91,N:14.94, found C:33.05,H:3.50,N:12.52.
Spectral data for the Compound-7h:
1-((1-(4-nitrophenyl-2-yl)methyl)-1H-1,2,3-triazol-4' yl)methyl)-3,3-di(1H-pyrrol-2-yl) indoline-2-one(7h), Yield 70%, M.P-154-1560C,IR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743-1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2,3-dione), 3100,2900,1620 ,1420,1000,850,800 cm-1 (Characteristics of 4-nitro phenyl signals), 3500, 1600, 1400,720cm-1(Characteristics of pyrrol signals),3030,1500,1100,810,720 cm-1 (Characteristics of1.2.3- triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of(( 1-(1-(4-nitro phenyl-2-yl)methyl)-1H-1,2,3-triazol'4- yl)methyl)-3,3-di(1H-pyrrol-2-yl) indoline -2-one (7h), was recorded in DMSO-d6 showed the signals at 8 PPm7.0- 7.50(m,9H,C6H4and C6H5 rings),5.90-6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2-flanked between indolone nucleus and 1.2.3- triazole system), 2.35 (s, 2H, CH2-flanked between1,2,3- triazole system and4-nitrophenyl ring). 7.42(s,H,1,2,3-triazole ring). Anal. Caled.(%) for C30H33N7O3: C:66.77,H:6.16,N:18.17, found C:33.54,H:3.03,N:16.05.
Preparation of Ag-NPs solution
Silver nanoparticlessolution was prepared using simple methodology by chemical reduction method reported, [24] using solution of AgNO3 and trisodium citrate was added with heating under magnetic stirring, then the solution turned to yellow colour. To confirm the forMicrobial Experimentationion of silver nanoparticles in this solution, we carried out an UV-visible absorption study and TEM imaging. In Fig. 1, a strong characteristic absorption peak around 400 nm is noted for the silver nanoparticles in the solution due to strong but broad surface plasmon peak has been well documented for various Ag-NPs size [25-27].
Transmission Electron Microscopy (TEM) images of the Ag- NPs solution which showed different size of Ag-NPswere recorded using a Zeiss Electron Microscope 10, operating at power 60 kV. TEM samples were prepared by dispersing 2- 3 drops of Ag-NPs solution on copper grid and dried at room temperature after removal of excess solution using a filter paper. A solution of compounds 7b,7a,7c,7h,7g,7f, and 7d were stirred with Ag-NPs solution, the residue products obtained in nano form were confirmed by TEM which showed different size of nano particles.
Microbial Experimentation
Microbial investigations were done to find the effect of some newly synthesized compounds against Gram +ve)Staphylococcus aureus ATCC (12600) and (Gram -ve) bacteria Escherichia coli ATCC (11775), in addition to their antifungal activity against Aspergillus flavus and Candida albicans with or without silver nanoparticles (Ag-NPs) solution. The preliminary studies of the biological assay were performed according to the agar diffusion method [28-31] at a concentration (25mg/mL) using DMSO as solvents. The results of the in vitro antimicrobial activity were recorded as average diameter of inhibition zone in mm, are given in Tables 1 & 2.
Anti bacterial activity:
The anti bacterial activity of 7b,7a,7c,7h were increased in presence of Ag-NPs solution against both test strains. There is no enhancing effect of 7f,7d,7g on the anti bacterial activities against Staphylococcus aureus and Escherichia coli. The highest fold increases in area were observed for 7b, 7a,7c and 7h in presence of Ag-NPs against both strains.
Anti fungal activity:
The anti fungal activity of 7b,7a,7c,7h were increased in presence of Ag-NPs solution against Aspergillus flavus. There is no enhancing effect of 7f,7d,7g on the anti fungal activities against Candida albicans. The highest fold increases in area were observed for 7b, 7a,7c and 7h in presence of Ag-NPs against both strains.
(Table 1 & 2)
Conclusion
The current study involved the design and synthesis of new heterocycles based on Bis-pyrrol indoline-2-ones moiety using simple synthetic route to evaluate their antimicrobial and antifungal activities, and study the effect of silver nanoparticles solution on their biological activities. Based on Bis-pyrrol indoline-2-ones triazole. Similar trends were noticed in anti bacterial and anti fungal studies of newly synthesized bis- pyrazoles in presence and absence ofAg-NPs.
Acknowledgement
One of the authors Dr. B. Saritha thank the authorities of S.K. University for providing necessary facilities for carrying out the work. Dept. Of Chemistry, and also the University grants commission for providing financial support (PDFW) Scheme 1.
To know more about Journal of chemistry,
Click here: https://juniperpublishers.com/omcij/index.php
To know more abour juniper Publishers,
click here: https://juniperpublishers.com/index.php
#Juniper Publishers Indexing Sites List#Juniper Publishers#Juniper Publishers group#Juniper Publisher Reviews#Juniper Publishers Indexing Sites#chemistry#organic chemistry#inorganic chemistry#chemistry journal#Open access Journal of chemistry#open access journals
0 notes
Text
Les hydrocarbures
Les hydrocarbures sont des composés organiques constitués d'une chaîne carbonée et d'atomes d'hydrogène. La chaîne carbonée peut être ouverte, linéaire ou ramifiée, ou peut comporter un ou plusieurs cycles. Lorsque tous les atomes de carbone de la chaîne sont reliés entre eux par une liaison covalente simple, on dit de l'hydrocarbure qu'il est saturé. Dans le cas contraire on dit qu'il est insaturé.
Les hydrocarbures saturés à chaîne ouverte sont appelés alcanes. Leur formule est CnH2n+2. Les hydrocarbures saturés en forme de boucle (formule CnH2n) sont appelés cycloalcanes. Les hydrocarbures insaturés à chaîne ouverte comportant une ou plusieurs liaisons carbone doubles sont des alcènes. Les alcènes en forme de boucle sont appelés cycloalcènes. Lorsqu'un hydrocarbure insaturé à chaîne ouverte comporte une liaison covalente triple on parle d'alcyne.
Hydrocarbures à chaîne cyclique
Comme nous l’avons dit plus haut, la chaîne carbonée peut comporter un ou plusieurs cycles. Lorsque les orbitales p des atomes de carbone du cycle se recouvrent, les électrons de ces orbitales sont délocalisés. Les hydrocarbures de ce type sont dits aromatiques. Les hydrocarbures à chaîne cyclique non aromatique (comme les cycloalcanes ou les cycloalcènes) sont appelés alicycliques. Les hydrocarbures alicycliques et les hydrocarbures à chaîne ouverte forment la sous-catégorie des hydrocarbures aliphatiques.
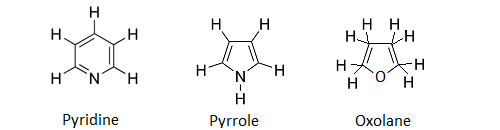
A noter qu’il existe aussi des hydrocarbure à chaîne hétérocyclique. Dans ces chaînes, un ou plusieurs atomes de carbone sont remplacés par un autre élément : azote (pyridine C5H5N, pyrrole C4H5N), oxygène (oxolane C4H8O) ou soufre (thiolane C4H8S). La purine (C5H5N4) et la pyrimidine (C4H4N2) sont des hétérocycles qui forment l’ossature des bases azotées constitutives des nucléotides.
Nomenclature des alcanes
Alcanes à chaîne linéaire :
méthane CH4
éthane C2H6
propane C3H8
butane C4H10
pentane C5H12
hexane C6H14
etc…
Alcanes à chaîne ramifiée :
Les alcanes à chaîne ramifiée comportent une chaîne carbone principale (la plus longue) et un ou plusieurs groupes alkyles. Un groupe alkyle est un groupe -CH2-(CH2)n-CH3. Ou tout simplement un groupe méthyl -CH3. Le nom de ce groupe s'obtient en remplaçant le suffixe -ane par le suffixe -yl. On donne à un alcane à chaîne ramifiée le nom de l'alcane correspondant à la chaîne principale précédé par le nom de l'alkyl. Par exemple, l’hydrocarbure dont la formule est CH3-(CH-CH3)-CH2-CH3 et appelé méthyl-butane. Le méthyl-butane est un isomère du pentane C5H12.
Cycloalcanes :
On donne aux cycloalcanes le nom de l'alcane comportant le même nombre d’atomes de carbone précédé du préfixe cyclo- (cyclopropane, cyclobutane, cyclopentane...).
Nomenclature des alcènes
On utilise les règles applicables aux alcanes en remplaçant le suffixe -ane par le suffixe -ène. L'éthène H2C=CH2 est plus connu sous le nom d’éthylène. Idem pour le propène H2C=CH-CH3 qui est aussi appelé propylène. Pour différencier les isomères entre eux on indique la position de la double liaison par un indice placé entre le radical et le suffixe -ène. Par exemple le pentène H3C-HC=CH-CH2-CH3 est appelé pent-2-ène.
Les composés H2C=CHR (R étant une chaîne carbonée) sont aussi appelés vinyles. (ou éthényles pour suivre les règles de l’Union internationale de chimie). Le chlorure de vinyle H2C=CHCl est un dérivé du vinyle très utilisé dans l’industrie. Il sert notamment à synthétiser le polychlorure de vinyle, autrement dit le PVC.
On donne aux cycloalcènes le nom de l'alcène correspondant comportant le même nombre d’atomes de carbone précédé du préfixe cyclo- (cyclopropène, cyclobutène, cyclopentène...).
Nomenclature des alcynes
Mêmes règles que pour les alcènes. L'éthyne C2H2 est plus connu sous le nom d'acétylène (H-CC-H).
Hydrocarbures aromatiques
Le plus simple et le plus connu des hydrocarbures aromatiques est le benzène C6H6. Les 6 atomes de carbone du benzène forment un hexagone et mettent en commun chacun un électron. Il existe de nombreux hydrocarbures dérivés du benzène, à commencer par le méthylbenzène C6H5-CH3 plus connu sous le nom de toluène. Le TNT (trinitrotoluène) dont la formule est H3C-CH6H2(NO2)3 est un dérivé du toluène.
Le nom des hydrocarbure dérivés du benzène suit la même règle que les hydrocarbures à chaîne ramifiée. Par exemple le C6H4-CH2-CH3 est appelé éthylbenzène. Les diméthylbenzènes (porteurs de deux groupes méthyl) sont appelés xylènes. Il existe aussi un triméthylbenzène (3 groupes méthyl) et un tetraméthylbenzène (4 groupes méthyl).
Il existe également des hydrocarbures dérivés du benzène portant un ou plusieurs groupes de type alcène. C’est le cas du styrène C6H5-CH=CH2 qui est le monomère du polystyrène.
Les hydrocarbures aromatiques polycycliques (HAP) peuvent former des motifs trés complexes. Le plus simple est le biphényle H5C6-C6H5. Le naphtalène C8H10 (la naphtaline de nos grands-mères) est un autre exemple de HAP. Les deux noyaux benzéniques qui le composent partagent deux atomes de carbone. La plupart des HAP sont toxiques. On donne le nom d’acènes aux hydrocarbures polycycliques aromatiques obtenus par adjonction linéaire de noyaux benzéniques (le naphtalène est un acène à deux cycles).
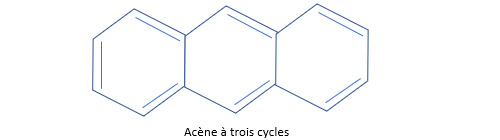
Remarque : la chimie du benzène est une chimie très riche. Le paracétamol HO-C6H4-NH-(C=O)-CH3 trône dans toutes les armoires à pharmacie à côté de l’acide acétylsalicilique (COOH) -C6H4-O-(C=O)-CH3, une molécule plus connue sous le nom d’aspirine.
Essence, kérosène et gazole
L'essence est un mélange d'alcanes à chaîne courte (octane, heptane...), de cycloalcanes, d'alcènes et de dérivés benzéniques auxquels sont ajoutés différents additifs.Le kérozène est un mélange d'alcanes comportant de 10 à 14 atomes de carbone (de C10H22 à C14H30).
Le gazole est composé de 75% d’alcanes (entre 10 et 16 atomes de carbone) et de paraffines (entre 18 et 30 atomes de carbone) et de 25% d’hydrocarbures aromatiques.
Essence, kérosène et gazole sont obtenus par distillation et raffinage du pétrole.
Pour en savoir plus :
post d’introduction à la chimie
post sur les composants élémentaires
post sur les électrons dans les liaisons covalentes
post sur les liaisons chimiques
post sur le carbone
post sur les propriétés chimiques des hydrocarbures
post sur les composés aromatiques
post sur la représentation topologique des molécules organiques
post sur les règles de nommage des molécules organiques
glossaire de chimie organique
post sur la classification périodique des éléments
glossaire de chimie générale
index
#hydrocarbure#chimie#alcane#cycloalcane#alcène#benzène#aliphatique#alicyclique#méthane#éthane#toluène#éthylène#acétylène#alcool#butane#propane#vinyle#polystyrène#styrène#gazole#essence#kérosène
0 notes
Text
Important Role of Chemical Industry
Chemical Industry Plays An Important Role In The Indian Economy. It Is The Most Diversified Of All Industrial Sectors. The Chemical Industry Covers More Than 70,000 Commercial Products. The Indian Chemical Industry Started Off With The Setting Up Of A Pharmaceutical Unit Near Calcutta In 1901. Buy online Chemicals and Chemical Products India's largest shopping store shopperbe.com for B2B Products and Equipment.
Chemical industry: India’s chemical industry is likely to touch $214 billion (approx ₹13,91,000 crore) in the next four years from $139 (approx ₹9,03,500 crores) in fiscal 2014 with estimated growth of around 9 percent a year amid growing demand scenario.

The Chemical Industry Is The Fourth Largest Industry In Size After Iron And Steel, Textile And Engineering Industries. Both Organic And Inorganic Chemical Industries. The Industry Manufactures A Wide Range Of Products Like Fertilizers, Drugs, Dye, Stilts, Pesticides, Paints And Plastic Etc.
Target Market: 1. Agriculture 2. Automotive & Transportation 3. Chemicals 4. Energy & Water 5. Industrial 6. Infrastructure 7. Construction 8. Electronics & Electric 9. Energy & Resources 10. Furniture & Wood 11. Home Care and I&I Cleaning 12. Nutrition 13. Packaging & Print 14. Paints & Coatings 15. Personal Care & Hygiene 16. Pharmaceuticals 17. Plastics & Rubber 18. Pulp & Paper 19. Leather, Footwear & Textile.

Chemical Type: 1. Methanol 2. Hydrated Phenol 3. Acetic Acid 4. Toluene 5. N-Hexane 6. Vinyl Acetate Monomer 7. MIBK 8. Styrene Monomer 9. Normal Butanol 10. Ethyl Acetate 11. Acetone 12. Molten Phenol 13. Butyl Acrylate 14. LAWS/MTO 15. Butyl Glycol 16. IPA 17. Mix Xylene 18. Cyclo-Hexanone 19. Butyl Acetate 20. MDC 21. C-9 22. MEG 23. Melamine 24. MEK 25. Crystal Phenol 26. Ortho-Xylene 27. PVC Resin K65 28. PVA Kuraray 173 29. MIBK 30. IPA Intact Drum.
Chemicals In Construction Industry
Chemicals Are Used In Construction Industry For Applications Like Waterproofing Solutions, Concrete Repair And Rehabilitation, Industrial Flooring, Sealants And Adhesives, Asphalt Modifiers, Protective Coatings Etc.
Though The Construction Industry Is Experiencing A Huge Growth, The Case Isn’t The Same With Construction Chemicals. The Lack Of Awareness And Training Regarding Its Usage Is A Major Threat To The Growth Of The Industry.
Concrete Admixtures Contribute To More Than Half The Share Of Construction Chemicals. The Share Is Expected To Grow In The Upcoming Years.
Concrete Admixtures Are Used To Alter The Properties Of The Mixture Like The Concrete Quality, Setting Time, Chemical And Physical Resistance, Concrete Workability, And Finishing. The Rising Demand For Customized Residential And Commercial Buildings Is Set To Boost The Use Of Concrete Admixtures.

Concrete Admixtures Can Be Then Classified Into Chemical And Mineral Admixtures. Chemical Admixtures Include Plasticizers, Accelerating And Retarding Agents, Air-entraining Agents, Waterproofing Admixtures. Whereas Mineral Concrete Admixtures Include Rice Husk Ash, Fly Ash, Granulated Blast Furnace Slag, And Silica Fume.
Sealants Are Substances That Are Used To Block The Passage Of Fluids Through The Surface, Joints Or Openings In Materials. Sealants Are Especially Used In Interior And Exterior Waterproofing, Fireproofing. Different Varieties Of Sealants Include Roof Sealants, Bathroom Sealants, Windows And Door Sealants, Joint Sealants Etc.
Join us: Facebook, Twitter, LinkedIn, Pinterest
#chemicals#shopping#buy online#construction industry#market#commercial products#Waterproofing Admixtures.#best quality#marketplace
0 notes