#astrocytes
Text
Secrets Revealed
Insight into the formation and maintenance of the blood-brain barrier and astrocyte-neuron interactions by analysing the brain cells' secretome – the molecules secreted by cells into the extracellular space
Read the published research paper here
Image from work by Miriam Burmeister and Annika Fraunenstein, and colleagues
Max Planck Institute for Biochemistry, Martinsried, Germany and Institute of Physiological Chemistry and Pathobiochemistry, University of Muenster, Münster, Germany
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Science Advances, July 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#immunofluorescence#blood brain barrier#astrocytes#brain#brain cells#neurobiology#secretome
31 notes
·
View notes
Text
Daily cell culture life be like:
Astrocytes are so 🥺🤏🏼

#astrocytes#neuroscience#science#university#undergrad student#cell culture#neuroscientist#stem research#women in stem#scienceinternship#daily life#workstation
6 notes
·
View notes
Text
Interesting Reviews for Week 49, 2022
Genetics and neurobiology of eating disorders. Bulik, C. M., Coleman, J. R. I., Hardaway, J. A., Breithaupt, L., Watson, H. J., Bryant, C. D., & Breen, G. (2022). Nature Neuroscience, 25(5), 543–554.
Anxiety and hippocampal neuronal activity: Relationship and potential mechanisms. Ghasemi, M., Navidhamidi, M., Rezaei, F., Azizikia, A., & Mehranfard, N. (2022). Cognitive, Affective, & Behavioral Neuroscience, 22(3), 431–449.
Control of complex behavior by astrocytes and microglia. Ortinski, P., Reissner, K., Turner, J., Anderson, T. A., & Scimemi, A. (2022). Neuroscience & Biobehavioral Reviews, 137, 104651.
#science#Neuroscience#computational neuroscience#Brain science#research#reviews#cognition#scientific publications#astrocytes
4 notes
·
View notes
Text
How sensory gamma rhythm stimulation clears amyloid in Alzheimer’s mice
New Post has been published on https://thedigitalinsider.com/how-sensory-gamma-rhythm-stimulation-clears-amyloid-in-alzheimers-mice/
How sensory gamma rhythm stimulation clears amyloid in Alzheimer’s mice


Studies at MIT and elsewhere are producing mounting evidence that light flickering and sound clicking at the gamma brain rhythm frequency of 40 hertz (Hz) can reduce Alzheimer’s disease (AD) progression and treat symptoms in human volunteers as well as lab mice. In a new open-access study in Nature using a mouse model of the disease, MIT researchers reveal a key mechanism that may contribute to these beneficial effects: clearance of amyloid proteins, a hallmark of AD pathology, via the brain’s glymphatic system, a recently discovered “plumbing” network parallel to the brain’s blood vessels.
“Ever since we published our first results in 2016, people have asked me how does it work? Why 40Hz? Why not some other frequency?” says study senior author Li-Huei Tsai, Picower Professor of Neuroscience and director of The Picower Institute for Learning and Memory of MIT and MIT’s Aging Brain Initiative. “These are indeed very important questions we have worked very hard in the lab to address.”
The new paper describes a series of experiments, led by Mitch Murdock PhD ’23 when he was a brain and cognitive sciences doctoral student at MIT, showing that when sensory gamma stimulation increases 40Hz power and synchrony in the brains of mice, that prompts a particular type of neuron to release peptides. The study results further suggest that those short protein signals then drive specific processes that promote increased amyloid clearance via the glymphatic system.
“We do not yet have a linear map of the exact sequence of events that occurs,” says Murdock, who was jointly supervised by Tsai and co-author and collaborator Ed Boyden, Y. Eva Tan Professor of Neurotechnology at MIT, a member of the McGovern Institute for Brain Research and an affiliate member of the Picower Institute. “But the findings in our experiments support this clearance pathway through the major glymphatic routes.”
How sensory gamma rhythm stimulation clears amyloid in Alzheimer’s mice
Video: The Picower Institute
From gamma to glymphatics
Because prior research has shown that the glymphatic system is a key conduit for brain waste clearance and may be regulated by brain rhythms, Tsai and Murdock’s team hypothesized that it might help explain the lab’s prior observations that gamma sensory stimulation reduces amyloid levels in Alzheimer’s model mice.
Working with “5XFAD” mice, which genentically model Alzheimer’s, Murdock and co-authors first replicated the lab’s prior results that 40Hz sensory stimulation increases 40Hz neuronal activity in the brain and reduces amyloid levels. Then they set out to measure whether there was any correlated change in the fluids that flow through the glymphatic system to carry away wastes. Indeed, they measured increases in cerebrospinal fluid in the brain tissue of mice treated with sensory gamma stimulation compared to untreated controls. They also measured an increase in the rate of interstitial fluid leaving the brain. Moreover, in the gamma-treated mice he measured increased diameter of the lymphatic vessels that drain away the fluids and measured increased accumulation of amyloid in cervical lymph nodes, which is the drainage site for that flow.
To investigate how this increased fluid flow might be happening, the team focused on the aquaporin 4 (AQP4) water channel of astrocyte cells, which enables the cells to facilitate glymphatic fluid exchange. When they blocked APQ4 function with a chemical, that prevented sensory gamma stimulation from reducing amyloid levels and prevented it from improving mouse learning and memory. And when, as an added test, they used a genetic technique for disrupting AQP4, that also interfered with gamma-driven amyloid clearance.
In addition to the fluid exchange promoted by APQ4 activity in astrocytes, another mechanism by which gamma waves promote glymphatic flow is by increasing the pulsation of neighboring blood vessels. Several measurements showed stronger arterial pulsatility in mice subjected to sensory gamma stimulation compared to untreated controls.
One of the best new techniques for tracking how a condition, such as sensory gamma stimulation, affects different cell types is to sequence their RNA to track changes in how they express their genes. Using this method, Tsai and Murdock’s team saw that gamma sensory stimulation indeed promoted changes consistent with increased astrocyte AQP4 activity.
Prompted by peptides
The RNA sequencing data also revealed that upon gamma sensory stimulation a subset of neurons, called “interneurons,” experienced a notable uptick in the production of several peptides. This was not surprising in the sense that peptide release is known to be dependent on brain rhythm frequencies, but it was still notable because one peptide in particular, VIP, is associated with Alzheimer’s-fighting benefits and helps to regulate vascular cells, blood flow, and glymphatic clearance.
Seizing on this intriguing result, the team ran tests that revealed increased VIP in the brains of gamma-treated mice. The researchers also used a sensor of peptide release and observed that sensory gamma stimulation resulted in an increase in peptide release from VIP-expressing interneurons.
But did this gamma-stimulated peptide release mediate the glymphatic clearance of amyloid? To find out, the team ran another experiment: They chemically shut down the VIP neurons. When they did so, and then exposed mice to sensory gamma stimulation, they found that there was no longer an increase in arterial pulsatility and there was no more gamma-stimulated amyloid clearance.
“We think that many neuropeptides are involved,” Murdock says. Tsai added that a major new direction for the lab’s research will be determining what other peptides or other molecular factors may be driven by sensory gamma stimulation.
Tsai and Murdock add that while this paper focuses on what is likely an important mechanism — glymphatic clearance of amyloid — by which sensory gamma stimulation helps the brain, it’s probably not the only underlying mechanism that matters. The clearance effects shown in this study occurred rather rapidly, but in lab experiments and clinical studies weeks or months of chronic sensory gamma stimulation have been needed to have sustained effects on cognition.
With each new study, however, scientists learn more about how sensory stimulation of brain rhythms may help treat neurological disorders.
In addition to Tsai, Murdock, and Boyden, the paper’s other authors are Cheng-Yi Yang, Na Sun, Ping-Chieh Pao, Cristina Blanco-Duque, Martin C. Kahn, Nicolas S. Lavoie, Matheus B. Victor, Md Rezaul Islam, Fabiola Galiana, Noelle Leary, Sidney Wang, Adele Bubnys, Emily Ma, Leyla A. Akay, TaeHyun Kim, Madison Sneve, Yong Qian, Cuixin Lai, Michelle M. McCarthy, Nancy Kopell, Manolis Kellis, and Kiryl D. Piatkevich.
Support for the study came from Robert A. and Renee E. Belfer, the Halis Family Foundation, Eduardo Eurnekian, the Dolby family, Barbara J. Weedon, Henry E. Singleton, the Hubolow family, the Ko Hahn family, Carol and Gene Ludwig Family Foundation, Lester A. Gimpelson, Lawrence and Debra Hilibrand, Glenda and Donald Mattes, Kathleen and Miguel Octavio, David B. Emmes, the Marc Haas Foundation, Thomas Stocky and Avni Shah, the JPB Foundation, the Picower Institute, and the National Institutes of Health.
#aging#Alzheimer's#astrocytes#blood#blood vessels#Brain#Brain and cognitive sciences#brain research#brains#cell#cell types#Cells#change#channel#chemical#cognition#data#direction#Disease#disorders#effects#Events#Experienced#express#fluids#Foundation#genes#genetic#Health#how
0 notes
Text
1 note
·
View note
Link
We measured markers of neurologic injury (glial fibrillary acidic protein [GFAP], neurofilament light chain [NfL]) and soluble markers of inflammation among a cohort of people with prior confirmed SARS-CoV-2 infection at early and late recovery after the initial illness...
Self-reported neurologic symptoms present approximately 4 months after SARS-CoV-2 infection are associated with elevations in markers of neurologic injury and inflammation at earlier time points. Some inflammatory pathways seem to be involved months after acute infection. Additional work will be needed to better characterize these processes and to identify interventions to prevent or treat this condition.
We found that those reporting CNS PASC symptoms approximately 4 months after initial infection had earlier elevations in several biomarkers, including GFAP, IL-6, and TNFα, suggesting that the acute infection resulted in direct CNS tissue injury and systemic inflammation, both of which might conceivably be causally related to the development of CNS PASC symptoms. Elevations in IL-6 and TNFα persisted through approximately 4 months of recovery. Replication of these findings in larger and more diverse cohorts may be a first step toward identifying interventions for their prevention and/or management.
...the fact that those reporting CNS PASC symptoms had a greater number of PASC symptoms overall makes it challenging to disentangle a more specific CNS PASC phenotype from more severe PASC in general. It suggests that CNS PASC might reflect 1 extreme on a spectrum of illness. Further work to compare those with distinct phenotypic clusters of symptoms, if they exist, could further elucidate the biology.
Dysregulation of IL-6 and TNFα is potentially deleterious in inflammatory disease states, related to systemic and localized tissue inflammation and endothelial dysfunction. The reason for elevations in levels of these markers among those with CNS PASC is not clear. One possibility is that they represent residual inflammation from the period of acute infection that is slower to resolve among those with PASC. However, the identification of persistent differences months after infection suggests other possibilities such as a delayed return to immunologic homeostasis related to a persistent immune response caused by an ongoing pathophysiologic process or processes (e.g., persistent antigenic stimulation,38 microvascular dysfunction,39 and autoimmunity40).
A handful of studies have explored the measurement of serum glial fibrillary acidic protein (GFAP), an intermediate filament protein found in the cytoskeleton of CNS astrocytes,10 and neurofilament light chain (NfL), a cytoskeletal protein expressed in the axons of neurons11 during acute COVID-19.12,-,19 In some cases, higher levels of these markers were identified in patients with neurologic symptoms during acute infection13,-,17; other studies have not identified such relationships.
We identified elevations in GFAP during early recovery that were associated with later CNS PASC. Although we did not observe elevations in NfL at either time point, we did identify a more precipitous decline among those reporting CNS PASC. Together, these observations lend support to the possibility of early injury that might resolve while clinical symptoms persist. Based on autopsy studies, acute SARS-CoV-2 can access the CNS. A correlation between severity of COVID-19 and levels of NfL and GFAP in the acute phase has been observed,13,-,17 and a recent study showed structural brain changes in those with prior COVID-19.20 However, studies of the trajectories of NfL and GFAP levels during the weeks after symptom onset have been inconsistent.19,29,34 Our findings are in line with studies that identified a correlation between NfL and/or GFAP with severity of reported neurologic symptoms during the acute phase, but found no association between the biomarker levels and persistence of neurologic symptoms after 6 months.
Astrocyte dysfunction, as reflected in increased plasma GFAP observed here, could relate to the emerging cognitive PASC complaints, which can encapsulate attention and working memory deficits.
0 notes
Text
Growing bad due to bad influences: here go guilty astrocytes hosing the cancer mass with cholesterol
Growing bad due to bad influences: here go guilty astrocytes hosing the cancer mass with cholesterol
Glioblastoma is an extremely aggressive and invasive brain cancer, for which there exists no known effective treatment. The tumor cells are highly resistant to all known therapies, and, sadly, patient life expectancy has not increased significantly in the last 50 years. A groundbreaking study at Tel Aviv University effectively eradicated glioblastoma by combining existing molecules. The…

View On WordPress
#astrocytes#blood-brain barrier#brain cancer#brain cortex#cancer cells#cellular energy#cholesterol#glioblastoma#macrophages
0 notes
Text
Some autism spectrum disorder symptoms linked to astrocytes -- ScienceDaily
Some autism spectrum disorder symptoms linked to astrocytes — ScienceDaily
Abnormalities in a type of brain cell called astrocytes may play a pivotal role in causing some behavioral symptoms of autism spectrum disorders, according to a preclinical study by Weill Cornell Medicine investigators.
For the study, published April 1 in Molecular Psychiatry, senior author Dr. Dilek Colak, assistant professor of neuroscience at the Feil Family Brain and Mind Research Institute…

View On WordPress
#astrocytes#autism#Birth Defects; Stem Cells; Brain Tumor; Nervous System; Autism; Learning Disorders; Disorders and Syndromes; Brain-Computer Interfaces#disorder#linked#ScienceDaily#Spectrum#symptoms
0 notes
Photo



Some of my favorite Destiny 2 helmets with a slightly alternative look.
#destiny 2#destiny 2 fanart#destiny hunter#destiny warlock#destiny titan#destiny the game#Graviton Forfeit#An Insurmountable Skullfort#Astrocyte Verse#reb3l musings#my art#reb3ls art
267 notes
·
View notes
Text
what the fuck even is a neuron. "describe how neuronal activity drives pathway-specific depolarization of peripheral astrocyte processes" i'll drive ur pathway-specific despoliation of astrocyte processes what the fuck i'm gonna fail this module idk what a brain is i feel so underqualified wdym test on 15 march i haven;t had a thought in my life
#i want a neuroscience-adjecent career and yet i am abt to run this module into the ground istg#are u ever so anxious that you end up doing nothing which u cant afford bc u have to catch up to many modules in a very short space of time#yeah#personal#studyrants#block this tag if u dont want to see these posts ill probably post a lot abt the horrors#GEVI expression colocalized with the astrocyte marker glutamine synthase 🤪#just tell me ur in love w me
12 notes
·
View notes
Photo
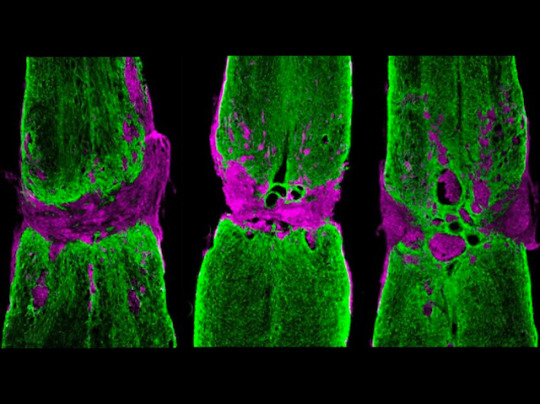
Barrier Breakthrough
Your body contains hundreds of nerves but they can't all regenerate. After injury, peripheral nerves replace damaged sections but nerves in your central nervous system (CNS) can’t. Instead, brain cells called astrocytes cordon off damaged tissue (lesions) to help preserve healthy nerve tissue. These lesions form a barrier, preventing regeneration. Transplants of neural progenitor cells (NPCs), made from stem cells, may help. Researchers investigate by tagging NPCs and transplanting them, via a hydrogel, into uninjured or injured mouse CNS. RNA analysis revealed NPCs in uninjured mice matured into cells resembling healthy astrocytes, while NPCs in injured mice matured into cells resembling ‘reactive’ astrocytes, which arise after injury to partition off lesions. Fluorescence microscopy of injured CNS (pictured) revealed that adding NPCs (right) reduced lesion size (magenta) and helped bridge lesions via astrocytes (green) compared with injured CNS without NPCs (left) or only hydrogel (middle). The injury microenvironment, therefore, directs NPCs towards wound repair.
Written by Lux Fatimathas
Image from work by T. M. O’Shea and colleagues
Department of Neurobiology, David Geffen School of Medicine, University of California, Los Angeles, CA, USA
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Nature Communications, September 2022
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#immunofluorescence#neuroscience#nerves#central nervous system#peripheral nerves#rna-seq#astrocytes#hydrogel#neural progenitor cells#stem cells
29 notes
·
View notes
Text
Today was a very special day for me, my first poster presentation!!
Considering that it was an international neuroscience conference I can say that I was a bit nervous but everything turned out to be a great experience! Therefore I advise you guys to persue your academic dreams and just go for it! It was a great opportunity for networking and learning! I'm so glad that I did it!
🧠🤍

#scienceinternship#astrocytes#neuroscience#science#university#federal university#ufsc#ufrgs#women in stem#stem research#undergrad student#cell culture#glial cells
3 notes
·
View notes
Text
Fixing Rogue Brain Cells May Hold Key to Preventing Neurodegeneration - Technology Org
New Post has been published on https://thedigitalinsider.com/fixing-rogue-brain-cells-may-hold-key-to-preventing-neurodegeneration-technology-org/
Fixing Rogue Brain Cells May Hold Key to Preventing Neurodegeneration - Technology Org
A team led by scientists at Case Western Reserve University School of Medicine has identified a new therapeutic approach for combating neurodegenerative diseases, offering hope of improved treatments for Alzheimer’s disease, Parkinson’s disease, Vanishing White Matter disease and multiple sclerosis, among others.
Astrocytes. Image credit: CWRU
Neurodegenerative diseases, which affect millions of people worldwide, occur when nerve cells in the brain or nervous system lose function over time and ultimately die, according to the National Institutes of Health. Alzheimer’s disease and Parkinson’s disease are the most common.
The research team’s new study, published online in Nature Neuroscience, focused on astrocytes—the brain’s most abundant cells that normally support healthy brain function. Growing evidence indicates astrocytes can switch to a harmful state that increases nerve-cell loss in neurodegenerative diseases.
The researchers created a new cellular technique to test thousands of possible medications for their ability to prevent these rogue astrocytes from forming.
“By harnessing the power of high-throughput drug screening, we’ve identified a key protein regulator that, when inhibited, can prevent the formation of harmful astrocytes,” said Benjamin Clayton, lead author and National Multiple Sclerosis Society career transition fellow in the laboratory of Paul Tesar at the Case Western Reserve School of Medicine.
They found that blocking the activity of a particular protein, HDAC3, may prevent the development of dangerous astrocytes. The scientists discovered that by administering medications that specifically target HDAC3, they were able to prevent the development of dangerous astrocytes and significantly increase the survival of nerve cells in mouse models.
“This research establishes a platform for discovering therapies to control diseased astrocytes and highlights the therapeutic potential of regulating astrocyte states to treat neurodegenerative diseases,” said Tesar, the Dr. Donald and Ruth Weber Goodman Professor of Innovative Therapeutics and the study’s principal investigator.
Tesar, also director of the School of Medicine’s Institute for Glial Sciences, said more research needs to be done before patients might benefit from the promising approach. But, he said, their findings could lead to the creation of novel therapies that disarm harmful astrocytes and support neuroprotection—perhaps improving the lives of people with neurodegenerative illnesses in the future.
“Therapies for neurodegenerative disease typically target the nerve cells directly,” Tesar said, “but here we asked if fixing the damaging effects of astrocytes could provide therapeutic benefit. Our findings redefine the landscape of neurodegenerative disease treatment and open the door to a new era of astrocyte targeting medicines.”
Source: Case Western Reserve University
You can offer your link to a page which is relevant to the topic of this post.
#Aging news#Alzheimer's disease#approach#astrocytes#Biotechnology news#Brain#brain cells#career#cell#Cells#development#Disease#Diseases#drug#effects#Featured life sciences news#Future#Health#Health & medicine news#Landscape#LED#Link#matter#medications#Medicine#nature#nerve cells#nervous system#neurodegeneration#neurodegenerative diseases
1 note
·
View note
Text

star shaped alien thingy i guess
2 notes
·
View notes
Text







Made a bunch of brain cells and a microscope lens for an Art in Science contest, won first place!
0 notes