#barium
Text
A new vibrant blue pottery pigment with less cobalt
Whether ultramarine, cerulean, Egyptian or cobalt, blue pigments have colored artworks for centuries. Now, seemingly out of the blue, scientists have discovered a new blue pigment that uses less cobalt but still maintains a brilliant shine.
Though something like this might only happen once in a blue moon, the cobalt-doped barium aluminosilicate colorant described in ACS Applied Optical Materials withstands the high temperatures found in a kiln and provides a bright color to glazed tiles.
Many of the brilliant blue pigments—like those in antique Chinese porcelain or works by Claude Monet—make use of cobalt-based compounds, including the famous "cobalt blue." Though the metal itself is toxic, in mineral form, it has high chemical and thermal stability, and those properties make cobalt aluminate one of the only pigments suitable for high-temperature applications, including pottery glazes.
Read more.
11 notes
·
View notes
Link
Out there in the wider Universe there are some really strange worlds that make the planets we have here in our Solar System seem almost mundane.
Super-Mercuries and mini-Neptunes, for instance, challenge expectations of planetary development. And then there's the hot Jupiters; and, for good measure, the ultra-hot Jupiters, worlds so close to their host stars that their atmospheres are thick with clouds of heavy elements, vaporized by the extreme heat.
Now, in two of the most hardcore worlds ever seen in the Milky Way, astronomers have detected the heaviest metal yet. In the atmospheres of exoplanets WASP-76b and WASP-121b drift clouds of barium – the 56th element on the periodic table.
Previous searches had found calcium, titanium oxide, and vanadium oxide in the atmosphere of WASP-76b, and vanadium, iron, chromium, calcium, sodium, magnesium, and nickel in the atmosphere of WASP-121b. That iron could even pelt down at the twilight horizon in a rain nobody would want to sing in.
But barium wafting through the air at high altitudes takes the whole shindiggery to another level.
"The puzzling and counterintuitive part is: why is there such a heavy element in the upper layers of the atmosphere of these planets?" says astronomer Tomás Azevedo Silva of the University of Porto and the Institute of Astrophysics and Space Sciences (IA) in Portugal.
"We were not expecting or looking for barium in particular and had to cross-check that this was actually coming from the planet since it had never been seen in any exoplanet before."
Continue Reading.
126 notes
·
View notes
Text
this was one of the few to survive the solar eclipse dunting dumpster fire, it's hard not to share;


and there's a few reasons why i was hesitant to let this one grow on me for further exploration...
it's a high Barium crystalline.
and here's a fun little piece from the msds take on it:

there's already plenty of stuff related to every part of the pottery making process that's actively trying to kill me, plus working out of a basement makes dealing with it a bit too much to be ok with. likely wouldn't be so hesitant on this end if there was proper aka very expensive industrial ventilation and/or being used on ground level [aka better air flow in general]
2. the ever-existing need to consider leaching once it's fired.
fun facts:
glazes undergo the high temps of a firing to melt and fuse the various components together. a stable glaze will have the proper ratio of fluxes, glass formers and stabilizers to enable vitrification, aka glass forming. there's some general info out there about a ballpark ratio of these but everything in this really comes down to testing every recipe.
crystals grow in a glaze when the right components and conditions break out of the glass forming bond to begin feeding the growing structure, aka devitrifying aka no longer a unified glass and all those things in the glaze can continue interacting with exposure. at a far faster rate, mind you, but still detectable with everyday use [acids and alkaline stuffs will have a heyday over time with this.] so in this setting, Barium can continue doing it's natural thing of being toxic af.
this is specifically why i use a reliably stable liner glaze on all functional interiors or food/mouth-contact surfaces for when you slurp backwash while making out with the mug you freaks.
not trying to be the reaper, rather, a considerate potter who wants everyone healthy, safe and inspired while using my work.
and for real, hard pass for dying on the job.
but it's so pretty!


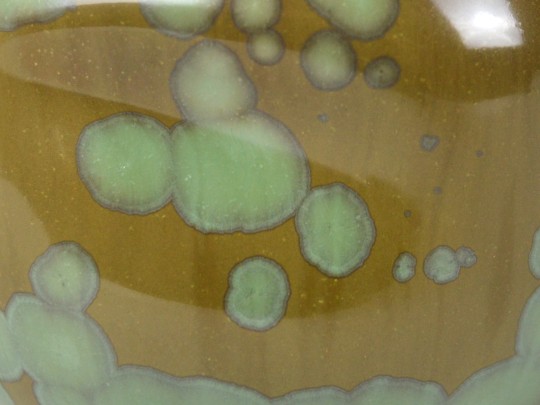
so when the time is right [aka safe] i'll be exploring more Ba crystalline glazes for sculptural projects
#pottery#ceramics#clay#wheelthrownpottery#porcelain#full time artist#glaze testing#crystal growing#chemistry#barium#msds#fun facts#the more you know
10 notes
·
View notes
Text
Round 1 - Part 4 - Matchup 10
Radium vs Barium


30 notes
·
View notes
Text
Round 2 - Alkaline Earth Metals
2 notes
·
View notes
Text

[Why would Munch want barium?]
6 notes
·
View notes
Text
my tummy hurts and I’m being so brave about it that I’m actually committing to the bit for once
barium sulfate is used in digestive imaging tests because it absorbs x-rays readily and also fluoresces green
2 notes
·
View notes
Text
ALKALINE EARTH METALS:
ROUND 1 POLL 3


BARIUM:
Used to make drilling mud
When barium sulfate was discovered, it was mistaken to be the philosopher's stone
RADIUM:
Known for its green glow and high radioactivity
Caused the radium girls incident
2 notes
·
View notes
Text

I’m finished 1 bottle, I need to drink another 🤢 i’ve puked 3 times in my mouth and had to swallow it EVERYTIME. I WISH I WAS DEAD BRO THIS SUCKS SO BAD
1 note
·
View note
Text

All eyes on you, Your Highness.
@poisonappletales
#poisonappletales#fanart#batw#beauty and the war#beauty and the war x playing pieces#x playing pieces#xpp#barium#the second ever barium fanart :') poor guy#hes only in the first demo so it makes sense
5 notes
·
View notes
Photo

Thinnest-ever freestanding film with ferroelectric properties
Researchers at the Institute for Future Materials and Systems at Nagoya University in Japan have successfully synthesized barium titanate (BaTiO3) nanosheets with a thickness of 1.8 nanometers, the thinnest thickness ever created for a free-standing film. Given that thickness is related to functionality, their findings open the door to smaller, more efficient devices. The research was published in the journal Advanced Electronic Materials.
The development of ever-thinner materials with new electronic functions is a highly competitive area of research. Such devices are especially important in ferroelectrics, materials that have a polarization that can be reversed by an electric field. This ability to reverse polarization makes these materials useful in memory and vibrational power generation.
However, as the materials used in these devices become smaller, they exhibit unexpected properties that complicate their industrial use. A big problem is the "size effect," as when the material's thickness is reduced to a few nanometers, its ferroelectric properties disappear.
Read more.
#Materials Science#Science#Ferroelectricity#Thin films#Barium titanate#Nanotechnology#Barium#Titanium#Electronics#Nagoya University
17 notes
·
View notes
Photo


Another LARGE flat color height chart commission for @lorna-rosefox! featuring star skins for several main and side characters in her fanfic. Of course, Oxide’s outfit is canon, and Lorna’s outfit is slightly altered from a pre-existing outfit of hers-- I changed the color of her arm straps and made it clearer that the thing on her leg is a communicator/radio.
As for the star outfits for the others, they are completely new! Lupin’s outfit is modified from an old sketch I planned to use for the other height chart he appears in. Zam’s outfit is based on coyote armor. Zem’s outfit is slightly biker-inspired, particularly his helmet. The gasmoxian trio’s outfits are meant to resemble space suits (kind of like the ones Koala Kong and Spyro wear in the Gasmoxia Grand Prix) but with shimmery star patterns decorating them. Also, Barium’s outfit colors are loosely based off wonder bread. LOL.
#commission#art#commissions#art commissions#height chart#lorna-rosefox#digital art#digital drawing#others' ocs#as usual left to right:#Lorna Rosé#Lupin Tremolo#Nitros Oxide#Zam#Zem#Cobalt#Barium#Pyro#gasmoxians#aliens#fashion design#outfit design#crash bandicoot#maybe calling this flat color is a bit of a stretch because of all the shiny parts but hey. thats just a bonus isn't it#anyways I worked on this for over 24 hours total apparently#holy shit#completed
6 notes
·
View notes
Text
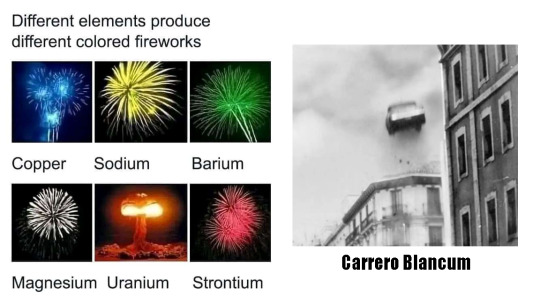
#petardos#petardo#fireworks#copper#cobre#sodium#sodio#barium#bario#magnesium#magnesio#uranium#uranio#strontium#estroncio#carrero#carrero blanco#blanco#meme
0 notes
Text
Dimanche
View On WordPress
#aluminium#Antenna#barium#Geo engineering#Nanoparticulate heavy metals#strontium#Weather manipulation
0 notes
Text
So my last post reminded me of this story so I'm going to tell it here
So when i was a tiny child, probably around 5 or so, i decided to swallow a coin, and not because of typical child reasons of putting everything in mouth and oops swallowed it, no this was a decision in which i knew exactly what i was doing and as a child with adhd and no impulse control i chose to ignore the consequences, sadly though my sibling was a snitch and immediately told my mother
Now this would normally be the end of the story, except my body doesn't like to behave and so after my mother didn't find the coin in my poop after a couple of days (i don't remember exactly how many but anywhere from 4-6), she brought me to my pediatrician who didn't quite believe that it hadn't yet left my body, but still she had me go to get an x-ray
Now when i went to get said x-ray they had me drink this horrendous concoction that had something called barium in it to help with the contrast in the x-ray or something like that, and after a reasonable reaction to the awful taste they added chocolate milk powder flavoring so i could actually get it down, which i was able to do, and after suffering through the barium they did the x-ray and low and behold the coin was in fact still inside me, albeit nearly out
#x ray#barium#coin#dumb child things#i cannot overstate how awful barium tastes#and in addition to the horrible taste it's also very thick#i hate it so much#i did get to keep the coin tho#so that's cool#my poor mother tho#she had to look through my poop to find that coin
1 note
·
View note
Text
These trends are illustrated in table 5.1, which gives some lattice energy values for ionic species.
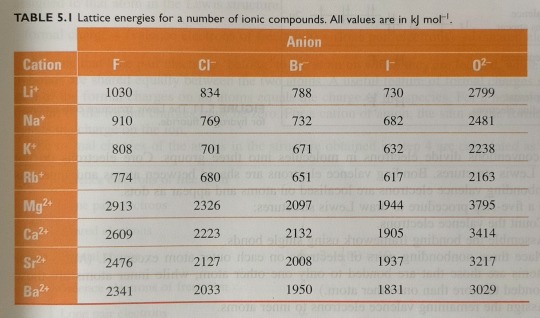
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#trends#ionic compounds#ions#cation#anion#lithium#sodium#potassium#rubidium#calcium#strontium#barium#fluorine#chlorine#bromine#iodine#oxygen
0 notes