#chem tips
Text
tips for acing chem ʕ•́ᴥ•̀ʔっ
(disclaimer: this worked for me. It may not work for you, and that’s okay! Keep trying, you can do it!)
Continuous exposure to chemistry: One of the biggest things that helped me in chemistry was the continuous exposure I had beforehand. I had plenty of books that I read about chemistry, watched documentaries, had an interactive book about all the elements, e.t.c. Continuous exposure allows you to retain a lot more information beforehand, and it is different from studying. Studying is BORING, continuous exposure and learning IS NOT. Allow yourself to have fun while learning chem!
Watch videos about things you don’t understand and apply them yourself: The most challenging thing in chemistry was probably equations. You should probably watch videos to learn this. There are tons of videos out on YouTube, Khan Academy and more. Not only should you watch these videos but try to follow along and apply them into questions. If, like Khan Academy, there are ways you can do a little quiz or test yourself, be sure to do that! It helps a lot.
Ask for help from peers: This, 100%, is a great way to learn. If I was feeling sluggish or tired, I would ask for help from peers, and write down what they said. I would also take pictures of their notes and learn from that as well, later on. It helps a lot to learn from someone just like you, so you can understand better.
Active studying: I think this is a very common tip, but it is so important!! You may have a textbook or something to follow on in classes, so here’s what to do: read the textbook chapters and summarize them in your own way. It helps so much. Writing about each section or chapter allows you to take that information, and write it in a different way while still having the most important parts. DO NOT highlight or read. That is passive studying, and trust me, it does not help. Write down, summarize, do a mind map but do NOT highlight or read. It’s okay to do that for help but it really isn’t the best way to handle a concept and info-heavy class like chemistry.
Interactive, do-it-yourself studying: I heard a lot of classes just have a teacher who drones on and on and most students just have to write the stuff down, a test about every 2 weeks and move on. That is a terrible way to learn. If you are not doing well on tests or anything, this is probably why. This is a chance to use other resources for interactive learning, like videos. Something I used to do was watch experiments on YouTube, which helped me a LOT to learn about chemistry, chemical experiments and more. If you have online resources which help in interactive learning, go ahead and do that. I would not pay attention in my physics class because this is what the class was like, but I would go back home and write everything I remembered, and learn from the web.
Revision tips: I think a great way to learn is to start from a blank page. Use that blank page to start writing everything you know about a topic, formulas, diagrams, e.t.c. Why is this such an effective studying style? Because in a test, you have a blank page. You don't have mind maps, your pretty studying notes or anything else. This helps you in tests so much because then you are skilled in pouring all that information onto the blank test page. After you finish, go ahead and look back on your notes: What are you missing? What doesn’t make sense? Now you can focus on what you don’t know, are confused about, and start honing on those spots.
Learn from tests: As soon as you get your test back, let's say you get a C. Instead of staring in shock, LOOK at the test. What did the teacher mark? What notes did they write? You can write short notes on a sticky note, and problems that you didn’t understand. Then, go back to your notes and figure out why you got that question wrong. Is it because you didn’t focus on this topic much? Silly mistake? Go ahead and write the answer and try the question again on a separate piece of paper. After that, go back and strengthen those spots so you don’t make that mistake again.
If you have other problems, like not being able to study for chem long, mind being somewhere far away, chemistry is boring.. Those are not chemistry problems, those are probably study problems. There are many tips out there on the web made by awesome, amazing people that can help you better. If you are truly struggling, maybe it's best to stop and think about the course as a whole. Ask for help. And remember, these tips helped me, but they may not work for you. And that is absolutely okay. Your worth is not determined by your grades.
#chemistry#chem#tips#studyblr#studytips#chemtips#notes#study#bujo#active studyblr#active studying#learning#school#grades#chem notes#studyblog#studyaesthetic#study aesthetic#study blog#chem tips#chemnotes#chemistry is fun
144 notes
·
View notes
Text
#I see this all the time and it drives me nuts#love this guy#cleaning#cleaning tips#chem thug#don’t mix vinegar and baking soda to clean#please#you’re just making water and gas#😫😫😫#not 1D
204 notes
·
View notes
Text
Notes for Metallic Bonding
METALLIC BONDING AND STRUCTURE
Delocalised electrons - electrons that are not associated with one specific atom and are free to move within the molecule structure
Metallic bond - the electrostatic attraction between a lattice of cataions and a sea of delocalised electrons.
In metals, state which electrons are the delocalised electrons present between positive ions in the lattice = valence electrons
Mg(s) has metallic bonding in the interaction between positive metal ions and delocalised valence electrons in a three-dimesional lattice structure. The metal itself is neutral and is made up of many, many atoms.
Identify the ways in which solid metals are similar to solid ionic and covalent network substances:
I. Lattice structures II. Non-directional bonding III. Electrostatic attractions between positive and negative species
Solid metals, ionic compounds and network covalent solids form three-dimensional lattices. In all three types of bonding there is an electrostatic attraction between positively and negatively charged species. Metallic – between cations and delocalised electrons, ionic – between cations and anions, covalent – between positive nuclei and shared electron pair.
PHYSICAL PROPERTIES AND APPLICATION OF METALS
Lustre (shiny appearance)
Delocalised electrons in a metal lattice interact with visible light. When visible light hits the surface of a metal, the electrons absorb some of that energy and vibrate. This vibration generates a second wave of light, which radiates from the surface.
Sonority (sound when struck)
When a metal surface is struck, the free electrons in the metallic lattice can move easily, propagating the incoming sound energy easily throughout the material.
Malleability (can be reshaped on compression) & Ductility (can be drawn out into a wire)
When stress is applied (for example, by bending, hitting with a hard object or pulling), layers within the lattice shift in response to that stress. As these layers shift, the cations in the lattice remain surrounded by delocalised valence electrons, meaning the metallic bonding also remains unaffected.
Electrical conductivity
The delocalised valence electrons can move throughout the metallic lattice. When a potential energy difference is applied to the metal, the delocalised electrons are repelled by the negative terminal and attracted to the positive terminal. This is why metals can conduct electricity in their solid state and why metals are used for electrical wires and cables.
Thermal conductivity
Thermal conductivity in metals is a result of the free electrons in the lattice.
STRENGTH OF THE METALLIC BOND
Strength of the metallic bond
The smaller the radius of the metal ion, the stronger the metallic bond. This is because of the shorter distance between the positive nucleus of the cation and the surrounding delocalised electrons. Dictionary
Charge of the metal ion
The higher the ionic charge, the stronger the metallic bond. This is because:
greater charge on the metal ion
greater number of delocalised valence electrons
The greater the ionic charge and the smaller the ionic radius, the stronger the metallic bond. The stronger the metallic bond, the higher the melting point.
TRANSITION METALS
As there are a large number of valence electrons from both the s and d orbitals, this results in a greater electron density within the metallic lattice. This increased electron density in turn increases the strength of the metallic bond.
Hardness
valence electrons (delocalised) increase attraction and increase metallic bond which results in greater hardness
Electrical Conductivity
Transition elements are electrically conductive. These metals form their metallic bonds through the delocalisation of electrons in unfilled d orbitals. The electrostatic attraction between metal ions in the lattice and delocalised electrons increases with an increasing number of electrons in d orbitals.
In comparison to s block metals, the melting point and electrical conductivity of transition metals are HIGHER and HIGHER
#academia#study#study tips#ib#student#study motivation#high school#studyblr#chaotic academia#studyspo#chemistry#notes#study notes#chem#stem#stemblr#school#college#metallic bond#organic chemistry#stem academia#stem student#stem studyblr
11 notes
·
View notes
Text

🦋
#frank iero#frank iero and the future violents#mcr#my chemical romance#my chem frank#my doodles#i hate to admit i had to trace more than i wanted#i want to issue a public apology to frank's grandmothers for how i depicted them in the tattooes#i tried#not sorry for skipping the sp*der tattoo tho#also sorry i made the tip of his nose too pointy... the problem with fineliners is that there's no second take 😭#mcr doodles
13 notes
·
View notes
Text
kisne iss saali (me) ko bola tha ki ye ghor paap (opting for coaching) kar le ab ghis rahi hai na (i dont think i'll survive this.) to ho ja khush (procrastination ki ma behen kar di hai)
#desiblr#desi teen#desi tumblr#just desi things#desi humor#pcmb lene ke faide#more like#coaching jaane ke nateeje#yes im a maths nerd and im proud of it#PHYSICS AND ORGANIC CHEM CAN SUCK MY D-#study tips ho to dena plz
8 notes
·
View notes
Text
I've realised i should have an introduction post :)
I'm izzy, and I'm a year 12 vce student based in melbourne, australia
this is my studyblr that i do try to stay active on, as it's hopefully going to provide some study motivation, which I severely lack.
The subjects I'm taking this year are physics, chem, general, english and viscom and i've already done methods 3/4
Some fun facts about me:
my favourite colour is purple but since thats kinda hard to pair with stuff my whole colour scheme for everything is blue/greens and neutrals :)
I'm not really sure what i want to do uni career-wise but we can worry about that later (that's next year's problem).
favourite music artists: new hope club, one direction, louis tomlinson, vansire :)
sports I like: i have like zero hand-eye co-ordination, so I swim (competed at my first ever nationals this year!) and run (casually) :)
I am a chronic, chronic procrastinator. I cannot emphasise this enough. i have written full 1000 word english essays on the day they're due which is good except for the part where that tells me procrastination is ok to do again...
I play the drums :)
favourite book is the secret history!
I draw sometimes (I'm not very good but it is fun)
I will add more if I think of more :)
Feel free to reblog with your own intros! I'm always open to make friends :)
#study space#study#student#studies#study tips#study hard#studying#aesthetic#study aesthetic#light aesthetic#aesthetic vibes#vce#biology#chem#chemistry#physics#maths methods#math#english#viscom#aboutme#maths#history
40 notes
·
View notes
Text





Goal for this fit was "something art school gerard would wear as drag in 1999"
#pro tip if you want hot goth girls to compliment you wear my chem shirts and jewelry with weapons on it. just trust me bro#selfie tag
18 notes
·
View notes
Text
Me accusing my mother of glorifying death when she teaches me life skills but because "you have to know this in case of war, little chick"
#i said i'd keep posting ab it if i kept getting jokes#the life skills in question were cooking mending clothes gardening and shooting#and my dad told me he'll teach me to drive stickshift because ''in case of war you might not have a choice''#my chem teacher in hs? also had ''this will be useful in case of war'' tips#listen. i'm balkan. death and pain and suffering is part of the lifestyle. we took memento mori and ran with it
4 notes
·
View notes
Text
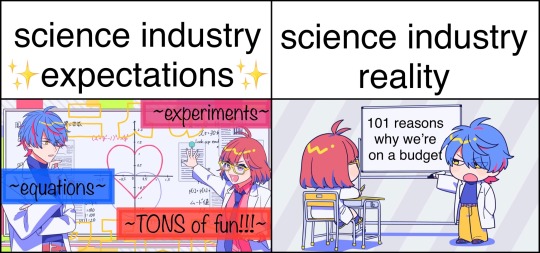
windows 98 is our one true #workbestie
#can’t get over how ancient the computer interface is tbh.#‘why are we using such an ancient version of windows?’ ‘because we’re on a ✨budget✨!!!!’#also features people advising against doing multiple repeats for the sake of saving consumables. like broooo they’re just glass vials </3#(and we reuse pipette tips and droppers too so… [scandalised bio noises])#so yeah. the science industry is awfully cheap and in this essay i will explain why—#(probably explains the high turnover rate too lmaoooooooooooooo)#also toluene eats gloves just so you know👀👀👀#come to think of it… i’ve worked in bio labs and chem labs but never physics labs…#maybe it’s for the best… i did eat that strange salt off the bench during a physics lab lesson a few years back after all—#it is suiyoubi my dudes
10 notes
·
View notes
Text
Alright boys saddle up
It’s loving Mikey Way Hours 😤🤠
#mcr#mikey way#my chemical romance#my chemical mikey#my chem#mcr5#my chemical meme#text post#it’s time for us country girls to do what we do best#make do#*tips sombrero because I am Hispanic and do not have a typical cowboy hat*
13 notes
·
View notes
Note
hiii I've thought u would be the most appropriate person to ask this ever since I saw u made that chemistry post! anyways they've started teaching the inorganic/ organic part of chemistry for the school semester and I really wanted to study some older basic parts of chem again ☹️ especially organic chem bc it gets quite confusing for me but I really don't wanna get behind the current work by doing this [btw I've a super tight schedule but really any tips would help :)]
hello! thanks for stopping by. i can understand your problem very well, and for some brush-ups, depending on where you are in your chemistry knowledge, i think taking a practice test or quiz can help! its great to know where you really are and see what topics you want to focus on. and it also helps to see if you need to completely master something or if its not as much of a priority. from there, you can try to fit in small study sessions based on your schedule. i really loved using those cram videos that cover a bunch of topics. for mastery, i'd reccomend using khan academy quizzes, or even chemistry by bbc bitesize. they are fairly easy to use and easy to get through.
2 notes
·
View notes
Text

He'll tap dance on your grave when you forget everything during your theory exam (it's me, I have a theory exam in 3 hours <(_ _)>)
#Wips with Leel#Your Majesty I am free on Tuesdays and Thursdays ◕_◕#You can't see it since it's cut off here but the crown is disintegrating (kind of??) and it looks really neat#Also. I'm loving how I used his stupid ''gloves that have the tips that let you touch screens'' and made then just fingerless gloves#sharp fingers#It's his transition state between power-tripped king and sad soul demon or whatever#(chem joke)#It's his birthday month he can be evil as a treat
6 notes
·
View notes
Text
My friends: we should do perfumes for fun
Me, who had to go for a full week to the lab to produce 20ml of perfume

#chem internals are my trauma#8h labs for a week is not my deal#paula rants#study motivation#academia#chaotic academia#ib#study#study tips#studyspo#student#studyblr#high school#stem#chemistry#organic chemistry#stem academia#stemblr#women in stem#stem student#ibdp#studying
10 notes
·
View notes
Text
brushed my teeth, did my skincare, ate breakfast, now ill do a 3 hour study session and then do my first workout of 2023 <3
3 notes
·
View notes
Note
What books do u recommend for neet chemistry and physics, I heard ncert isn't enough ( not attending my coaching ATM but I have their books(aakash) )
if you have aakash test series ke books, then follows those qs they're pretty good
i use my aakash modules mostly for chem and phy and sometimes hc verma for questions, i have modern abc for chem but im not sure if a lot of people use it
please do not take my opinion as the only suggestion, kindly do take it up with others so that you have a common consensus
wishing you all the best with your prep💚
5 notes
·
View notes
Text
I signed up for tutoring at my college and goddamn the imposter syndrome is kicking in
#if anyone sees this and has tips i will gladly take them#i havent tutored anyone since gr 8/9 maths#much less advanced bio and chem concepts#god give me strength#rambles
0 notes