#drug safety
Text
Research first published informally by Vancouver’s Drug User Liberation Front has now been published in a peer-reviewed international journal.
DULF bought heroin, cocaine and methamphetamine from online sources known to sell purer forms of these drugs, rigorously tested the drugs and then sold them at cost to its 47 compassion club members. Members lived in Vancouver’s Downtown Eastside neighbourhood, were 19 years old and up, were members of a drug user group in Vancouver and were at high risk of overdose.
The study, published recently in the International Journal of Drug Policy, found the 47 compassion club members were less likely to die from overdose while they were able to access this safer supply.
Continue Reading
Tagging @politicsofcanada
#cdnpoli#canada#canadian politics#canadian news#british columbia#drug user liberation front#substance use#harm reduction#drug safety
240 notes
·
View notes
Text

Save a life: bring NARCAN! ⛑️🕊️
#MLP FIM#my little pony#rarity#pony art#narcan#PSA#public service announcement#kawaii cute#pastelcore#pastel art#menhera kei#yami kawaii#pastel goth#fairy kei#dark kawaii#digital art#info graphic#drug safety
460 notes
·
View notes
Text
I don't know how to start this post, but. When it comes to drug use, there's something called harm reduction, and it's well... exactly what it sounds like. Harm reduction is about improving safety in a given situation, and it stands in opposition to the abstinence-only approach. It accepts that, for better or worse, drug use is part of society, part of life. No matter what we do, there are always going to be people who use drugs. The most any of us can do is offer support, advice, and resources.
Examples of harm reduction practices are: offering free drug testing, clean needles/syringes, having someone to watch over/take care of you during drug use (a tripsitter), having naloxone for opioid overdoses... It's educating people about the drugs they're on, the risks involved, potential drug interactions, signs of a medical emergency and what to do in one... that kind of thing. But I want to further explain the whole "given situation" part.
In most cases, the immediate goal of harm reduction isn't to have someone not use drugs. In fact, in the case of withdrawal, having someone not use may be dangerous than them not using at all. There are also situations where, if someone doesn't have access to drug A, they'll go take the more dangerous drug B. They may even combine multiple drugs to try to achieve similar effects as drug A... which can also be dangerous. All of it can be deadly. This is to say, sometimes harm reduction means giving people drugs - especially drugs that aren't cut (laced) with other substances (commonly, fentanyl).
There are many situations where someone is going to do something dangerous no matter what we do - but maybe, we can help change how dangerous it is. Some of the practices I mentioned earlier will do that, but further examples are taking a lower dose, taking a break to lower tolerance, taking one drug at a time, spacing out how often you use a drug... Hell, even making sure you're fed, hydrated, and as comfortable as possible helps. Or maybe, when it comes to cravings, you distract yourself with something for a bit, even if you still end up using.
Instead of "everyone needs to not use drugs ever," which doesn't actually help anyone who's using... harm reductions strategies are based on an individual person/population's needs. It's not coercive. It accepts people know their needs/wants better than others, and that bodily autonomy is a human right even when that autonomy leads to someone harming themself. And this premise can be (and is being) applied to other things, like behavioral addictions, self harm, and disordered eating.
Basically, abstinence-only may work for some, but they're only a small portion of the population. Applying a single approach to everyone who does/will do drugs is not only ineffective, but deadly. Harm reduction has saved and improved countless lives through its acceptance and versatility, and more people need to know about it.
#softspoonie#harm reduction#drug use#substance use#substance abuse#substance use disorder#drug safety#drug abuse#addiction#self harm#disordered eating#eating disorder#addiction awareness#addiction recovery#severe mental illness#serious mental illness#mad liberation#<- feel like these go together#mental health#mental health awareness#physical health#chronic illness#chronically ill#mental illness#mentally ill#drug usage#substance misuse#substance abuse disorder
120 notes
·
View notes
Text
General psa to not just go for natural supplements claiming to boost testosterone or estrogen without looking into whats in it and what the ingredients actually do, because a lot of them will just boost LH and increase the more predominant hormone in your body or depend on what gonads you have. Im not sure how it would vary for different intersex conditions but unless its a drug that actually increases estrogen or testosterone or progesterone etc. (or is straight up the hormone) for anyone with any organs, its not going to give you your desired result.
Do research into what a drug or supplement does before taking it. Chances are, some boost hormones depending on the organs in your body and are not inherently masculinising or feminising(whichever effect preferred), and may have other unrelated effects on the body. I feel like people often ignore that herbal supplements can cause harm too, if taken improperly or with no knowledge of what they do to the body.
#just saw a “testosterone boosting” vitamin thing in a grocery store the other day w like fenugreek or some shit and#if you want your T levels to increase and you dont have testes organs in your body that function as expected you wont get any increase in T#if you take that#you may even get an increase in E.#so like. do your research#hopefully the “hrt pill” scam that was made to doxx transfems made ppl more aware of this but just in case#safety#drug safety#medication safety#trans#gnc#hrt#we're trans and intersex and dont mean this as fearmongering#we also know that its not easy to get hrt especially testosterone in most countries#but basically be careful w herbal supplements and other supplements that may have a cis perisex focused idea#of what the drug does written on the label
60 notes
·
View notes
Text
Say no to opiates, kids. Liking cocaine is a slippery slope to liking jazz music.
42 notes
·
View notes
Text
Frances Kelsey: Thalidomide's Unsung Hero

In 1937, Frances Kelsey, at the age of 23, witnessed the tragic consequences of an unsafe drug during the "Elixir Sulfanilamide incident." Over twenty years later, in 1960, she joined the FDA and faced her first task: reviewing the morning sickness drug Thalidomide.
Thalidomide was touted as a safe option for pregnant women, but Kelsey, aware of potential risks, demanded thorough safety data, ultimately preventing its approval in the U.S. Despite global tragedies caused by Thalidomide, Kelsey's steadfastness spared the U.S. from similar devastation, earning her recognition as a silent hero.


#drug safety#drug#thalidomide#chemblr#stemblr#molecule#interesting stuff#amazing facts#organicchemistry#chemistry#science#compound#kingdraw
8 notes
·
View notes
Text

Mainline Magazine, winter 2020 issue, "P Freaks" poem contributed by "Suzie".
P Freaks
Brain leaks
Carnage they seek
Then get weak
Coz that's what they seek
Week after week
wank as stupid geeks
They get exactly what they reap
Shame should've taken the back seat
Before getting in too deep
Just get some fucken sleep!
#harm reduction#drugs cw#tw drugs#poem#mainline#magazine#methamphematine#meth#needles#drug safety#drugs#war on drugs#dopesick#zine#aotearoa#new zealand#nz zine#addiction#writing#drug poem#addict#poetry#nz writing#nz poetry#nikfiendluvr666
20 notes
·
View notes
Text
Apropos of a recent post, I want to talk a little about Francis Oldham Kelsey.
Born in Canada in 1914, she studied pharmacology at McGill University in Montreal, and went to a PhD program at the university of Chicago.
When she arrived in Chicago the FDA was dealing with a national emergency; a series of deaths had followed the use of the antibiotic "Elixir Sulfanilimide" made by Massengill Co., and as a grad student she worked in the team that identified diethelyne glycol (DEG) as the toxin. This wasn't malicious on the company's part; it wasn't widely known that DEG was toxic at the time and safely testing new drugs or preparations wasn't required prior to 1938. Over 100 people died in that incident, and the owner of the company denied that they had any responsibility because they "not once could have foreseen the unlooked-for results". And that was precisely the problem; you SHOULD look and make sure you aren't producing poison before you sell a medication to the public.
That's why we require safety testing. Federal regulations are often written in blood; remember this the next time someone tells you they are "burdensome to innovation."
Dr. Kelsey went on to medical school, taught pharmacology, worked as a primary care physician for awhile, then was hired by the FDA as chief of the Division of New Drugs. Her job was to decide whether to approve new medications for use in the US, and as we noted, this was a relatively new concept at the time and there were no formal requirements around how safety testing was done. Companies just submitted an application and expected it to be taken at face value. Kelsey received an application for thalidomide from F. Joseph Murray, an executive from the William S. Merrill Co. that looked on paper like a wonder drug for insomnia and treatment of morning sickness in pregnancy (among other things). It was already approved and in use in Europe and Australia, where it was becoming wildly popular, but some parts of the application stood out; they claimed it had no side effects and no lethal dose (even water has a lethal dose). The "data" was a collection of anecdotes (data is not the plural of anecdote). She denied it. They resubmitted.
She noticed an article linking thalidomide to nerve damage in England and asked why they hadn't addressed that, and requested safety data for the fetus since this was supposed to be given to pregnant women. Denied. Resubmitted (still without safety data).
Murray went on to pressure Kelsey by writing an angry letter to her boss, and resubmitting the application a total of five times. Around the same time he was pushing for approval, reports of birth defects involving missing or deformed limbs started emerging in Europe and Australia. It took some time to connect these to thalidomide.
Kelsey had rejected the approval of thalidomide four times and it was withdrawn on the fifth. Around 8,000 babies were born with severe birth defects, and thousands more were miscarried. Only 17 of these were in the US, from samples Merrill gave out as an "investigative trial".
One result of this was that now safety trials have to be based on adequate, controlled studies and the FDA can inspect labs to ensure that this is happening. It also requires any human participants in those trials to give informed consent; if you are taking a drug that isn't approved you have to be told, and understand the risks. This was not the case before 1962!
Again for the folks in the back: Federal regulations are often written in blood. The reason we require safety testing is 100+ deaths from an antibiotic elixir and 8,000 babies with no limbs. Also, if you were born in the 60s with all 4 limbs, thank Frances Kelsey.
5 notes
·
View notes
Text
“If only I could tell how many of my patients are law students trapped in the self-medicated vicious cycle of stimulants in the morning and benzos to sleep...”
#medicine#med school#medblr#psychiatry#university#college#student#law school#pharmacology#drug use#drug safety#and remember#just because it’s legal doesn’t mean you should do it
17 notes
·
View notes
Text
CVS Pharmacy employees walked out in KC on September 20-21 because the status quo under-staffing was making it impossible for them to work safely. These non-unionized pharmacy workers are organizing a second walk-out on Wednesday, Sep 27, to force CVS and other large retailers to improve working conditions. The current chronic under-staffing is untenable. It is not safe for workers nor for customers.

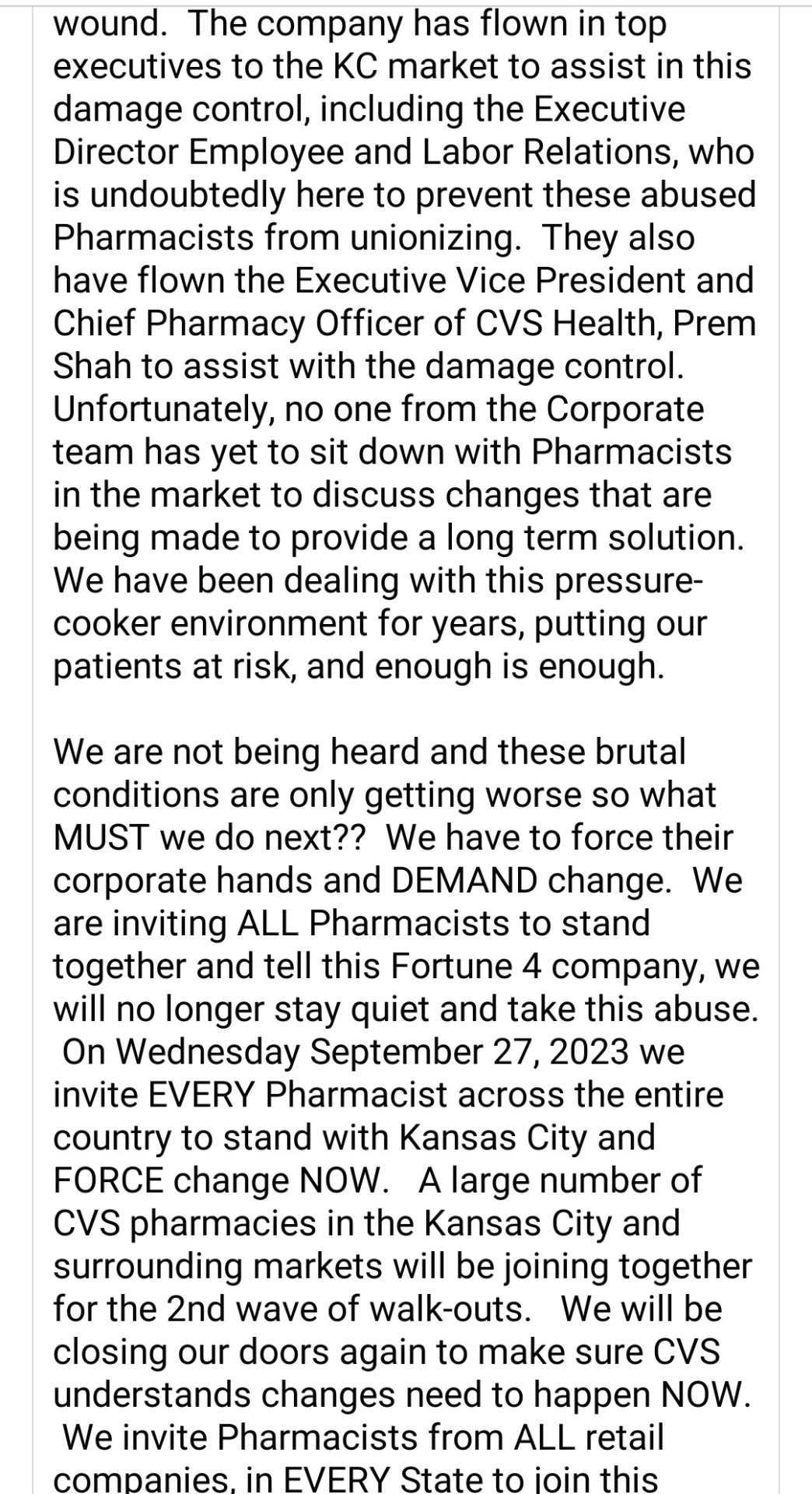


#walgreens#cvs#pharmacy#pharmacist#pharmacy technician#solidarity#labor rights#worker safety#drug safety
2 notes
·
View notes
Text

Good ponies carry NARCAN! ⛑️🕊️
etsy.com/shop/danneroni
#mlp fim#my little pony#rarity#rarity fan art#rarity art#pony art#NARCAN#kawaii cute#pastel core#pastel art#menhera kei#yami kawaii#pastel goth#fairy kei#dark kawaii#digital art#drug safety#harm reduction#glitter sticker#vinyl sticker#waterproof sticker#stickerapp
86 notes
·
View notes
Text
hey med taking and drug doing folks. don't take multiple CNS depressants at once, or combine them with other drugs that affect your vital signs. it can kill you - and will, if you don't pay attention to what you're taking and how it's affecting your body.
for example. I used to take seroquel XR, topiramate, and prazosin all at once. every night. while having sleep apnea and dysautonomia. i was used to sedation because i'd been on seroquel for years - but with that combo, it genuinely felt like my heart was gonna stop every time i took my meds, and i was too sedated to do anything about it once it started.
i did not realize that is. absolutely not a normal side effect and could kill me (because of trauma from gaslighting). so yeah. don't do it, trust your body, and always check for drug interactions - no matter where you got the meds/drugs from, you may not have been given accurate information. nothing is more important than your life.
#softspoonie#disabled#disability#drugs#meds#harm reduction#mentally ill#mental illness#physical disability#gaslighting#mental disorder#mental health#drug safety#medication#anti psychotics#psychotic#schizospec#schizophrenia#schizophrenic#pots#postural orthostatic tachycardia syndrome#sleep apnea#dysautonomia#spoonie#spoonie tips#drug usage#ptsd#cptsd#complex ptsd
149 notes
·
View notes
Text
About Me
My name is Upton Sinclair, and I am here to quarrel with the dangerously unregulated state of the US food and drug manufacturing industries. I have been made aware that in the nearly 120 years since I first attempted to bring awareness to the hazardous nature of manufactured foods and medications in this nation, there has been little progress.
In my day, anyone could sell anything and say it was a miracle cure, without disclosing the ingredients and their respective quantities or the safety concerns associated with the product. The manufacturer had no obligation to inform the consumer if the product even provided its promised benefits (which it rarely did). One could brand oneself a doctor and sell whatever bunk panacea they so chose, making whichever claims they felt like making, regardless of the verity of those claims. It was miserable. Americans were falling ill to deadly foodborne microbes present in their groceries, overdosing on untested, adulterated, and potent drugs in their cough remedies, sickened by heavy metals and radioactive materials in their cosmetics, and wasting money on do-nothing elixirs.
All of this spurred President Theodore Roosevelt to sign the 1906 Food and Drug Act, which granted Harvey Washington Wiley's Bureau of Chemistry the ability to oversee various areas of consumer safety. While threatened by idiotic judicial action and arbitrary divisions of regulation into multiple agencies (the Board of Food and Drug Inspection and the separate Referee Board of Consulting Scientific Experts), this was a major improvement. In 1927, the regulatory practices were reorganized into a new USDA body, the Food, Drug, and Insecticide Organization, which was later shortened to just the Food and Drug Administration. The FDA was later granted more powers with the 1938 Food, Drug, and Cosmetic Act, further guarding the American consumer from toxic materials.
For a brief, glorious era the American consumer was finally able to rest knowing that the foods they ate and the treatments they took were regulated by a disinterested federal agency. It was never perfect, with concerns existing regarding supplements and compounded pharmaceuticals, but various amendments increased the government's ability to monitor the medications produced in the United States. The FDA could ensure that powerful medications were only available under the watchful eye of a trained professional, that marketed drugs had been proven to be safe and effective in scientific trials, and that drug studies conformed to safety guidelines. Later, the FDA made procedures for the approval of generic medications, giving consumers the ability to buy their needed treatments from different companies than those that invented the medication for a cheaper price, and started allowing promising new medications to safely bypass standard testing protocols in instances where speed was of the utmost importance.
Then came the supplement and compounding lobbies. The government caved to the demands of the multi-billion dollar supplement industry to have less regulation with the Dietary Supplement Health and Education Act of 1994. DSHEA has made it possible for "herbal medications" and "natural healing products" to be sold and advertised despite making false claims regarding health benefits, and has prevented the FDA from inspecting and regulating manufacturing conditions at supplement plants. The supplement lobby was able to turn the American public against the organization that secured their health and safety through ads laden with logical fallacies and ad hominem attacks, and, under the pressures of public opinion, a huge industry lobby, and corrupt politicians with personal stakes in the supplement industry, such as Orin Hatch (R-UT) and Tom Harkin (D-IA), the legislatures soon caved and passed this blow to American wellbeing.
Another blow was dealt to the FDA in 2013 with the Drug Quality and Security Act. This act was drafted as a response to a deadly 2012 outbreak of fungal meningitis caused by compounded spinal steroid injections made by the New England Compounding Center, which struck 798 injection recipients, typically people with chronic pain, and killed at least 100. Compounded medications are medications historically made in small-scale family pharmacies and therefore subject to lesser oversight. Compounding is often used in cases where different ingredients or dosages are needed for a particular patient, but loopholes in the laws and the political force of the compounding lobby have lead to a massive shadow industry of the semi-legal mass-production of compounded pharmaceuticals, made in factories that aren't subject to regular safety inspections, being shipped across state lines, with devastating effects. While the intentions of this act were pure, the initial draft was twisted by supporters of the compounding industry into a nothing law. The compounders are allowed to compound, overseen by inattentive and underfunded state governments, only they may now choose to be approved and regulated by the FDA. This is completely useless, as it's optional, and doesn't much benefit the compounding manufacturers. What does benefit them are the repeals of some restrictions that their industry had previously been subject to- now, compounding pharmacies are allowed to promote their wares through advertisements.
These legal changes have destroyed the FDA's ability to monitor two major components of the American medical product landscape. We were on the path of improvement, but we have now turned ourselves back around. The compounding and supplement industries are dangerous, and my goal is to continue my mission of keeping the everyman safe from experimental treatments, do-nothing money-pits, and predatory business practices.
It's time to rejuvenate the FDA.
Main Sources:
Dearen J. KILL SHOT : A Shadow Industry, a Deadly Disease. Avery Pub Group; 2022.
2. Offit PA. Do You Believe in Magic? : The Sense and Nonsense of Alternative Medicine. Harper; 2013.
#drug safety#food safety#FDA#politics#public health#third party pharma manufacturing#healthcare#supplements#dietary supplement#compounding pharmacies#DSHEA#public safety#health and wellness#medicine#usa#us
1 note
·
View note
Text
Tragic Lessons: The Elixir Sulfanilamide Incident and the Birth of Drug Safety Regulation

In 1937, the US-based Massengil pharmaceutical company's chemists developed a new sulfonamide oral preparation named "Elixir Sulfanilamide." At that time, there was no requirement for safety review before launching new drugs. Consequently, this untested drug went on sale in September of that year. In October, the American Medical Association received reports of fatalities caused by the medication.

In November, the US Food and Drug Administration (FDA) intervened, recalling the drug. The investigation found that "ethylene glycol," used as a solvent in the drug, was the main culprit of poisoning. Ethylene glycol is highly toxic to mammals, with a minimum lethal dose (LD₅₀) of 786 mg/kg in humans. This issue, easily detectable through a simple animal experiment, resulted in over 100 deaths that fall, with 30% being children.
The following year, under immense public pressure, the US Congress passed the landmark "Federal Food, Drug, and Cosmetic Act" (FFDCA), mandating safety reviews for all new drugs before market approval and granting the FDA regulatory oversight authority.
#fda#us fda#drug safety#drug#ethylene glycol#chemblr#stemblr#science#molecule#chemistry#kingdraw#incidence#toxicity
5 notes
·
View notes
Text
Ethylene Glycol in Cough Syrups Tragedy – Asrar Qureshi’s Blog Post #900

View On WordPress
#Asrar Qureshi#Blogpost900#Children#Contamination#Cough Syrups#Deaths#Drug Safety#Gambia#India#Indonesia#Pharma Industry#Pharma Veterans#Toxic Substance#Uzbekistan
0 notes