#glowing
Text
What can I say?
I love her 🥰
15 notes
·
View notes
Photo





magnets and an old mac classic
27K notes
·
View notes
Text



Georgie Porgie's Ice Cream Parlor - Westport, CT (1987?)
Designed by Arnold Kaye
Scanned from a 1988 issue of Contract Interiors Magazine & the book, Food Presentation & Display (1992)
#80s#design#interior design#interiors#architecture#1980s#colorful#pink#glowing#neon#metallic#ice cream parlor#reflective#westport#connecticut#my scans#purple
5K notes
·
View notes
Text
When sodium hypochlorite (bleach) solution is added to luminol, a chemical reaction occurs that releases energy in the form of light. This is called chemiluminescence. The bleach solution acts as an oxidizing agent, which means it takes electrons away from the luminol molecule. This causes the luminol molecule to become excited, and it releases the energy as light.
🎥 Courtesy: Kendra Frederick
The luminol molecule is made up of two amino groups, a carbonyl group, and an azo group. The amino groups are electron-rich, while the carbonyl group is electron-poor. The azo group is a conjugated system, which means that the electrons in the double bonds can move freely from one atom to another.
When sodium hypochlorite (bleach) solution is added to luminol, the bleach molecules react with the amino groups of the luminol molecule. This reaction takes electrons away from the luminol molecule, which causes the luminol molecule to become oxidized. The oxidized luminol molecule is in an excited state, which means that it has more energy than it normally does.
The excited luminol molecule then releases the extra energy as light. This light is called chemiluminescence. The light emitted by the chemiluminescence reaction is blue because the luminol molecule has a blue fluorescence.
The chemiluminescence reaction between luminol and sodium hypochlorite is catalyzed by the presence of a metal ion, such as iron or copper. The metal ion helps to stabilize the excited state of the luminol molecule, which makes it more likely to release the extra energy as light.
The chemiluminescence reaction is very sensitive to impurities, so it is important to use pure chemicals. The reaction can also be affected by the pH of the solution. The optimal pH for the reaction is around 9.
The chemiluminescence reaction between luminol and sodium hypochlorite can be used to detect blood, as the iron in hemoglobin can catalyze the reaction. The reaction is also used in some commercial products, such as glow sticks and emergency lights.
I hope you enjoyed learning about this. ❤️🙏
#chemiluminescence#luminol#bleach#science#chemistry#scienceexperiment#glowing#light#excitedstate#oxidation#chemicalreact
5K notes
·
View notes
Text



gale romance + greetings in act ii
bonus:

#bg3edit#baldur's gate 3#bg3#baldurs gate 3#gale dekarios#gale of waterdeep#gale x tav#tav x gale#dailygaming#videogameedit#ch: gale dekarios#otp: a soul that steels my own#vg: baldur's gate 3#series: baldur's gate#gif: mybg3#GLOWING#DELIGHTED TO TALK TO THE PLAYER#and also sadness ofc bc it's gale
1K notes
·
View notes
Text
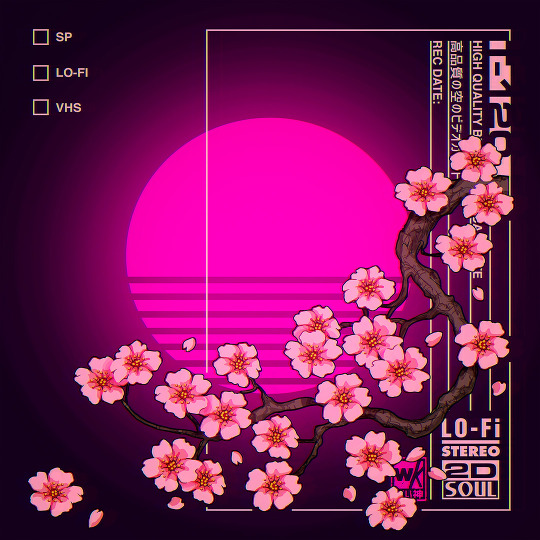
VHS Sakura Rewind (Midnight)
#cherry blossom#sakura#vaporwave#retrowave#lofi#illustrators on tumblr#artists on tumblr#retro anime#sakura blossom#pink flowers#neon#v a p o r w a v e#japanese aesthetic#nostalgia#90s aesthetic#anime#aesthetic#glowing#art#1000
2K notes
·
View notes
Text
Cosplay Glow Up

#cat cosplay#cosplay#cats#kitty#cats in costumes#cat#cats of tumblr#aww#cat costume#fan art#glow up#meme#joke#nightmare before christmas#oogie boogie#glowing#paint#pics
2K notes
·
View notes
Text




#glowing#grateful#gratitude#growing#i am grateful#black girl aesthetic#black girl moodboard#black femininity#black women#black women in luxury#soft black women#soft life#soft woman#divine feminine#feminine#femininity#feminine energy#women#feminine journey#feminine energy life#brown skin#melanin#black beauty#black girl magic#black excellence#black girls
709 notes
·
View notes
Text






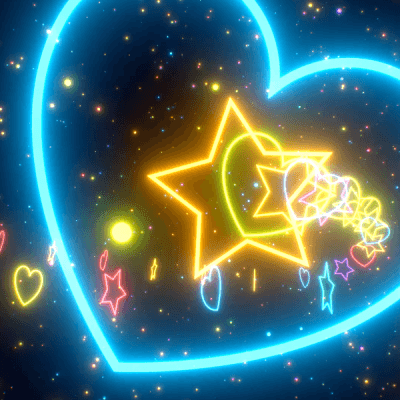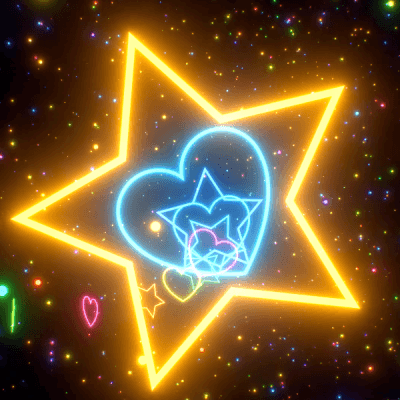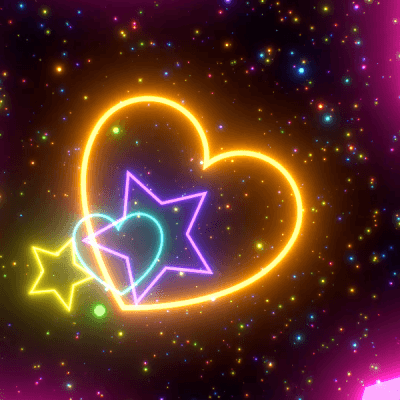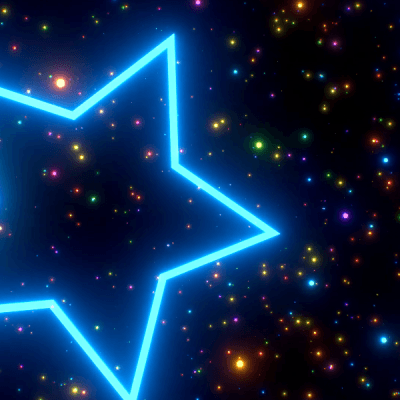



STIM - Rainbow VFX 🌈
Link back to this post if used.
[ x x x - x x x - x x x ]
1K notes
·
View notes
Text

Bertrand Gadenne: Les Papillons (2015)
3K notes
·
View notes
Text

SDJ- Hey there Friend~
📺👻🤡
#alizera62#my artwork#yandere#fanart#sdj#sdj jack#sunny day jack#ghost#clowns#sunnydayjack#sunny day jack fanart#somethings wrong with sunny day jack#glowing#tv#glitch#comic
446 notes
·
View notes
Text

522 notes
·
View notes
Text


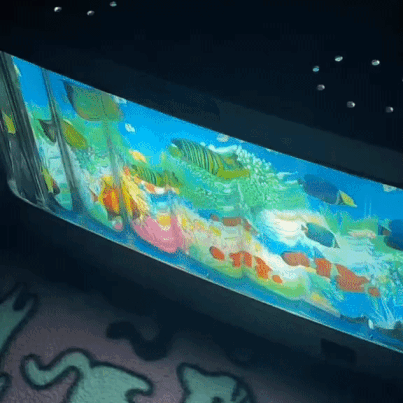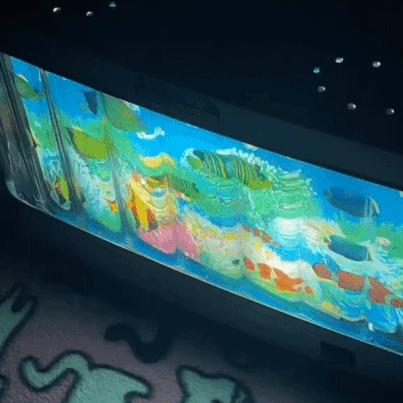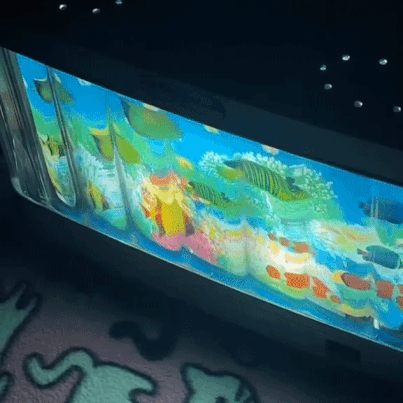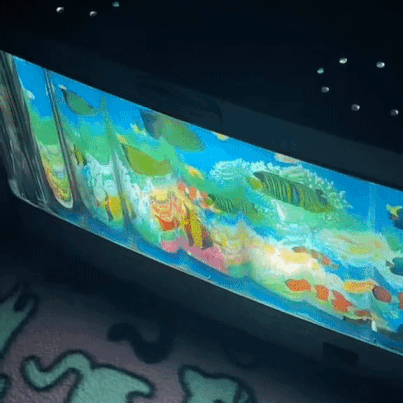

moving aquarium lamp
2K notes
·
View notes
Text



💜💿 ~ Life Flow ~ 💿💜 (choisonny)
(Credit if you use) (ko-fi)
#rainbows#technology#LED lights#pc build#custom build#techcore#pretty lights#pretty gifs#tech stim#tech#purple and blue#blue and purple#blacklight#black light#neon#glowing#rainbow#rainbow lights#led lights#rainbow leds#neon colors#stim#tw pulsing lights#slight flashing#flashing lights#glowing lights#rainbow aesthetic#rainbowcore#rainbowwave#LEDs
454 notes
·
View notes
Text

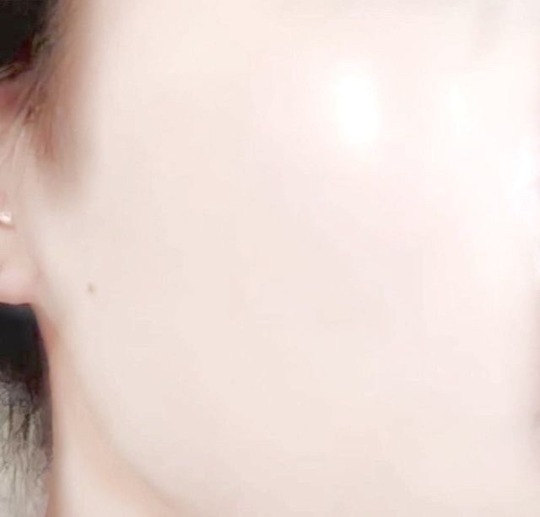


✨glass skin✨
#cute#aes#aesthetic#girly#kawaii#adorable#korea#kpop#wonyoung#skincare#glass skin#jang wonyoung#idols#kpop idol#korean#korean skincare#glowing#glowing skin#asia
419 notes
·
View notes
Photo

~ Purple and Red ~
599 notes
·
View notes