#iso 13485 qms
Text
Exploring the Importance of ISO 13485 Certification in the Medical Device Industry

In the domain of healthcare services, the creation of medical devices requires severe quality guidelines to guarantee the safety and adequacy of these items. ISO 13485 certification remains as a foundation in the medical gadget industry, framing the requirements for a thorough quality management system.
This article digs into the meaning of ISO 13485 Certification, explaining its role in maintaining quality, compliance, and patient safety inside the area.
What is ISO 13485 Certification?
Inside the ISO 13485 standard areas and provisions exist that address the parts of the plan, advancement, creation, audit, testing, bundling, marking, establishment, overhauling, fixing, maintain, removal, and documentation of medical devices. The reason for the ISO 13485 standard is to assist makers with creating protected, solid, and compelling medical devices.
How does ISO 13485 Certification in Dubai help medical device manufacturers?
By observing the rules in this standard, producers can:
Guarantee products are manufactured by industry-acknowledged accepted procedures.
Lessen the risk of item reviews because of imperfections or breaking down parts.
Avoid expensive claims from patients harmed by faulty equipment.
Work on quiet results.
Increment in customer satisfaction.
Meet unofficial laws.
Keep a decent corporate picture
Regulatory Compliance:
ISO 13485 fills in as a globally perceived standard explicitly custom fitted for medical gadget producers. Compliance with ISO 13485 shows adherence to administrative prerequisites across different wards, including the European Union's Medical Device Regulation (MDR), the U.S. Food and Drug Administration (FDA) guidelines, and other worldwide regulatory systems.
By lining up with ISO 13485, organizations can explore complex regulatory scenes all the more actually, guaranteeing market access for their items.
Why Is an ISO 13485 Certification Important for Medical Devices?
To sell your items in different nations, then you should be consistent with nearby regulations and guidelines. For instance, most European nations require medical gadget makers to acquire CE marking before they can advertise their products in those areas.
Furthermore, many states require medical gadget makers to enroll their items with state facilities. Consequently, having an ISO 13485 accreditation can assist you with remaining on the ball while showcasing your items around the world.
What happens when you don't have an ISO 13485 certification?
Without an ISO 13485 accreditation, you will not have the option to trade your item product beyond the US. Furthermore, the FDA expects that all medical devices sold in the U.S. deliver an ISO 13485 certificate.
Enhanced Quality Management:
Integral, the certification is the foundation of a hearty ISO 13485 Quality Management System (QMS) customized to the extraordinary necessities of the medical gadget industry. The standard system requirements, for example, plan control, risk management, restorative and preventive activities (CAPA), and recognizability, cultivate a culture of constant improvement and item excellence.
Executing an ISO 13485-certified QMS empowers organizations to smooth out activities, limit threats, and reliably deliver superior-grade, safe medical devices to end customers.
Focus on Risk Management:
ISO 13485 Certification puts major areas of strength for on-risk management all through the item lifecycle. By incorporating risk-based approaches into configuration, assembling, and post-market exercises, associations can proactively distinguish and alleviate potential perils related to their medical devices. This proactive position upgrades item security as well as supports regulatory compliance and encourages partner trust.
Worldwide Market Access:
ISO 13485 certificate fills in as an identification to global business sectors, working with exchange and market acknowledgment of medical devices around the world. Numerous nations and locales require the ISO 13485 standard as an essential for item enrollment or market passage, highlighting its significance in worldwide market access techniques.
By acquiring an ISO 13485 certificate, producers show their obligation to quality and regulatory compliance, consequently upgrading their seriousness in the worldwide commercial center.
Customer Confidence and Reputation:
ISO 13485 certificate ingrains certainty among clients, medical services experts, and regulatory specialists the same. It fills in as a substantial show of an organization's obligation to item quality, security, and administrative compliance. Moreover, the ISO 13485 QMS improves the branding and validity of medical gadget makers, cultivating trust and dedication among partners.
Why Should My Organization Care About ISO 13485 Certification?
Producing medical devices implies a great deal of threats. You might confront issues like:
Product reviews in light of an imperfection or failing part
Claims from patients harmed by damaged parts
Loss of business or income due to unfortunate client care
Inflated costs related to product reviews
Inflated costs related to getting an ISO 13485 accreditation
These dangers can hurt your reputation, your business, and your primary concern.
Note: Minor deviations from the standard are ordinarily rare. Most of the time, these can be immediately found and fixed, despite the fact that they seldom quickly affect the eventual outcome. Minor non-conformance can include single issues like the beginning and stop runs of plastic infusion molding machines or errors in machine calibration.
This sort of resistance is brought about by a one-time setting source, and it probably won't endure during the large-scale manufacturing of a product. Yet, on the grounds that this sort of non-conformity is seen as “minor” doesn't infer it ought to be dismissed. Minor infractions often heighten to larger ones.
Conclusion:
All in all, ISO 13485 Certification holds an essential part in the medical device industry, filling in as a foundation for quality management, regulatory compliance, and market achievement. By embracing ISO 13485 principles, associations can lift their quality systems, relieve dangers, and improve their competition in the worldwide commercial center.
As healthcare services keep on advancing, ISO 13485 stays fundamental for guaranteeing the security, viability, and reliability of medical devices, at last helping patients and medical care suppliers around the world.
#ISO 13485 Certification#ISO 13485 Certification in Dubai#ISO 13485 Quality Management System#ISO 13485 QMS
0 notes
Text
ISO 13485 Certification - ISO 13485 Medical Device Consultant
#iso 13485 consultant#iso 13485 qms#iso 13485 implementation#iso 13485#iso 13485 certification#benefits of iso 13485#iso 13485 medical devices#consultant iso 13485#13485 medical device#iso 13485 standard
0 notes
Text
#iso 13485 certification in usa#iso standard in usa#iso certification services in usa#qms for medical device in united states#iso 13485 certification#sis certifications
0 notes
Text
🌐 Explore the world of ISO 13485:2016 - Your path to excellence in quality management. 🏆
🔒 ISO 13485 Certification Services: Elevate your business with the gold standard of quality.
🧙♂️ Our ISO 13485 Consultants are your trusted partners, guiding you through the QMS maze.
💡 Uncover the secrets of ISO 13485:2016 by reading our comprehensive guide. >> https://ndgcs.com/a-deep-dive-into-iso-134852016/
🚀 Join us on the journey to better quality and safety. Let's thrive together!

#ISO13485#QualityManagement#QMS#CertificationServices#ISOConsultants#SafetyFirst#ISO 13485 Consultant
0 notes
Text
When should you start a QMS?
“When do I need a Quality Management System (QMS)?” is the most common question that we get from new clients who are just entering the medical device field. The answer depends on your target market and your exit plan.
QMS is a quality management system as the name suggests this is essential for any simplest medical device too. There are two aspects of QMS. One initial is to build a system wherein you will document the system mostly as per ISO standard 13485. System build means, writing down the procedure for each section of ISO stating what, how, when and where you are going to do. At this stage, you are only developing a strategy or system but not doing anything in action.
In the second stage, you will carry out the action and create evidence of action through records of your system. This is an explanation of what QMS one must do.
This is just a basic need. Then comes regulatory requirements which are different for different countries. These are must requirements, and you have no choice to follow them or not.
In theory, a medical device is supposed to be developed using design control as described in ISO 13485 and 21 CFR 820. In the US once clearance or approval is granted for a device it can be legally placed on the market and the FDA may inspect the manufacturing facility to ensure the required quality system is in place. During an inspection, the auditor looks for evidence that the QMS is being obeyed and this most often comes in the form of records, or more specifically manufacturing records.
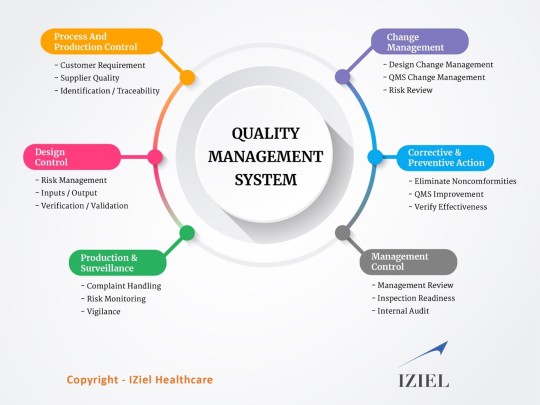
IZiel covers all the product-specific requirements for all components, manufacturing processes, verification & validation along with corrective and preventative actions (CAPA) for assessing customer satisfaction, product non-conformance, assessing and improving quality policies and procedures, carrying out and assessing the results of internal audits, and implementing systems for continuous improvement.
IZiel works with clients to make the QMS simple yet effective and flexible to allow changes to keep up with the changing regulatory requirements.
#Quality Management System#QMS Certificate#QMS Documentation#ISO 13485 Certification#ISO 13485 Consultants
0 notes
Text
What is the ISO 13485 Certification in Saudi Arabia? Its requirements?
What is the ISO 13485 Certification in Saudi Arabia? Its requirements?

What is the ISO 13485?
Specifically designed for the medical device sector, ISO 13485 is a global standard for quality management systems.
The purpose of ISO 13485 is to ensure that medical devices and services consistently meet customer expectations and relevant regulatory requirements. This standard was first published in 1996 and has since been revised in 2003 and 2016, with the current version coming into effect in March 2016. Companies involved in designing, producing, installing, servicing, or manufacturing medical devices should become ISO 13485 certified if they wish to follow this blueprint for quality system compliance.
What are the requirements for ISO 13485 Certification in Saudi Arabia?
1. A quality management system
A quality management system (QMS) is a formal system that documents the structure, processes, roles, responsibilities, and procedures required to achieve effective quality management.
The requirements of ISO 13485:2016 for a QMS include: documenting the structure and processes required to achieve effective quality management; defining roles and responsibilities for all relevant parties; establishing documented policies and procedures related to the standard's requirements; creating records of activities performed following these policies/procedures; establishing key performance indicators (KPIs) for measuring the success of processes within the QMS; using an eQMS software tool that is validated according to 21 CFR Part 11 guidelines before use; monitoring the effectiveness of the QMS through Deming Cycle methodology or other methodologies.
To be certified under ISO 13485 certification in Saudi Arabia, organizations must put in place a quality management system (QMS) that meets certain requirements. These requirements cover various aspects of quality management, including the organization's ability to provide medical devices and services that meet customer and regulatory requirements, as well as its commitment to continual improvement.
2. Management responsibility
Management responsibility refers to the role of executive management in ensuring that your quality management system (QMS) is effective.
It is important for ISO 13485 because it ensures that executive management has the necessary authority to implement and maintain your company's QMS. This includes appointing a management representative who has the power to oversee and manage QMS efforts. Management should also provide evidence of its commitment to this process and ensure that it is working effectively. By fulfilling these responsibilities, managers can ensure that their organization meets all regulatory requirements related to quality assurance standards such as ISO 13485.
3. Scope
ISO 13485 is a quality management system standard that sets out the intended outcomes of a modern medical device quality management system, including the significance of the process approach and continuous improvement.
The standard contains eight clauses that outline its requirements, including principles such as defining objectives and responsibilities, identifying risks, establishing processes to manage those risks, and ensuring continual compliance with regulations. Additionally, it emphasizes the importance of implementing a systematic approach to quality management that involves continuous improvement efforts.
4. Processes and procedures
For ISO 13485, you need to establish quality requirements, define the required processes and supporting documentation, and outline company infrastructure such as work environments and employee qualification and training requirements. You also need to define processes for verification, validation, measurement, monitoring, handling inspection storage distribution, and traceability. Additionally, you need to preserve and protect medical devices during processing storage handling distribution by specifying packaging shipping containers' environmental criteria such as temperature and humidity.
1 note
·
View note
Text
PT 264: MD-QMS ISO 13485:2016 Internal Auditor Course
We provide Internal Auditors with both the confidence and ability to apply the audit assurance tools and techniques, methodology, and desired behaviours to assess the organization’s QMS Medical Device processes and process controls to consistently provide safe & effective medical device products and services that meet customer and applicable statutory and regulatory requirements.
2 notes
·
View notes
Text
13485 audit
13485 audit
Did you know that the ISO 13485 standard is the international quality management system (QMS) standard for medical devices? As a result, any organization that manufactures or sells medical devices must comply with this standard. One of the key requirements of the standard is to have an independent audit of your QMS. This blog post will discuss what to expect during an ISO 13485 audit and how to ensure your organization is prepared.
What is ISO 13485?
ISO 13485 audit is an international standard that specifies requirements for a quality management system for the design and manufacture of medical devices. The standard is designed to help organizations ensure that their medical devices are safe and effective.
There are many benefits to implementing ISO 13485, including:
• improved product quality
• reduced risk of recalls and other problems
• increased customer satisfaction
• improved efficiency and effectiveness of the organization
To learn more about ISO 13485, please visit the ISO website.
The benefits of ISO 13485
There are many benefits of ISO 13485 certification, including:
1. Improved quality management system - ISO 13485 certification can help improve your quality management system, making your organization more efficient and effective.
2. Enhanced market access - Many customers and suppliers require ISO 13485 certification as a prerequisite for doing business. Certification can help you gain access to new markets and customers.
3. Improved organizational efficiency - A well-run quality management system can help streamline your organization, improving efficiency and effectiveness.
4. Cost savings - An effective quality management system can help reduce costs associated with rework, scrap, and warranty claims.
5. Increased customer satisfaction - Certification can help show your commitment to quality and customer satisfaction, leading to increased loyalty and repeat business.
The requirements of ISO 13485
When it comes to the requirements of ISO 13485, there are really two main sections that you need to focus on. The first section is devoted to quality management system requirements while the second part focuses on medical device-specific requirements. Let’s take a closer look at each section:
Section 1: Quality Management System Requirements
This section contains all of the general requirements that any organization looking to implement ISO 13485 must meet. This includes things like establishing a quality management system, ensuring top management commitment, and conducting risk assessments.
Some of the key highlights from this section include:
Establishing a quality management system : Your organization will need to put in place a quality management system that meets all of the requirements laid out in ISO 13485. This system will need to be tailored to your specific organization and business needs.
: Your organization will need to put in place a quality management system that meets all of the requirements laid out in ISO 13485. This system will need to be tailored to your specific organization and business needs. Top management commitment : Top management must be fully committed to the success of your quality management system. They must provide resources, support, and direction as needed. Additionally, they should establish policies and objectives that ensure continual improvement of your quality management system.
: Top management must be fully committed to the success of your quality management system. They must provide resources, support, and direction as needed. Additionally, they should establish policies
The audit process
The audit process is designed to provide assurance that financial statements are free from material misstatement, whether due to fraud or error. The auditor expresses an opinion on the fairness of the financial statements in accordance with Generally Accepted Accounting Principles (GAAP).
There are four phases to the audit process: planning, fieldwork, reporting, and follow-up.
Planning: During this phase, the auditor develops an understanding of the client's business and internal controls. The auditor also establishes materiality levels and assesses risk.
Fieldwork: This is the phase where testing is performed to obtain evidence about the assertions made in the financial statements. The auditor will use a variety of techniques, including inquiry, observation, and analytical procedures.
Reporting: After completing fieldwork, the auditor will issue a report detailing their findings and opinions. If there are any material misstatements identified, these will be disclosed in the report.
Follow-up: Following up after an audit is important to ensure that any recommendations made by the auditor have been implemented by management. The auditor may also perform additional procedures to assess changes in risk or control deficiencies.
How to prepare for an ISO 13485 audit
When preparing for an ISO 13485 audit, it is important to understand the requirements of the standard and how your organization's quality management system (QMS) meets those requirements. You should also review your organization's procedures and records to ensure they are up to date and accurate.
The auditor will likely want to see evidence that you have a robust QMS in place, so be sure to have all relevant documentation ready. This may include your quality manual, quality policy, procedures, work instructions, and records. The auditor will also want to see how your QMS is implemented throughout the organization, so be prepared to give a tour of your facilities and show how the QMS is integrated into day-to-day operations.
It is also important to be aware of the auditor's expectations. Be polite and professional, and be prepared to answer any questions the auditor may have. By being open and cooperative, you can help make the audit process as smooth as possible.
Conclusion
We hope that this article has given you a better understanding of what is involved in a 13485 audit. As you can see, there are a lot of moving parts to consider, but with the right preparation and understanding, the process can be immensely valuable for your business. If you have any questions or would like help getting started, please don't hesitate to contact us. We're here to help!
2 notes
·
View notes
Text
How to Use ISO 13485 Standard For CE Marking Approval of Medical Devices?
If the medical device is manufactured outside of the European Union, the CE marking provides access to European markets for products. Before putting the product on the market in the European Economic Area, the manufacturer shall be required to get and display the CE mark on the product (EEA). This responsibility shifts to the importer if the medical device is brought in from outside the EEA. The abbreviation “CE” stands for “European Conformity,” which is the English translation of the French word “Conformité Européene.
Since ISO 13485 is in line with European medical device directives, putting it into practise aids in meeting their requirements. As a CE mark medical device consultant , we provide guidance to medical device manufacturer for the proper and valid ISO13485 standard certification.
Important steps for getting CE marking on your Medical Device:
Getting the CE marking on your device involves some logical and formal procedures. The “old technique” demanded that incredibly stringent technical requirements be met. The following steps will be useful as you move through the “new way,” which includes more fair and standard requirements for safety and functionality:
Identify medical device status:
Recognize regulatory requirements and their fulfil-ment:
Development and preservation of the technical files:
Review for product conformity:
Declaration of conformity:
As medical device regulatory consultant we assured that, Companies operating outside of the European market that already have a Quality Management System based on ISO 13485 standard can more easily obtain the CE mark for their products because the ISO 13485 standard already satisfies many of the directives’ requirements for conformity assessment evaluation. In some instances, a manufacturer’s certificate of declaration of conformity is sufficient to get a CE mark for medical devices produced using ISO 13485-compliant systems.
Read More - ISO 13485 Standard For CE Marking Approval
#ce mark approval medical device#iso13485 standard#iso 13485 certification#en iso 13485#13485 certification#iso 13485 medical devices#iso 13485 requirements#iso 13485 qms#iso 13485 quality management system
0 notes
Text
#iso 13485 certification in USA#ISO 13485 Standard in USA#SIS Certifications#QMS For medical devices in united states
0 notes
Text
Understand the Management Responsibilities in ISO 13485 Quality Management System for Medical Devices

ISO 13485 is a Quality Management System standard, consequent from the internationally recognized and recognized ISO 9000 quality management standard series. ISO 13485 familiarizes the previous version of ISO 9001, ISO 9000:2008 process-based model for a regulated medical device manufacturing environment. While ISO 13485 is based on the ISO 9001 process model concepts of Plan, Do, Check, Act, it is considered for regulatory obedience; therefore, it is more prescriptive in nature and needs a more thoroughly ISO 13485 documents QMS. ISO 13485 support medical device manufacturers in designing a quality management system that establishes and sustains the effectiveness of their procedures.
#iso13485documents#iso13485standard#managementresponsibilities#iso13485certification#iso13485medicaldevices#qualitymanagementsystem
2 notes
·
View notes
Text
Medical Device Registration in Argentina | OMC Medical Limited
Ministry of Health
Ministry of Health
Regulatory Authority
Administracion National de Medicamentos, Alimentos y Tecnologia Médico (ANMAT) (The National Administration of Drugs, Foods and Medical Devices)
Medical Device Regulation
Disposicion N 2124/2011
Mercosur Resolution No. 40 (Classification)
Disposicion N 2318/2002
Disposicion N 727/2013
Official Language
Spanish
Classification
Class I, II, III and IV
Medical Device Registration in Argentina
– Determine the Classification of the product
– Appoint Argentina Authorized Agent (AAR)
– AAR submits the documents to ANMAT through the HELENA portal and pays registration fees.
– AAR submits a notification to ANMAT for Class I devices prior to placing the device on market.
– Once approved,
ANMAT gives a notification number for Class I devices.
ANMAT provides clearance for Class II, III and IV devices for placing on the Argentina market.
Documents Required
– Declaration of Conformity
– Device classification information
– Manufacturer Information
– Labelling & IFU
– Technical File
– Certificate of Free Sales or Foreign Govt. certificate
Post-market Requirements
Adverse events must be reported to ANMAT
Legalized/Notarised Documents (if any)
Certificate of Free Sales (FSC) required from recognised countries – Australia, Canada, Japan, US & EU.
Applicable QMS
GMP or ISO 13485:2016
Registration Timeline
Class I & II – 15 working days
Class III & IV – 110 working days
Authorized Representative
Yes
License Validity
Class I – Lifetime validity
Class II, III & IV – 5 years
Special Notes
All submission documents must be in Spanish
Originally Published at: https://omcmedical.com/medical-device-registration-in-argentina/
0 notes
Text
What is the Importance of ISO 13485 Certification in Tanzania
/ Uncategorized / By Factocert Mysore

ISO 13485 Certification in Tanzania
ISO 13485 Certification in Tanzania healthcare location is experiencing notable increase. As the choice for for medical devices rises, ensuring their brilliant and safety turns into paramount. This is wherein ISO 13485 certification in Tanzania plays a critical function. This entire guide explores the importance of ISO 13485 for Tanzanian scientific device agencies, outlining the stairs inside the route of certification and its capability benefits.
Understanding ISO 13485 certification in Tanzania: The Global Benchmark for Medical Device Quality
Developed through the International Organization for Standardization (ISO), ISO 13485 certification in Tanzania is the internationally diagnosed elegant for a Quality Management System (QMS) unique to scientific gadgets. It outlines the necessities for an entire framework that guarantees regular production of ordinary and powerful medical gadgets within the direction in their complete lifecycle—from layout and improvement to production, distribution, and located up-marketplace surveillance.
What is the Importance of ISO 13485 Certification in Tanzania
Obtaining ISO 13485 certification in Tanzania offers severa benefits for Tanzanian medical tool corporations, impacting various factors of their operations:
Enhanced Patient Safety: The middle principle of ISO 13485 certification in Tanzania is prioritizing affected person protection. By imposing a strong QMS, organizations can restriction the threat of defects and malfunctions of their devices, number one to greater normal healthcare transport.
Regulatory Compliance: Tanzania has guidelines governing clinical device protection and exceptional. ISO 13485 certification in Tanzania demonstrates compliance with the ones recommendations, facilitating smooth regulatory interactions and marketplace get right of get right of get right of entry to to to.
Improved Product Quality: The amazing emphasizes stringent superb manipulate techniques ultimately of the product lifecycle. This interprets into typically dependable scientific devices that carry out as supposed.
Increased Market Access: ISO 13485 certification in Tanzania is a globally recognized image of exquisite. This opens doors to international markets, allowing Tanzanian groups to boom their advantage and compete globally.
Enhanced Brand Reputation: Certification indicates a strength of mind to superb and affected man or woman safety, fostering hold in thoughts with healthcare specialists, patients, and customers. This strengthens the brand popularity and creates a competitive gain.
Improved Risk Management: The QMS framework emphasizes proactive chance identity and mitigation techniques. This minimizes the capability for product recollects and associated charges.
Streamlined Internal Processes: Implementing a primarily based totally completely in reality actually in truth QMS improves normal universal overall performance and consistency inside the path of numerous departments inside the industrial company enterprise organization.
The Path to Certification: Implementing an ISO 13485 certification in Tanzania-Compliant QMS in Tanzania
Achieving ISO 13485 certification in Tanzania generally includes a tough and fast up and phased method. Here’s a breakdown of the crucial steps:
Gap Analysis: The first step is to conduct an extensive hollow assessment to assess how your present superb control practices align with ISO 13485 certification in Tanzania’s requirements. This assessment identifies regions in which your modern-day device desires improvement.
Developing a Quality Manual: A whole tremendous guide serves due to the reality the roadmap for your QMS, outlining your superb suggestions, techniques, and strategies particular to scientific gadgets.
Implementation and Documentation: The subsequent degree consists of implementing the middle elements of an ISO 13485 certification in Tanzania-compliant QMS. This includes putting in vicinity risk manage strategies, developing exceptional control plans, and documenting layout control, manufacturing manage, and positioned up-marketplace surveillance techniques.
Training and Awareness: All personnel worried inside the clinical device lifecycle ought to investigate on the QMS, which incorporates their roles and duties in preserving super necessities. This fosters a manner of life of splendid interest within the enterprise employer agency commercial enterprise organisation.
Internal Audits: Regular internal audits are critical to assess the effectiveness of the QMS and find out any areas for development. This ensures non-prevent tracking and refinement of the device.
Management Review: Senior control performs a important characteristic in the QMS’s ongoing fulfillment. Regular manage reviews take a look at the tool’s effectiveness, discover regions for improvement, and make sure continual improvement within the path of excellent desires.
Tanzanian Resources for ISO 13485 certification in Tanzania
Several assets are available in Tanzania to help clinical tool organizations on their journey in the direction of ISO 13485 certification in Tanzania:
Tanzania Bureau of Standards (TBS): The national requirements body can offer information on relevant pointers and ordinary certifications of our bodies in Tanzania.
Accredited Certification Bodies: Several certified certification our our our our our bodies in Tanzania can decide your QMS and award ISO 13485 certification in Tanzania upon very last touch.
Consultancy Services: Consulting groups focusing on medical device tips and ISO 13485 certification in Tanzania implementation can provide valuable steering and useful beneficial beneficial useful useful resource in the end of the device.
Integration with Existing Management Systems:Organizations with modern-day-day control systems like ISO 9001 (Quality Management System) can leverage the ones systems at the equal time as enforcing ISO.
Conclusion:
In surrender, ISO 13485 certification in Tanzania offers a strategic possibility for Tanzanian clinical device agencies to raise their operations and merchandise. The blessings increase past affected individual safety, encompassing regulatory compliance, advanced emblem popularity, prolonged marketplace get right of access to, and a aggressive detail in the worldwide market.
Why Factocert for ISO 13485 Certification in Tanzania
We provide the best ISO consultants Who are knowledgeable and provide the best solution. And to know how to get ISO certification. Kindly reach us at [email protected]. work according to ISO standards and help organizations implement ISO certification in Tanzania with proper documentation.
For more information, visit ISO 13485 Certification in Tanzania.
Related links
ISO 9001 certification Tanzania
ISO 14001 certification Tanzania
ISO 45001 certification Tanzania
ISO 13485 certification Tanzania
ISO 27001 certification Tanzania
ISO 22000 certification Tanzania
CE Mark certification in Tanzania
0 notes