#iziel
Text
Do medical devices need to be approved by the FDA?
Medical Device Manufacturers require USFDA Approvals to sell their products in USA. USFDA differentiates product approvals in Class I, II &III depending upon the risk associated. The submissions include self-certification, 510(k) and PMA depending upon the class of the product.
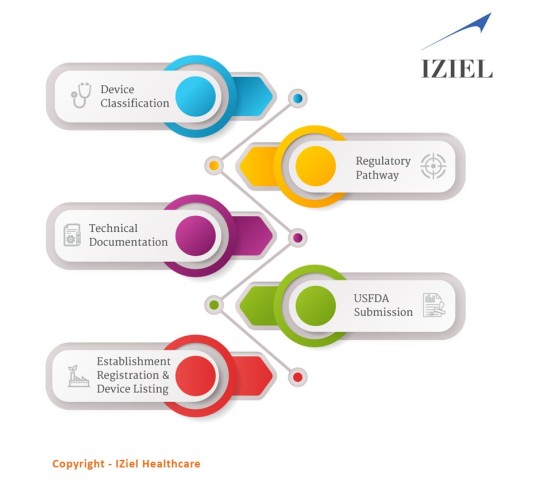
The FDA medical devices have been classified into 3 classes.
Class I: They are low-risk devices.
Class II: They are medium-moderate risk devices.
Class III: They are of high risk, generally life supporting and life-sustaining.
IZiel adopts an analytical mindset thus enabling us to root out all possible non-conformances in a regulatory submission. IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) for USFDA Approvals.
0 notes
Text
What is Post-Market Clinical Follow-Up?
PMCF study is carried out for CE certified medical devices that are placed in the market. It’s a method of proactive collection of clinical data to analyze the emergent risks/side-effects of the medical device to demonstrate the safety and performance as per the intended purpose, throughout the expected lifetime of the device.
Performance and safety parameters are predefined with acceptance criteria based on the benchmark devices data, and milestones are designed for several PMCF studies and activities. The literature screening and registries study also supports PMCF findings. The sample size is predefined as per the sales of the device to study the emergent risks. Benefit-risk ratio analysis is performed as per the PMCF findings, for the acceptability of the device in the market. The PMCF findings are also documented in Technical Documentation, Risk File, SSCP, CER and PSUR
PMCF Includes
PMCF Procedure
PMCF Plan
PMCF Report
PMCF Plan
PMCF Protocol
Sample Size
Design of Milestones with follow-up period
Design of measurable endpoints for performance and safety
Ethical Committee approval
Questionnaire/Survey form
Patient/subjects consent form
Clinical Investigation/Trial procedure (if any)
Real World Evidence
Statistical Significance
PMCF Report
Milestones completion
Real World Evidence analysis
Literature Screening
Registries
Questionnaires and Surveys results
Feedback from users/end user
Clinical Investigations/Trials results (if planned any)
Outcome of performance and safety endpoints
Risk benefit analysis
Residual Risks (if any)
Statistical Analysis
CER citation
Frequency of PMCF update
IZiel Healthcare offers PMS services to our clients where we perform end to end Post Market Surveillance activities. These services can be PMS Plan, PMS/PSUR Reports, PMCF Plan and Reports, Trend Reporting, Complaint Handling, Clinical Evaluation, Risk Benefit Management and many more.
We have expertise in designing PMCF Plan and drafting reports for our clients for all the Classes of Medical devices – Class IIa, Class IIb and Class III.
You may contact us at Contact Us – Iziel.
0 notes
Text
Iziel

An adoptable I own from a closed species
Interested? Toyhou.se link
#adoptable#original character#original art#digital drawing#oc#air-horn#ref sheet#clip studio paint#xppen#cieran art#cieran oc
1 note
·
View note
Text
"I don't understand!" Selene paced frantically, raking her hands through her hair. "I should- this doesn't happen!" She hissed.
Iziel shrugged, swirling his chalice of wine. "The course of love is strange."
"Shut up you old fool, you've never known love-" She spat the word- "The concept is as foreign to you as it is me."
"I've known love, and despair, and hatred." His eyes narrowed. "Love invokes many emotion, but they all share one familiar trait, no matter what, to feel them is agony in the best way. There is no stronger sensation than one drawn from love, no adoration, no grief, no betrayal-"
"Will you stop going on and on. Your a selfish, heinous, coward, and the fact that I can speak the words makes them true!" She sneered.
"It makes what you think true Selene, you truly believe you aren't in love, like you truly believe I am nothing more than a cruel monster-"
"That's because I'm not, and you are!"
Iziel sighed, glancing at the little wine in his chalice, perhaps forgoing the chalice and just stealing the bottle away would have been wiser. "You've grown attached to him them, you've not had anyone for company apart from wolves for the past three centuries, as immortal as you are you are not immune to the basic desire for contact and company, and all your toiling with him meant he's been the only person you've truly bothered to dedicate any time to."
Selene paused then, actually considering the words, and Iziel swallowed the drabs of his wine, playing the grimace off as a bi-product of the taste and not her shocking obliviousness. For someone who had spent the past three hundred years raiding his personal library for knowledge she certainly was inept at understanding basic emotional responses.
2 notes
·
View notes
Photo



I’m lazy with comics but I do have very few
Sil having a nightmare 0.0
16 notes
·
View notes
Text
When Tesla Met Vivekananda
When Tesla Met Vivekananda
By- Krishanu Bajani
“Mr. Tesla was charmed to hear about the Vedantic prana and akasha and the kalpas. He thinks he can demonstrate mathematically that force and matter are reducible to potential energy. I am to go to see him next week to get this mathematical demonstration. In that case Vedantic cosmology will be placed on the surest of foundations. I clearly see their perfect union with modern…

View On WordPress
1 note
·
View note
Photo

The Crystal Exarch strayed a bit too far from his tower, so Iziel gave him a lift home~
(Shadowbringers was absolutely incredible by the way!)
#final fantasy xiv#ffxiv#shadowbringers#crystal exarch#Iziel Kayri#WoL#warrior of darkness#fanart#my art
61 notes
·
View notes
Text
Strange Outline
Chapter One - A Tiny Woman!

“This is so…boring!” Misthel exclaims in annoyance as she crosses her arms and leans back nearly sliding off the seat. The carriage moves slowly over a open green field, it hopefully wouldn’t take too long to reach Mirkwood, Misthel thinks to herself as she gazes out the small window by the carriage door. It almost seemed like some sort of a punishment by her ada...
Her being alone, trapped inside of this moving carriage without any company… haunted by her own thoughts about the arranged marriage and her duties of being a Lady of Rivendell.
He could at least have accompanied her! She thinks to herself as she remembered back on her departure from Rivendell…
“Now remember what I have told you… you represent us! So don’t miss behave and try your best to fight off some of your natural instincts...such as being overdramatic, clumsy, stubborn and... “ Misthel's ada lectures her. While his list goes on and on, Misthel gets easily distracted by their servants loading off all of her luggage onto the carriage. Her ada sighed defeated after realising that Misthel was already being distracted and wasn’t really paying any attention to what he was saying. He took a hold onto her and pulled her into a loving embrace, which caused her focus to switch back on him. “I will miss you my méla daughter…”
The memory of her Ada slowly fades… as she looks at her own reflection in the small window. She needs to be perfect, huh? She thinks to herself and her stare hardens as she searched for any visible flaw on her petite face. She sighs as her gaze lands on her braided hair crown. This wasn’t a very comfortably hairdo at all !
Being all dressed up and polished to impress her future father-in law. He had the last say… before they could make the engagement official. Not that she felt like getting married any time soon… but according to her Ada, its for her own good and of course for Rivendell… their homes future. It all depends on her…
Why her? Why not her older sister Arwen? Couldn’t she fulfill this duty instead of her? Not that she had anything against Legolas… But something inside her, tells her that she isn’t ready...to go through with it just yet, not to mention that the young prince was more of a brotherly figure than a romantic interest in her eyes. The doubt and fear begins to fill her mind… her body tenses and her grip on the door handle tightens.
However her worried thoughts gets interrupted by a sudden loud sound coming outside from the front of the carriage. Wait… was that the warhorn ? She thinks to herself.
At once the carriage stops, causing Misthel to fall from her seat and land on the floor. She gets up on her knees and takes a peek outside the window, all the guards stands ready with their weapons drawn, prepared for a fight.
For a second everything is quiet and Misthel almost thinks that it was just a mistake, but then….. a small pack of Orcs appears in the distance, rushing towards them in an ambush from the forest on her right.
The guards made quick work of them, but some tried to make a retreat, causing everyone except one of the guards to chase after them. The one guard who was left behind, stood right in front of the carriage door, which Misthel opens as she hears the sound of the others chasing the ambushers, accidentally knocking out the only person left to protect her.
As she starts to panic, she then realises that now there is no one to force her to Mirkwood….. if she misses the finalization of the engagement she would be free!
Sure her ada would be angry for a while... but that would only be a temporary thing... she hoped.
With this she takes her chance and sets off, running into the woods in the opposite direction of where the orcs disappeared into, she did not want to run into those foul creatures…!
As she starts her escape, she ends up running for quite a while before slowing down to a calm walking pace, her shoes and dress making it quite hard for her….and they were quite nice so she did not want to ruin them especially, because her ada would be even more disappointed in her, if she returned and looked like she had rolled around on the forest floor!
One thing Misthel did NOT give much thought when she made her escape, however was the fact that she had no idea were she was!
She had already walked for what must have been hours since the sun hadn’t set yet, but it felt like days!
Just as she’s about to give up all hope, she gets distracted by something shining and glistening in the distance. Quickly she rushes in the direction its coming from, completely forgetting everything else.
The shininess seemes to never get any closer, no matter how much she hurries towards it.
Finally after what felt like an eternity, she reaches the opening of a cave from where the light seemes to come from. Slowly she makes her way in there only to be faced with an ice crystal that appears to be very cracked.
Not being able to do as her ada said and fight her instincts…… she pokes it…. this causes the ice crystal to crack even worse and more light shines from it. As the pieces starts to fall off... the light gets brighter, and it's too bright, especially for Misthels sensitive elven eyes and temporarily blinds her…
Once her vision returns, she looks down a bit to only to see a tiny woman kneeling in front of her surrounded by some small chunks of ice.
Misthel is very confused, why was there a tiny woman in the ice? How long had she been in there? And most importantly who-or even WHAT is she? The questions fills up her mind and leads to her once again giving into her natural instinct and squats down in front of the tiny woman, before asking a small “Hello?” not receiving any response she tries again “Are you alright?”.....now not being able to stop herself she again raises her hand…. and pokes the tiny woman!
This causes the small creature to lift its gaze, first to the hand that poked her still reached out, ready to give another poke if needed and then to lock eyes with Misthel. “Where am I...? Who are you...?” comes the small and slightly raspy voice of the woman, clearly not having talked in quite a while. Just how long had she been frozen?!
Both of them stands up, The small woman wobbling a bit from not using her legs for a while. She then looks at Misthel, who was already staring intrigued at her with big wide eyes. “Well...you were encapsulated in ice...i think…. when i found you and well, now you’re not?” Misthel says, not really sure herself as the situation she found herself in was very strange to say the least.
She did after all just find a person...in ice...in the damn woods!
The small woman looks at Misthel before giving a bow as a “thank you” to Misthel for freeing her. Misthel not sure if she did or not decided to just accept it.
Misthel sees the small one still looking at her questiongly “Who were you again?...Do I know you?” she asks with a small tilt of her head, her voice finally starting to return to normal.
“OH Right! Sorry, I’m Lady Misthel of Rivendell.” Misthel says with a quick and small curtsy “Thank lord's my ada isn't here, he would’ve given me a lecture for not remembering my manners” Misthel mutters to herself while rolling her eyes slightly, still loud enough for her new companion to hear her.
“And who...and what are you tiny woman?” Misthel then asks, leaning forward slightly in curiosity. “I-I’m….” The small woman stops up and stares blankly at Misthel. “To be honest I’m...not sure” she then mumbles.
For once Misthel actually paying attention and her elven hearing being useful. “ Well then, I’ll just give you one then! Since you can't remember and you don't seem to have label with it on!” Misthel says excitedly, which made the small woman to take a step back in worry.
“A what?.....label?” she worriedly asks the elven woman, only getting more and more confused by her, but she seem kind so far. “A name! And you know sometimes the elven mothers attaches a label to their children at gatherings with the child's name and who the parent is!” Misthel explains, not realizing she just compared the small woman with a child…
After all her ada still sometimes does it to her, knowing how easily distracted she gets…
“Hmmmmmm you were trapped, nuzzled into the ice…..Iziel!-no nevermind that's no good.” Misthel Rambles to herself “Nuzzileas? no, not that either...OH! Oh, oh, oh, oh, oh I know! Nyzziel! Nyzz or Nyzzie for short!” She suddenly exclaims, startling the small woman, who’s name was now Nyzziel, causing her to let out a cheerful squeak. Misthel just looked at her for a second..
“That was…..ADORABLE! DO IT AGAIN!” she yells grabbing Nyzz’s shoulders and making her release another squeak involuntarily.
After talking to Nyzz shortly it is clear to Misthel that she has some sort of memory loss, and Misthel therefore decides to help out, what she had deemed to be her new friend.
Misthel’s ada had taught her a lot over the years, one thing being about different races. Nyzz was tiny and had a lot of hair on her head and she seemed kind and welcoming…. short, hairy and welcoming…. she MUST be a hobbit!
Or at least Misthel believes her to be one, based on her adas teachings. Although Misthel feels like she has forgotten one key feature about hobbits…. couldn’t be that important then!
She did however know that they lived in the Shire and therefore she confidently sets off in the direction of said place with Nyzz alongside her.
_____________________________________________________________________________________________________________________
!❤ ! (>’o’)>”Bootylicious”<(’o’<) !❤!
_____________________________________________________________________________________________________________________
We hope you've enjoyed the beginning of this adventure and look forward to the next part!
2 notes
·
View notes
Link
Iziel Healthcare is offering complete solution in Process Validation for Medical Devices. Our team is Design for Six Sigma (DFSS - Black Belt Level) trained and is fully competent to conduct requirements management, process validations, that are ISO13485, US FDA & CE approval compliant
0 notes
Text
Job opportunity for Recruiter @ IZiel Group
Job opportunity for Recruiter @ IZiel Group
Job opportunity for Recruiter @ IZiel Group
#OmAccounting.in #GstAccounting #GstReturns #accountingServices #accountingOutsoucing #onlineAccounting #gstecommerceaccounting
Details: About Role & Company End to End Recruitment Engineering domain Recruitment experience is preferred. 2-4 years Experience Proven recruiting experien…
Source: careerbuilder
View On WordPress
0 notes
Text
Greetings.
Hello. I am Valak Iziel Michaelis, and I've come here to roleplay. I use to do Wolf roleplay on Instagram, but thought it had gotten dead over time. So-that being said. I do human or wolf roleplay. Doesn't matter. (My model is Kai from EXO, so EXO-Ls please be-friend me.) I will had more, if you liked to, feel free to message and say hello. Or if you just want to roleplay. :)
0 notes
Text
Things You Need to Know About Medical Device Software Validation
Software Verification and Validation (Software V&V) is an integral part of software design that spans all the development stages as specified in IEC 62304 which addresses the Software Development Life Cycle (SDLC) of medical software and software embedded within medical devices. Software Verification is to test if the software was designed as per requirements and Software Validation is to test if the right product was built for the user. Medical device software validation generally occurs during or at the end of the development cycle.
The difference between Software Validation & Software Verification can be answered by asking these mentioned questions:
· Verification: Are we building the product, right?
· Validation: Are we building the right product?
Software validation is a process of checking if the product will meet the customer’s actual needs, while verification involves procedures for making certain that the software is well-engineered, free of errors, and functional.
Following are the steps for Software Validation for Medical Devices
· Create a software validation plan
· Determine system requirements
· Create a validation protocol and test specifications
· Conduct and document tests
· Establish procedures and write your final report
IZiel has highly trained software engineers with multiple years of experience in software coding, software verification and software validation. The team consists of senior engineers who have worked in the design and development of highly sophisticated implantable devices at industry-leading companies, with direct expertise in software V&V.
0 notes
Text
What is the IQ OQ PQ validation protocol?
Process Validation IQ, OQ, and PQ is conducted to ensure consistent delivery of quality products meeting its predetermined specifications/requirements and quality characteristics. This enables to ensure the complete safety & efficacy of medical device.
IQ, OQ, and PQ process validation are sequential activities that manufacturers carry out to validate their manufacturing processes. IQ stands for Installation Qualification, OQ for Operational Qualification, and PQ for Performance Qualification. The purpose of process validation is to establish documented evidence that the production equipment is correctly installed, operates according to requirements, and performs safely. It is also to demonstrate that the manufacturing process under normal operating conditions will consistently produce conforming products.
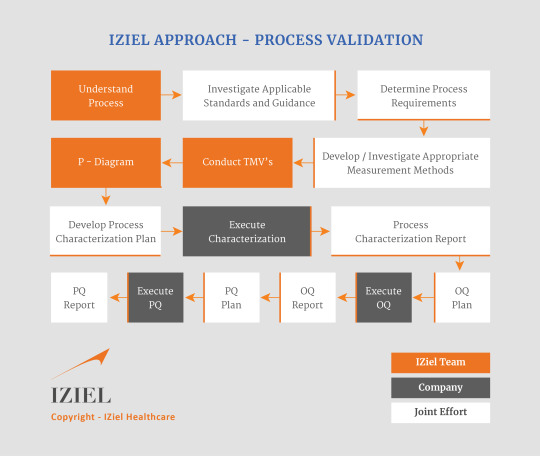
IZiel works with your team to assist you to complete all the following process validation activities
· Process Validation Master Plan (PVMP)
· Process Characterization
· Equipment Installation and Qualification (IQ)
· Operational Qualification (OQ) –
· Performance Qualification (PQ)
· Test Method Development (TMD) & Validation (TMV)
0 notes
Text
Are you making these mistakes with FDA 21 CFR PART 820 made for medical devices
Manufacturers are expected to follow the quality system requirements described in FDA 21 CFR part 820 This document guides to govern the design, manufacture, packaging, labelling, storage, installation, and servicing of medical devices intended for human use. The requirements in 21 CFR Part 820 are meant to ensure the safety and efficacy of medical devices sold in the US marketplace.
FDA conducts regular inspections of medical device manufacturers to ensure compliance with these regulations. The inspection process, known as the Quality System Inspection Technique (QSIT), evaluates a company’s internal quality processes to determine whether they are in alignment with or in violation of these regulatory requirements. If any violations are discovered, the inspecting agent from FDA will issue in the form of 483 Inspectional Observations, Warning Letters what is applicable
Here are the most common mistakes companies run into with FDA 21 CFR Part 820:
1. CAPA Procedures and 21 CFR Part 820.100(a) CAPA not followed adequately and repeat failure is detected
2. Complaint Handling and CFR Part 820.198(a) Parameters described in procedure are not fulfilled and closure is not timely and adequate.
3. Nonconforming Product and CFR Part 820.90(a) Investigation is not proper, CAPA is not applied conclusion is not enough, preventive action is not proper, repeat failure is detected.
4. Purchasing Controls and CFR Part 820.50 control for specification and artwork s not controlled purchase found with old label artwork and specification. PO does not mention specification or art- work reference
5. Process Validation and CFR Part 820.75 System is not adequate, not followed, re-validation where defined is not done or done late, changes are not captured in validation. Protocols are not properly and timely approved before starting activities.
6. Quality Audit and CFR Part 820.22 Audit done randomly, annual plan is not available, Audit closer is not proper, audit compliance are not reviewed in Management review meeting.
7. Device History Records and CFR Part 820.184 Incomplete, completed in hurry just before audit, quality and regulatory are not in full control of DHR.
8. Design Validation and CFR Part 820.30(g) Protocol is not adequate; all aspects of validation are not covered. Documentation is not proper.
9 PMS (post marketing surveillance) System is not followed. Some reportable incidents are not informed to FDA. Some critical or major complaints related to product not covered in PMS
10 UDI (Unique Device Identification) system documentation and records are poorly maintained.
11. Production, process control and quality control and assurance: System inadequate, documentation is not timely, and records are filled late or improper. Critical values are not verified by another independent person.
IZiel adopts an analytical mindset thus enabling us to root out all possible non-conformances in a regulatory submission. IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) for USFDA Approvals.
0 notes
Text
Healthcare Merger & Acquisition: What Decision Makers Need to Know Before Partnering
Mergers & Acquisition in the healthcare industry has been strong in recent years and is expected to grow. Acquiring a new company or product line can have a positive business impact, but it also comes with implementation, process, quality & regulatory challenges. Especially, in the medical device industry, failure to comply with regulations can result in the loss of authority to deliver products and services.

Because of this, it can be difficult for hospitals and healthcare organizations to know whether they should partner, when to partner and what type of organization to partner with.
There are a few things every organization needs to think about before they get started -
· Before considering a partnership, first step back and evaluate your organization’s needs, goals, and long-term strategy.
· After a thorough self-review, deciding what type of partnership to pursue should consider multiple factors.
· Knowing when you’ve found the right match can be a complex process.
· Understanding when to engage legal counsel is crucial.
IZiel enables the company to ensure compliance with the product specific standards & requirements by performing high-quality due diligence to mitigate risk and thereafter provide support to complete documentation as per USFDA / EUMDR requirements. IZiel has successfully worked with one of the world’s largest medical device manufacturers to update more than 2000 documents, 500+ approvals with release in Agile in a short span of 8-9 months.
Strong Project Management, Medical device Expertise, Quick Scalability of Skilled Resources are the key enablers of our success for Acquisition Integration Projects. As depicted above, IZiel’s approach has been very comprehensive and methodical.
IZiel team can support your team in various ways, including but not restricted to –
· Post-Acquisition Assessment
· Manufacturing Process Instructions
· Process Optimization & Validation
· Labelling
· Software & Tool Validation
· Documentation Support
· Configuration Management
· Component Remediation
· Commercialization
· Supplier Quality
0 notes
Text
Configuration Management in the Medical Device Industry
Configuration management is “a process for establishing and maintaining the consistency of a product’s performance, functional and physical attributes with its requirements, design and operational information throughout its life.”
It is imperative to develop product configurations and manage effectively. A structured configuration management program ensures accurate and consistent product documentation (e.g., requirements, design, test, and acceptance documentation) along with the actual physical design of the product. Configuration Management plays an important role to ensure thorough product variations to enhance customer satisfaction, competitiveness, profitability & continuous changes / requirements. Therefore, it’s critical that the product and process configuration is unified across the organization, which minimizes handoffs of specialized information.
Configuration Management must include –
1. Enterprise or Unified Supply Chain Configuration Solution
2. Error-Proofing
3. Intuitive and Flexible
IZiel has highly trained configuration managers who will be able to create, coordinate and implement the Configuration Management Plan (CMP – includes responsibilities and resources, (including personnel), training requirements, administrative meeting guidelines (including a definition of procedures and tools), baselining processes, configuration control and configuration-status accounting, naming conventions, audits and reviews, subcontractor / vendor configuration management requirements, regulatory requirements) for Product Creation Process (PCP) projects in co-operation with the Project Managers and Operations Department.
0 notes