#purple irises
Text
Iris violets






photos perso
#iris#iris violet#iris violets#purple irises#iride viola#iris morado#lila iris#fleurs#flowers#fiori#flores#blumen#photo#photos#photographies#photography#my photos#nature#chemin#vero emilie
105 notes
·
View notes
Text

Irrevocably Attached
Watercolor on Black Cotton Paper
2023, 10"x 14"
Purple Iris
#artists on tumblr#flowers#floral#art#nature#plants#artwork#watercolor#painting#minimalism#watercolor painting#flora#purple irises#purple iris#iris#irises#purple flowers#purple#flower#florals#floral art#watercolor floral#floralls#watercolor art#watercolors#watercolour#watercolour art#watercolour flowers#watercolourpainting#artists
167 notes
·
View notes
Text

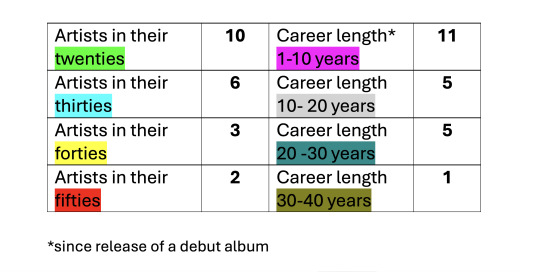
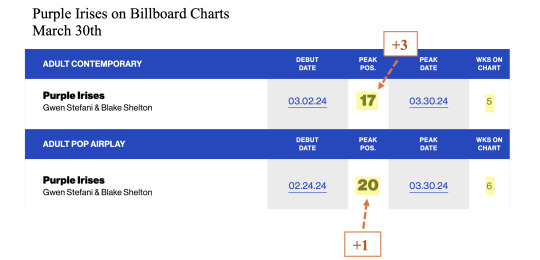
I wanted to put the success of PI in perspective: Gwen has by far the longest career of the artists who have songs charting in the top 25 of the HOT AC chart. Together with JLO, she’s also by far the oldest artist on the chart. As you can see, most of the artists on the chart are in their twenties and in the prime of their career. Gwen's versatility and longevity is truly unmatched.
31 notes
·
View notes
Text

Shopping for more?
13 notes
·
View notes
Text
I've been meaning to make more posts about this, but there's so much i have to say/images to show that this will be a part 1 out of ??? and this one focuses mainly on the first batch of Purple Iris watercolors and the pH mystery they made me unravel through chaotically-organized researching.
Basically, I've been messing around with anthocyanins, a common class of plant pigments that are pH sensitive/can be used as a pH indicator. The first source I've tried has been purple irises, which i've only vaguely been familiar with in the past. The ones I picked were the ones that had begun to shrivel slightly, to the point where they were still a deep purple but picking them they would almost be leaking a purple liquid that stained my hands. I put them in a thing of hot tap water (not boiled, just the hot setting on the faucet), enough to cover the flowers, and let them steep. they began changing the color of the water almost immediately, with the fresher ones not losing their color as quickly as the ones that had begun to wilt on the plant. within 30 minutes i decided it was extracted enough.

This left a strong purple in the water, which i then poured off into three other containers, two of which i would alter the pH of.
The purple is due to delphinidin, a type of anthocyanidin that forms the building blocks of anthocyanins. Note i italicize the word anthocyanidin just so it's easier to tell apart the two.
there are anywhere from 16-31 anthocyanidins depending on what source you find, but they are basically the backbone structure of anthocyanins, of which there are over 600 something. The main thing that turns an anthocyanidin (aglycon) into an anthocyanin (glycoside form) is a sugar attached to it.
Realistically, that distinction isn't useful when simply extracting things from flowers in hot water, but i thought it was a fun fact to note. Anthocyanidins also come in handy for knowing what builds the anthocyanins in your flowers/plant part;
cyanidin (30%), delphinidin (22%), and pelargonidin (18%) make up the base for a good majority of all the anthocyanins in plants (~60% collectively),
peonidin, malvidin, and petunidin being runnerups (20% collectively)
the 20-something remaining anthocyanidins make up the rest
So basically, they all have slightly different colors that are pH reactive, and can provide anything from red to pink to orange to purple to blue. But, for our purposes, if you have a blue/purple flower, that likely means it has some amount of delphinidin-based anthocyanins in it! there can also be more than one anthocyanidin type present in the same plant.
Other well-known sources of anthocyanins are grape skins, red cabbage, red onions, butterfly pea tea, and purple violets. However, they're also very abundant in many many other plants, these are just the common ones i can think of that lots of people are probably familiar with to some degree.
Fun fact, grape skins are actually really well-studied as far as anthocyanins go (i believe they mainly have malvidin-based ones) because they're so important for the coloration of wine!
Anthocyanins as a whole are also studied as a natural source of food dyes, along with other flavonoids such as carotenoids.
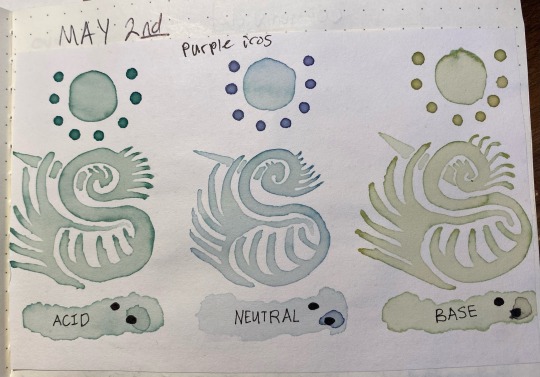
As for why it turns colors, this is because of the way the anthocyanin changes structure in different pHs. The short answer is it turns red/pink in low pH (acidic) conditions, purple in slightly acidic/neutral conditions, and blue/green in slightly high pH conditions.
The long answer is something I'll explain in a moment, but for now here's the acid/base colors:


(on the left, i altered it with vinegar and it became a bright magenta color; on the right, i altered it with baking soda and it became a sea green/blue cyan that refused to show up accurately on camera). That's one thing I've noticed, and others have too, is that when working with pigments (especially natural ones) the color accuracy of the camera often just completely fails. there's only so many colors a digital camera can capture!
Here's a slightly more accurate color due to different lighting, note how it's more a malachite green than a pure blue. off the bat this was interesting becuase I wasn't expecting as green of a liquid as i got.

Anyways, the first thing i did with them was use them as-is, no alterations past the addition of the respective vinegar and baking soda. I painted with them just as one would paint with watercolors, and interestingly enough, when i put them onto paper, they began to change from their pink/purple/malachite colors to a teal/indigo/emerald set instead.
This seems to be the result of something in the paper itself, likely calcium carbonate (which i only recently learned is added to "buffer" paper against acidic substances; the cellulose in paper is more stable long term when there's no acid present, and the calcium carbonate neutralizes any acids applied to a degree).
It's still interesting that even though the acids are neutralized, they give a unique color when compared to the basic paint.
I also tried soaking some of the same paper in vinegar water, which got rid of that buffer and let me paint with the pinks intact, but that's for another post.
Also, note that i said "neutral" for the middle, this is just what i wrote down for the tap water sample; in actuality the tap water is actually a bit closer to pH 6 instead of a true neutral 7, which i only found out after i had gotten this far. So whenever i say "neutral," i mean "tap water that's slightly acidic"
Here's something interesting that happened, though. Overnight i left the jars on my desk, and while the acid and neutral colors were the same when I came back ~24 hours later, the basic had degraded into a murky brown. this was interesting since that meant the instability was pH-dependent.
So, i made another color swatch with the acid/neutral/base, and used that as a comparison to look at how it had changed. Surprisingly, it painted out a yellowy-green instead of a murky gray-brown
here's the murky water that the once-malachite-green turned into:

I also poured off a bit into another container, and shifted it back into a low pH with a bit of vinegar to see if it would still change color. Surprisingly, it turned a slight pink, like pink lemonade, which means there were still anthocyanins in there but they were likely a lot less concentrated than they used to be.
Here's the pink, with a few leftover bubbles from the baking soda/vinegar reaction:

Here's the results of painting with these:
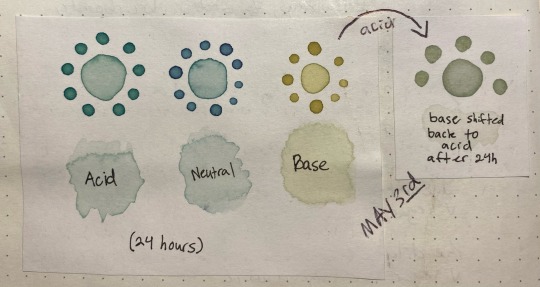
the acid was basically unchanged after sitting in a jar for 24 hours, the neutral had lost a bit of its purple color but was still about the same, and the base was now a lot yellower/tanner with a bit of green still showing through. The shifted sample was a pale stormy gray that ended up taking on a green color as it dried, as though following the trend of pink shifting to a bluer color but on a much more muted scale.
Now obviously, i wanted to figure out what caused this, so i dug around both on wikipedia and other sites but found myself eventually reading into scientific papers on the topic, at which point it became very clear that i would need to learn like, organic chemistry and such to be able to say for sure what was happening.
I did eventually manage to figure out a few things despite the dense terminology; for one thing, anthocyanins are more unstable than other plant pigments such as carotenoids. there are plenty of things that can affect their stability, including the pH of the substance they're stored in. Any higher than pH 7 (basic pHs) and theyll begin to degrade. This explains why the high pH sample lost its blue/green color, and why there was very little left to be shifted back to a pink color.
I also found out that the pH color shift isn't as simple as it seemed. Rather, there are multiple chemical forms of anthocyanins.
At the lowest pHs, basically all of them are in the "flavylium cation" state, which basically means it's positively charged and this is what gives a red color
still at a low pH (2-4), there's anothe chemical form that appears, the "quinoidal" structure that gives a blue color. Note that the red cation is still present, just no longer the only form
the more the pH rises, the more forms start to coexist, with some of those forms being colorless (one of which is a "colorless carbinol"
so, between 4 and 6 there are the cations (red) quinoidal (blue) carbinol (colorless) and something called a chalcone that gives a pale yellow
and then past that, I'm unsure, but of course that's around when the anthocyanins begin to degrade
There are also a lot more than these that i've encountered in various contexts but these seem to be the basic ones.
Do note that i do not fully understand these terms (flavylium, quinoidal, carbinol, chalcone, etc.) and have only recently begun to actually try to learn what they mean and the context surrounding them as i only had a class of basic high school chemistry under my belt prior to this. The main paper i combed over to try to find info on it seems to be behind a paywall but the DOI is:
doi.org/10.1016/j.foodchem.2008.09.001
for anyone curious and able to access it, whether through legit means or what have you.
That being said, to me, the takeaway here seems to be that there's a yellow form that appears around the time that other color forms begin to disappear, and as those degrade it makes sense that the resulting degraded forms also contribute to a murky color. This helps explain why it changed color in the jar and also retained a bit of yellow and green.
This also explains why the blue form seems to also be slightly green, it's got the blue quinoidal chemical form as well as the yellow chalcones.
There are also interesting things of note that I will get into at a later date, such as the texture/reflectivity of the way it dries, the differences in extraction ease between this and purple violets, the addition of a genuinely neutral/pH 7 sample later, a sample from a plant that doesnt seem to have delphinidins, and sample the seems to genuinely sparkle??? Much more of interest to come soon!
#art#traditional art#anthocyanins#watercolors#purple irises#painting#science#natural dyes#pigments#dyes#pH indicators
61 notes
·
View notes
Text
Gwen Stefani & Blake Shelton - Purple Irises
9 notes
·
View notes
Text
youtube
#purpleirises lyric video by #BlakeShelton #GwenStefani
2 notes
·
View notes
Text




Plant of the Day
Friday 1 March 2024
It was only a few days ago I wrote about Iris 'Blue Note' (Reticulata) but my friend had such a lovely display of this small, early bulb, protected by a frame of Cornus alba (dogwood) stems, that here it is again!
Jill Raggett
#Iris#irises#cornus#dogwood#purple flowers#red stems#plants#horticulture#gardens#garden#containers#bulbs#bulbous perennial#orkney
214 notes
·
View notes
Photo

~ Black in Back ~
230 notes
·
View notes
Text


Ogata Körin
Irises
Early 18th century
#ogata korin#irises#japanese artist#japanese painter#flowers#floral art#purple#art history#aesthetictumblr#tumblraesthetic#tumblrpic#painted screen#screen#japanese art#tumblrpictures#tumblr art#tumblrstyle#artists on tumblr#tumblrposts#aesthetic#asian art#japanese aesthetic#asian aesthetic#japanese beauty
357 notes
·
View notes
Text

Day Dreams
Watercolor On Cotton Paper
2024, 41"x 30"
Purple Bearded Iris
Amethyst and Sugilite
#art#nature#flowers#watercolor#floral#artists on tumblr#painting#minimalism#artwork#plants#botany#spring#flora#iris#irises#sugilite#purple#plant#flowercore#garden#gardencore#gardens#minimal#cottagecore#amethyst
170 notes
·
View notes
Text
youtube
7 notes
·
View notes
Text

Two Irises Painterly
91 notes
·
View notes
Text
Every time the lil nutrition radar chart comes up in dungeon meshi I feel this mounting sense of foreboding, because their food (while relatively balanced, and certainly (very) high in iron most times) never has vitamin C in it! The vitamin C axis is there, so it must be there for a reason, and the way this story has committed to grounding everything in such a cool way so far has me dreading the consequences of their fruit-free dungeon spree.
So anyway, I’m looking forward to Chapter 70: everyone’s teeth fall out and then they die of scurvy.
#dungeon meshi#hoping praying etc they find a giant walking orange tree soon and get some fruit in their diet#sometimes the radar chart also looks like the 8 shape of ol mate mage’s irises which hopefully means nothing#purple SAVs
82 notes
·
View notes