#spinal cord
Text
A YOUNG GOD'S WRATH

#gore#spinal cord#blood#cw gore#extreme gore#cult of the lamb#the lamb#cotl fan art#cotl#cotl au#cult of the lamb au#lambi-au
242 notes
·
View notes
Text
y'all thought i wasn't gonna do it huh . here's the guys in harry's brains who don't show up as much, now set to music! i didn't reach my goal of 10 for all of them but I still think that these are pretty okay playlists lmao thank you to everyone who gave me suggestions and the rest of the main skills will be coming shortly!!
#disco elysium#ancient reptilian brain#limbic system#tutorial agent#spinal cord#playlists#fan playlists#Spotify
60 notes
·
View notes
Photo
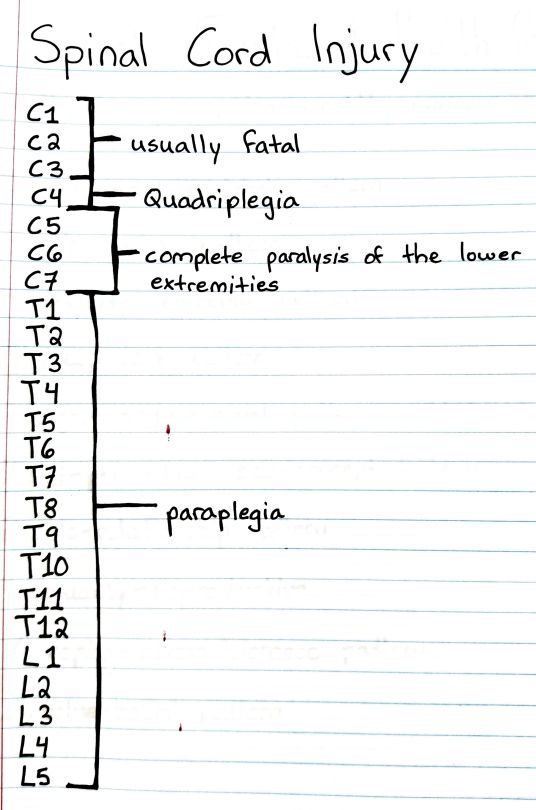
#studyblr#notes#medblr#medical notes#med notes#emergency medicine#emergency medicine notes#ems#ems notes#emt#emt notes#emergency medical technician#emergency medical technician notes#spinal cord#spinal cord injuries#spine injuries#paraplegia#quadriplegia#paralysis#paralysis notes#paralysis levels#levels of paralysis
326 notes
·
View notes
Text
#this is science#100% confirmed by 9/10 dentists#disco elysium#spinal cord#harry du bois#harrier du bois#harry dubois#harrier dubois#kim kitsuragi
37 notes
·
View notes
Text
*unsheathes a sword hidden inside a spinal cord*
“Alright! Which one of you bitches wants to dance?!”
#dougie rambles#personal stuff#sword#spinal cord#spine#spinal#my poor attempt at a joke#unreality#what#no context#this sounded funnier in my head#shitpost#highbrow shitposting#swords#weapons
13 notes
·
View notes
Text
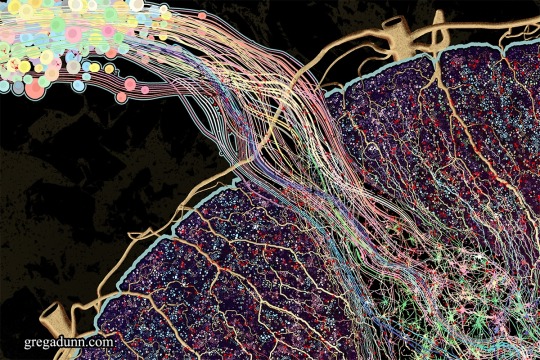

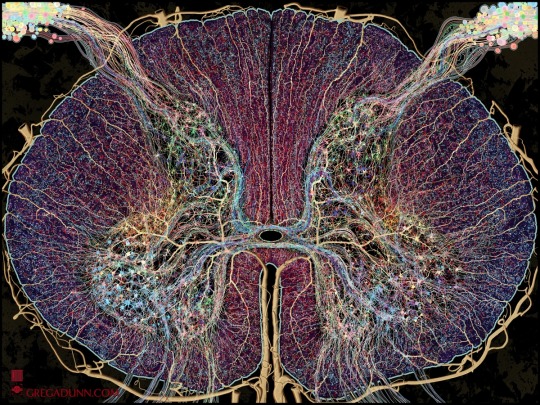
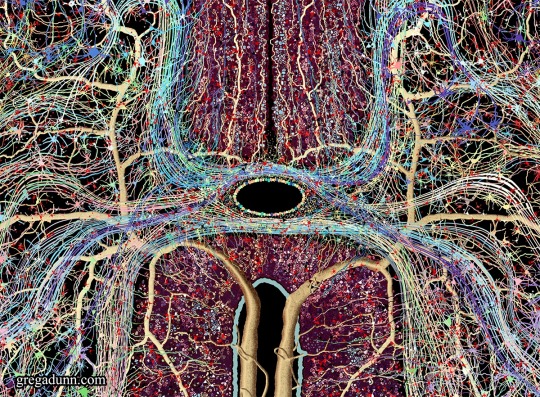
[Greg Dunn Neuro Art]
After a year's work here it is, the new piece Spinal Cord!
The spinal cord is the information superhighway connecting our brains to our bodies, an indispensible organ and masterpiece of evolution as fundamental to our existence as our brains themselves.
It is available as:
1. Fine Art Prints (pictured here)
2. Microetched Prints (I'll post more about these soon, these animate the neural activity of this region)
3. Gold Microetchings (also have reflective animations, highest end option)
This image was made entirely from scratch, deeply informed by the neuroscientific literature and my background in neuroscience and designed to be an attractive piece of fine art as well as an accurate atlas of both anatomy and connectivity. It is the most ambitious project that I’ve undertaken since the creation of Self Reflected.
This artwork and associated images, GIFs, and video are designed to give the viewer a comprehensive understanding of the complex anatomical details of this region and how information travels through the cord. In particular, I wanted to emphasize the complexity of the transverse white matter regions and how signals travel into and out of these areas. Information travels through the afferent inputs in the dorsal ganglia, is processed in the butterfly shaped gray matter regions in the central cord, and through computations with interneurons and afferent/efferent signals from adjacent regions of the transverse white matter tracts leads to signal outputs through the ventral cord that execute movements.
* * * *
This could be enough for me or you today, place your hand on another and melt it on, every square millimeter of your hand relishing contact, fingers as hoses of energy, filling the room with the other, and the horizon.
Alan Bowers
#anatomical#spine#spinal cord#neuroscience#mind#brain#anatomy#nervous system#anatomical art#Alan Bowers#quotes#bodywork#energy#touch
16 notes
·
View notes
Note
Sorry if this is an awkward question, but I can’t find anyone else on tumblr who seems to have good knowledge on this and you said you studied the nervous system for a while
I had this problem where my hands would randomly get pins and needles and lose coordination for a while, I got checked for tennis elbow, nerve damage, and other repetitive strain injuries. Nothing ever came up on a nerve conduction test, no inflammation markers ever showed up in bloodwork, and it would come and go at random
Then I got covid
Now I can’t feel half of my hand at all, it’s gotten even more stiff and my doctors refuse to run new tests. This seriously interferes with my ability to function, as I am now frequently dropping objects and struggling to use that hand
I am sorry if this ask is too much and I know you aren’t a doctor, but I was wondering if you uhm… I’m not sure, might know something? You seem very knowledgeable about nerves and stuff. Do you think this is just one of those ‘little problems’ I’ll have to get used to, or is this a warning sign of something bigger?
I know you mentioned you’ve had some tests done but I didn’t see you mention the cervical spine
The discs in your cervical spine (from your collar to your skull) correspond with different nerve areas in your hands, arms, shoulders, & skull
When I damaged C5-C6, I lost sensation and use in half of my hand. I was having neck aches too but brushed them off as muscle pain or something. Well it wasn’t. I got a CT and my herniated disc was squishing my spinal cord !!!

Here is a helpful illustration about which discs correspond to which areas of sensation. You can see C5-C6 in yellow. That is where the crushed nerve in my neck was affecting me.
if you’ve already had tests/imaging I would disregard this, but if not it might be worthwhile to try and get a MRI of your upper spine to rule out cervical stenosis/pinched nerve
22 notes
·
View notes
Text
This is Me. This is You. This is what We are.
4 notes
·
View notes
Text
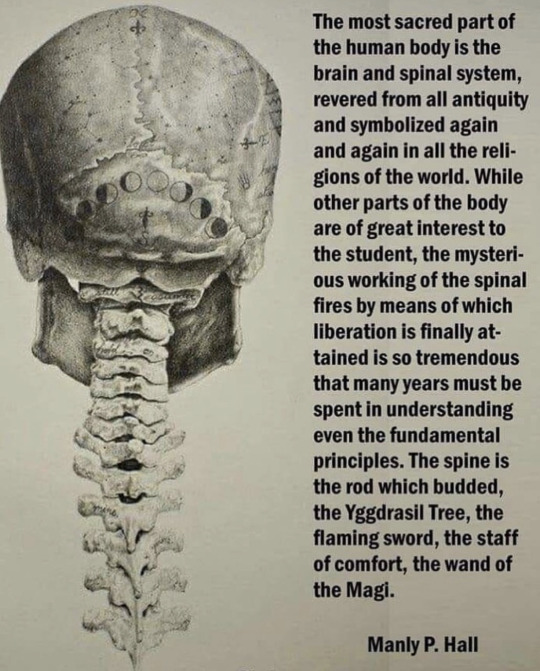
#spinal cord#manly hall#aesthetic#vintage#old school cool#consciousness#liberation#free your mind#nervous system#sacred#spirituality
15 notes
·
View notes
Text
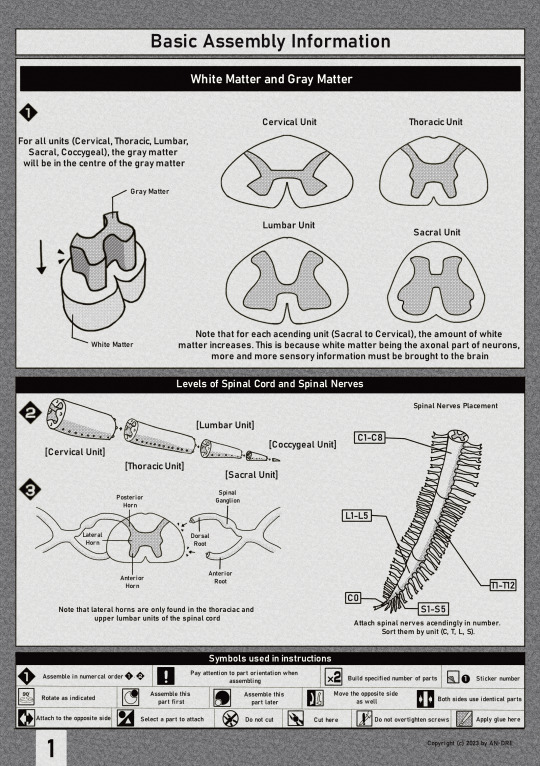

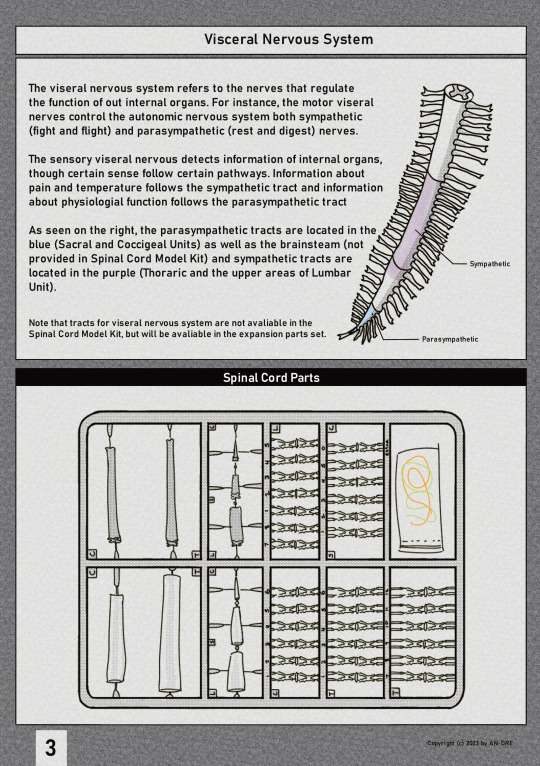
Here's some mock gundam set model instruction manual of the spinal cord that I made for my neuroanatomy class. I was pretty proud of it.
4 notes
·
View notes
Text
Severely Disabled
Sometimes it's so isolating being so disabled by a condition that doesn't affect other people as much as it affects me. I can't walk. Half the days I struggle to get out of bed with my transfer board into my chunky powerchair (which I use full time) before 4 PM. I wear braces on almost every joint in my body, experience extreme fatigue, and am functionally a paraplegic (including my trunk) due to spinal problems (that typically don't get this severe because they are typically caught before they progress this much) but sometimes my hands are affected too. I can only microwave meals and have struggled to arrange care for myself even with a decent support system. I feel like I don't understand how all of this can be true while I work two part-time jobs (totaling to like 20 hours a week) plus school. It feels like because I can work and go to school I should be fine but I'm not. Without my meals preprepared by dining halls and without skipping multiple classes every week, I cannot function. I need weekly IV fluids in a port because I only had two good veins to begin with (which are now occluded) in a condition for which IV fluids are a "hot topic" aka a highly contested treatment that in the end denies patients any sliver of quality of life. I have only been recently able to travel long distances in cars without having to bear ridiculous symptoms (as I finally was given a neck brace which helped my symptoms induced by motion). Honestly, I just feel so alone. I know people have a lot going on in their lives you can't always see, but the nature of my disability is the opposite of invisible. I guess I just want to hear from other severely disabled people. That our experiences are not so common. That our experiences are hard. That our experiences are unique. To be understood in the fact that severe physical disability is different from moderate or ambulatory physical disabilities. That not being able to hold yourself up and get out of bed is a different life from being in an active chair and able to master wheelies. I feel alone. My disability is hard. I am proud of it mostly because I am proud of who I am. I'm just struggling to feel in community with others who don't need so much support, who don't feel so removed from it all or feel so unseen.
4 notes
·
View notes
Text
does anyone who's had a longer mri have any advice for me ? I'm getting a full spine scan tomorrow and they said its gonna be about 2 hours and I'm really stressed lol
5 notes
·
View notes
Text
The Full Awareness of All Nerves
A Deep Dive into Our Body’s Silent CommunicatorsThe human body, a marvel of nature, operates with a complexity that often goes unnoticed. Central to this intricate system is the network of nerves, the silent communicators orchestrating our every move, thought, and sensation. Let’s embark on a journey to understand these fascinating elements of our biology.Firstly, consider the brain and spinal…

View On WordPress
#Brain Health#Consciousness#Human Anatomy#Kundalini Awakening#Nervous System#Neurons#neuroscience#Peripheral Nervous System#Sciatic Nerve#Sensory Perception#Spinal Cord
2 notes
·
View notes
Photo
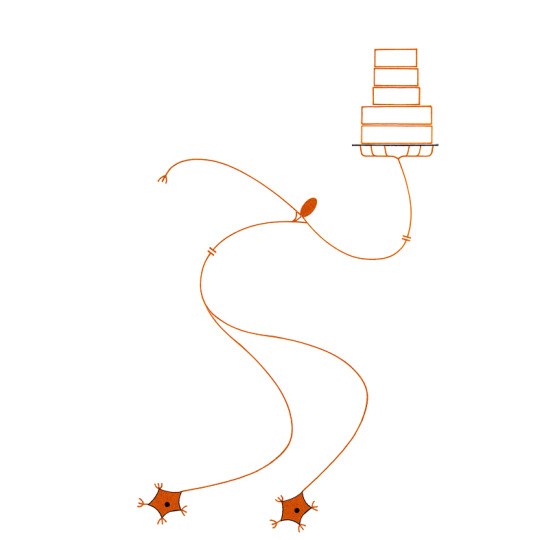
page 180 - my dream job, a roller skating server at a retro diner celebrating an america that has been lost or possibly never was. I was fired because I kept asking people what it was like to be trapped inside a collective national dream.
Also, missed a bunch of shifts, dropped hot fries and cold shakes on a congressman, etc.
That right there is a nice looking cake though. DAMN I love frosting.
#biology#biologist#zoology#zoologist#neurophysiology#dorsal root#dorsal#receptor#afferent#efferent#effector#gray matter#synapse#white matter#spinal cord#spinal#diagram of a simple reflex arc as postulated by early students of neurophysiology#service industry#service job#restaurant#restaurant life#roller skating#roller skates#cake#pastries#boulangerie#sweet treats#sweets
22 notes
·
View notes
Link
“All we know at this point is that bipedality evolved long before brain enlargement and tool use,” said paleoanthropologist Yohannes Haile-Selassie, the director of the Institute of Human Origins at Arizona State University who wasn’t involved in the latest study.
One of the distinctive features of the Toumaï skull is that the hole for its spinal cord is placed forward of similar holes in apes that didn’t walk upright, which suggests its skull was on top of its spine, rather than in front of it.
16 notes
·
View notes
Text
I need to see a spinal specialist. Feel as though my spine has gotten worse but I need a referral from the doctor. I can barely call them over the phone. idk how I am going to be able to go in and speak to someone. I'm very afraid
2 notes
·
View notes