#studyblr class of 2018
Text
Baie | she/they | unlabled | minor

— currently listening to: 'who we are' by hozier and 'hate to be lame' by lizzy mcalpine feat. finneas
— currently reading: 'hamlet' by william shakespeare

hello :> welcome to my little blog! I mainly just reblog things that i love or that resonate with me. Otherwise i’m talking about current reads or school. i’ve kinda got my foot in the door of studyblr and bookblr.
about me: australian!! 2006. lover of love and life. infj 4w5 unlabeled and very much in love <3. chaotic good! and recently chronically ill. rediscovering and building my faith :)
academia: graduating class of '23. atar + headstartx2, [english, general math, legal studies, psychology, chemistry] I love learning languages!! currently self-teaching japanese [mother tongue is english, small talk in japanese, very minimal french and korean]
the goal: double bachelor of criminology and criminal justice + psychological science

fandoms here!
they don't have huge relevance here, but i love them dearly <3
sherlock!! acd and bbc (currently consuming all other adaptations like a starving victorian child)
rwby!! yes my favourite character is qrow, moving on.
bts!! army since 2018, bias: v :)
bee and puppycat!! seriously in love
the osemanverse!! esp loveless, radio silence <3 and ofc heartstopper
arcane!! obsessed.
monster high!! grew up on gen1 :)
it's honestly kinda hard to think about these off the top of my head ngl
now books!!
favourites that have stuck with me :>
the midnight library by matt haig good 4 the soul
the inheritance games by jennifer lynn barnes NEWBOOKSOON!!
i fell in love with hope by lancali
song of achilles by madeline miller no elaboration needed.
embassy row series by ally carter reread too many times
hamlet by william shakespeare
sparrow by sarah moon beautiful

⇢ i love you whoever you are, and i'm always here for you :)
thank you for reading all this <3
Source: cafekitsune
#intro post#about me#chaotic academia#literature#i’ll add my personal tags here later <3#my tags ->#ineptias loquor#non necessaria notitia#that one type of green#poems and quotes#media#music#meme#aesthetic#places#chaotic academic#chronically ill#on life#on faith#on love#on death#on solitude#on humanity#on girlhood#on society#on identity#on permanence#dearest honeybee#<3#the little forest cryptid speaks
3 notes
·
View notes
Text
for my pinned; WIP
studyblr & bookblr
motivation & inspo reblogs
basics…
rae (she/her)
1999
non-traditional degree seeking*
*i have some general education credits from high school and from 2017/2018. since i’m going back to school not directly after graduating high school, that is what is called ‘non-traditional.’
pet(s): - dogo argentino (born 2022 December)
learning…
language(s): WIP
trait(s): sleeping earlier / waking up with a better attitude
hobby(-ies): hiking
lifestyle: better eating habits / stretching for five mins. each morning / skin care
mental health: gratitude / self-compassion / speaking positively to the universe and to myself
…
- study inspo - law & order - academia - misc. -
hobbies: - studying - thrifting - books - health/exercise - travel - ttrpgs
fandoms: - a24 films - law & order (svu, oc) - anna taylor-joy - d20
currently…
reading: - a touch of darkness by scarlett st. clair
favorite youtuber: - stephany andrea
going to school for: - police science tech AAS (summer 2025 & fall 2025)
dnd level/class/race: - level 13 bard (college of lore) fire genasi
songs:
- all too well (10 minute version) [taylor’s version] by taylor swift
- victoria’s secret by jax
1 note
·
View note
Text
studying in a haze; introduction.

hiii thereee!!!! it's me, studyinginahaze, here to make my debut onto tumblr!
somethings about me:
i'm 14
i am in 10th grade
classes:
AP world history
advanced algebra honors
chemistry honors
history/english accelerated
msl 4 chinese
hobbies/interests:
reading!! my favourite books are the night circus, the shadowhunter chronicles, any book by kerstin gier, and wuthering heights
i loveeeee to paint!
i like listening to taylor swift, lana del rey, marina, snow patrol, lorde (on occasion), and fall out boy
i play(ed) 3 instruments: violin, piano, flute!
why a studyblr?:
i've been watching studytube since 5th grade, 2018, and i only recently decided to become a studytuber as well! i also decided to be a part of studygram and studyblr because i loveeee talking about school and studying and reading! i love being motivated by others and inspired!
fav studyblrs:
@studyquill @minijournals @emmastudies @studyblr
thank you so much for reading! <3 have a good day :+)
#study hard#studyblr#study#study blog#inspo#notes#reading#studying#study motivation#school#student life#reach#lana del rey#taylor swift
1 note
·
View note
Text
How to get 5 on AP exam
1) become best friends with Khan academy (if they have your specific AP course)
2) spend around an hour studying that subject every. single. day.
3) take multiple practice exams (start at least 2 weeks ago)
4) take the actual AP exam and gET THAT MOTHAFUCHING 5!!!
#studyblr#studyblr tips#studyblr motivation#studyblr college admission#studyblr class of 2018#studyblr seniors#studyblr high school#studyblr ap#studyblr inspiration#studyquill
12 notes
·
View notes
Text
.
#just to keep in touch#not me being brought to tears at 930 in the ay em bc one of my fave professors emailed me#i haven't had a class with him since like 2018 but i just love him so much 🥺#not studyblr
1 note
·
View note
Text

27.12.18
Hey studyblr, it’s been a while! 👋🏽
Christmas has been a lot of fun this year - I cherish spending time with my family a lot more now that I’ve moved out for uni.
Getting back to the grind with some pure maths revision! 📚
#studyblr#mathblr#motivation#you can do it#study motivation#studying#studyspo#university#college#class of 2018#inspo#study inspiration#studyspiration#chakrastudies#stem#maths#computer science
462 notes
·
View notes
Text

That Thanksgiving work flow 📚💻📝
#thanksgiving#break#study#studyblr#bookblr#homework#college life#senior year#class of 2018#history#major#political#science#bloggerspeaks
19 notes
·
View notes
Text
AQA CHEMISTRY PAPER 1 (Higher Tier, Triple Science) - KEY POINTS FOR GCSE 2018
(Forgot to specify tier etc on the last post, sorry!)
So like I did for Biology Paper 1, here’s a list of things for Chemistry Paper 1 which I find a good idea to remember/tend to forget! I hope it’s useful!
How Fuel Cells work:

Moles = mass/Mr
Avogadro’s constant = 6.02 x 10^23
1 dm3 = 1000 cm3
Concentration in mol/dm3 = moles/volume
Concentration in g/dm3 = mass/volume
Remember to convert volume in cm3 to dm3 (divide by 1000!!) if it’s given in cm3
Moles of a gas = volume (dm3)/24
Periodic table development went: tiny spheres arranged by atomic weights (Dalton), triads where middle element was avg. mass (Dobereiner), period similarities noticed but ignored due to lack of symbols, law of octaves but left no space for discoveries (Newlands), elements placed by mass and properties with spaces (Mendeleev), order of atomic numbers after discovery of nucleus (Moseley)
The size of an atom is approximately 1 x 10^-10
The size of the nucleus is approximately 1 x 10^-14
If percentage yield is more than 100%, there may have been a greater mass of the reactant, or the sample was not dry
If the percentage yield is less than 100%, it may be because the reaction was incomplete, other unexpected reactions took place, or some of the product was lost
History of the atom goes as: Thomson discovered electrons so developed plum pudding model, Geiger & Marsden’s experiment showed a nucleus (because some of the alpha particles were deflected rather than going straight through meaning mass was concentrated in the centre) which had a positive charge, Bohr’s experiments showed electrons orbited at a distance or they would spiral inwards, Chadwick provided evidence of neutrons, later experiments then showed the positive charge could be split up into protons
Metallic bonding is a lattice is positive ions held by electrostatic attraction to negative delocalised electrons
Ionic bonding is strong electrostatic forces of attraction between non-metal negative ion and metal positive ion
Conduct when liquid as ions are not fixed and can therefore move around
Simple covalent structures have weak intermolecular forces between individual molecules
Fullerenes are good lubricants because they are round and therefore roll
Course particles are between 1 x 10^-5 m and 2.5 x 10^-6 m
Fine particles are between 1 x 10^-6 m and 2.5 x 10^-7 m
One nanometre is 0.000000001 (billionth) of a metre as is written as 1 x 10^-9 m
Nanoparticles have a diameter between 1nm and 100nm
Boiling points of noble gases increase as you go down the group
Alkali metals have lower melting/boiling points, are softer, less dense, and are weaker than transition metals
A more reactive halogen will displace a less reactive halogen from a solution of its salt
This is the reactivity series:

A more reactive metal will displace a less reactive metal from a solution of its salt
The easier it is for a metal to lose an electron, the more reactive it is
In the electrolysis of a molten compound, metals go form at the cathode and non-metals form/are released at the anode
In the electrolysis of an aqueous solution, hydrogen is released from the cathode if the metal is MORE reactive than it, and oxygen is released from the anode UNLESS the solution contains halide ions (in which case the halide ions are released as halogens)
For bonds to be broken, reacting particles must collide with sufficient energy
This is what Energy profile diagrams look like (remember the products of an exothermic reaction will have LESS energy):
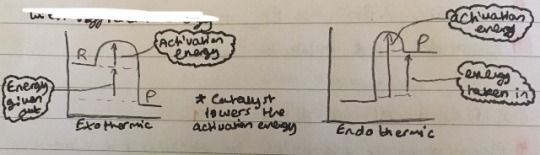
Overall energy change = bonds broken - bonds made
A negative energy change is exothermic because the products had MORE energy, as it was released
Bases are insoluble alkalis and can include metal carbonates, metal oxides, and metal hydroxides
Filtration is used to separate a soluble solid from an insoluble solid
Crystallisation is used to obtain a soluble solid from a solution
Simple distillation is used to obtain a solvent from a solution
Fractional distillation is used to separate mixtures in which the components have different boiling points
Chromatography is used to separate the different soluble, coloured compounds in a mixture
GOOD LUCK ON YOUR EXAM TOMORROW EVERYONE!! Get plenty of sleep, especially since it’s an AM exam!
548 notes
·
View notes
Text
January 26, 2019 | I just realized that I kept writing the dates wrong and no one told me 🙃 I kept writing 2018 instead of 2019, good job to me ahaha 😅 so today I’m trying to get this simulation done and I can’t seem to find an error.. so that’s going well. And yes I still have a christmas mug even if it’s the end of january. Btw don’t worry, the informations showed is not real, it’s just for practice purposes 😊📚
#studyblr#studying#inspiration#french canadian#french student#studyspo#frenchstudyblr#school#studymotivator#cute#accountingblr#accounting#class#apple#macbookpro#acomba#christmasmug#2019#2019 not 2018#simulation#help
5 notes
·
View notes
Text


12.01.2018.
48/100 days of productivity
Came home late yesterday, so here's what I should have post. 😀
wrote a handwritten letter to one of my best friends for her birthday
read Mrs. Dalloway
went to art class
December is here you guys! These are some of my bujo spreads for it. Last month of 2018, crazy!!
"I want everything - love, adventure, intimacy, work." Virginia Woolf
#movie magus#studyblr#film student#student#university#productivity#100 days of productivity#art class#mrs dalloway#virginia woolf#literature#bujo#english literature#bullet journal#bujo spread#december#last month of 2018#habit tracker#virginia woolf quote#study#reading#planning#read#book#plans#writing#letter#handwritten#friends
11 notes
·
View notes
Photo


a little peak into my planner for the next school year⭐️🌼🌻
#class of 2018#biochemistry#planner#agenda#calendar#studyblr#college freshman#freshman tips#studyblr off school#studyblr community#study life#student things#study motivation
85 notes
·
View notes
Photo

I can’t even believe it yet!
The past posts were often abt me “being back” - which I failed to be, because I had so much stress with my graduation exams.
But yesterday at 12:45 I got my gratduation results.
And I did it! I graduated with a GPA of 1.5 (the best being 1.0), being on honor’s roll, holding the valedictorian speech for my year and 60/60 points in my written English exam! Alles Gute, Absolvia 2018!
How my graduation exams worked
- Two oral exams (Colloquium; for me: Biology and Ethics)
- Three written exams (For me: English, Maths and German)
- Exams started on May 2nd and ended on June 12th
- You get to choose your third written exam (English) and both of your oral exams (Biology, Ethics)
- The written exams usually last for three to five hours, I took tons of food, a pillow and a polaroid of me and my mom with me, but I also saw people taking blankets with them lmao
- The oral exams are made up of 30min for working with the questions and the material they give you in an envelope, then you hold a 10min presentation about what you found as an answer for the questions and the following 20min are filled with questions about your presentation and two out of four semestres of the past two years
What posts you can expect now that I’m free!
- How I wrote my graduation speech
- How I chose my university and my majors/minors
- How I studied for graduation
- What to do with your free time once you leave class
CONGRATS TO ALL MY FELLOW GRADUATES OF THE CLASS OF 2018 ♥ WE DID IT!
#studying#studyblr#Abitur#studyspo#german studyblr#platosnotes#meins#school#gymnasium#class of 2018#absolvia 2018
50 notes
·
View notes
Text
Fall 2018
Here are my classes for the upcoming semester:
1) Astronomy 1
2) Marketing Research
3) Consumer Behavior
4) Business Law
5) Management Information Systems
Here are my goals:
1) Get a 4.0
2) Get some experience
3) Get some more business contacts
I hope everyone has a lovely semester!! :D
16 notes
·
View notes
Text
Hey friends,
Sorry for being so absent lately! Vivi and I are currently going through our final exams, so we didn't really had the time to run this little blog actively. We are both finally finishing school after 12 long years, which is both exciting and terrifying (some of you guys can prob relate to that) and hopefully we can be a little bit more active in the future.
Besides that, we both are hoping to start university this year and study psychology. If somebody has any tips or tricks for starting university in this particular field, please message us. We would truly appreciate it :)

#psychology#university#college#vienna#class of 2018#school#studyblr#studyinspo#inspiration#study inspiration#study motivation#studyspo#inspo#Berlin#notebook#bullet journal
63 notes
·
View notes
Photo


only took 20 minutes to do November spread because i’m tired of doing things the same way and getting the same unsatisfactory result.
#studyblr#studyblr bujo#studyblr bullet journal#studyblr motivation#studyblr inspiration#studyblr class of 2018#my post
4 notes
·
View notes
Photo
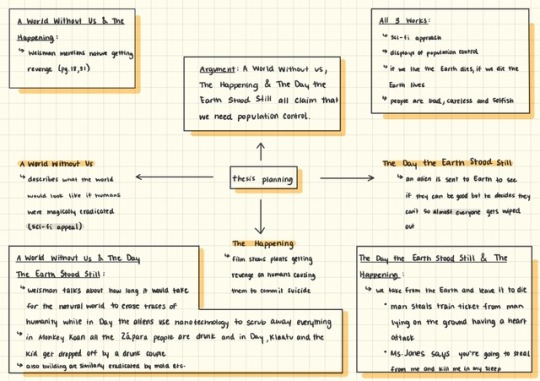
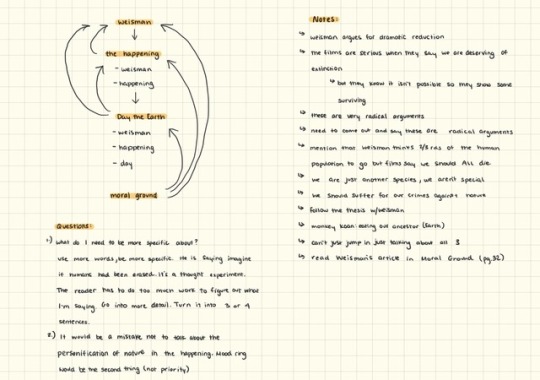
are you any of you guys taking a summer class? If so, what class is it? Are you enjoying it? . . Essay planning. I got a synthesis essay due this Sunday and it’ll be my last essay. Yippee! After that I will be finished with my last summer college English class. Whoop whoop! Then my summer fun will officially begin.
#studyinganna#studyblr#studyspo#English#essay planning#college essay#college student#collegeblr#synthesis paper#syhtesis#writing a paper#summer class#notes#iPad#digital notes#GoodNotes#goodnotes app#student iPad#iPad 2018#mind map#mindmap#mind mapping#thesis#moral ground#the happening#the day the earth stood still#a world without us#environmentalism#environmentalist
19 notes
·
View notes