Text

Research progress in thermal expansion characteristics of TATB-based polymer bonded explosives
Under complex temperature variations, the irreversible thermal expansion of polymer-bonded explosives (PBXs) containing 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) leads to diminished shape stability. This, in turn, directly impacts the mechanical properties and safety performance during storage and use. In recent years, extensive and thorough research has been conducted to investigate the thermal expansion characteristics of TATB-based explosives.
In a study published in Energetic Materials Frontiers, a group of researchers from China explored the distinctive crystal structure of TAT and the thermal expansion mechanism of TATB-based PBXs. Additionally, they summarized the microstructural evolution during the thermal expansion process and analyzed the consequential effects of thermal expansion on the overall performance of these explosives.
"More attention was paid to the influencing factors of thermal expansion and control methods. Evidently, designing a new structure of negative thermal expansion binding system, through the design of negative thermal expansion polymers or fillers and positive expansion TATB crystals, can reduce the linear expansion coefficient of PBXs," explained the study's lead author, Cong-mei Lin. "This approach not only suppresses material thermal expansion but also holds broad application prospects."
Read more.
8 notes
·
View notes
Text
as a child being told "the moon controls the tides" with no additional explanation was like. oh okay. you want me to believe in magic? you're talking about magic right now? okay. fine
128K notes
·
View notes
Text
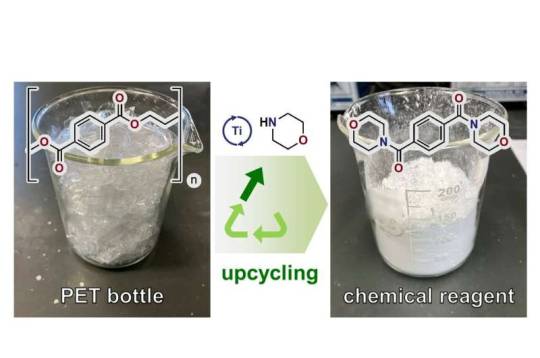
Scientists upcycle polyesters through new waste-free, scalable process
Researchers from Tokyo Metropolitan University have developed a new chemical process which upcycles polyesters, including PET in plastic bottles, to morpholine amide, a versatile and valuable building block for synthesizing a vast range of compounds. The reaction is high yield, waste-free, does not require harmful chemicals, and is easily scalable. The team have successfully broken the, often costly, closed-loop recycling loop of plastic waste, allowing upcycling to more valuable products.
Recycling plays an indispensable part of our fight against plastic waste. But at what cost? The recycling of polyesters, for example, including polyethylene terephthalate (PET) in plastic bottles, often requires power to get the required chemical reactions hot enough, or strongly alkaline conditions which generate chemical waste. At the end of it all, we get intermediate compounds which are used to make the same products they came from. Not only can this be wasteful, it can also be economically unviable.
This is where upcycling comes in. Scientists have been working to break this closed loop and create compounds from plastic waste which are more valuable and useful for society. An open-loop scheme like this is a vital part of practical strategies to help us transition to a greener society.
Read more.
74 notes
·
View notes
Video
youtube
Gallium: Did you know?
Gallium has the ability to diffuse into the lattice of other metals, such as aluminum-zinc alloys or steel, and turn the once ductile material very brittle. In the video above, NurdRage on YouTube coats a sheet of aluminum with gallium, greatly weakening the aluminum sheet.
43 notes
·
View notes
Text
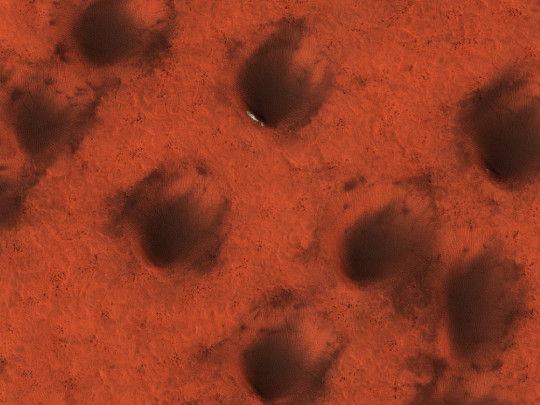
HiPOD 18 Oct 2023: Late Summer on Northern Dunes
Northern dunes are made of dark basaltic sand, causing them to stand out against the rougher redder surface. No seasonal carbon dioxide ice remains other than the few bright cold spots where the ice (which may be water ice) has been protected. (Enhanced color cutout is less than 1 km across.)
ID: ESP_079927_2550
date: 15 August 2023
altitude: 318 km
NASA/JPL-Caltech/UArizona
45 notes
·
View notes
Note
What’s your thoughts on sexual relations with robots?
i turned this computer on like 5 minutes ago can i have a brief moment of peace before i have to read these things please
60K notes
·
View notes
Text
me every time i overcome something i thought i wouldnt:
17K notes
·
View notes
Photo


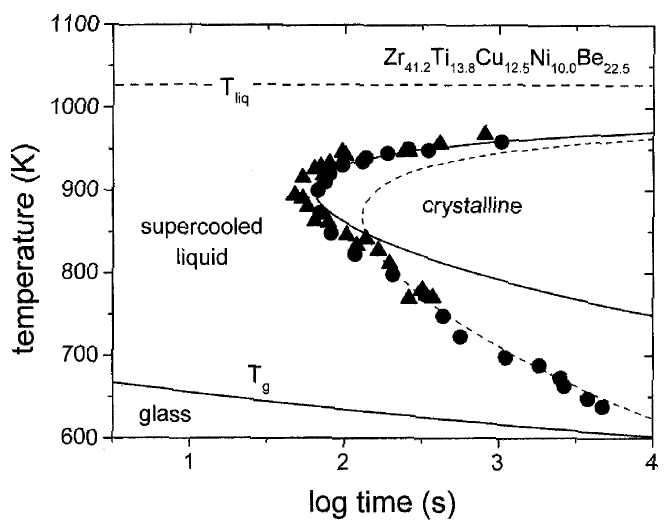


Amorphous metal, metallic glass
In nature they prefer to form crystal structures but if liquid metals are cooled down fast enough then, just like glass, the atoms will arrange into a disordered amorphous structure. These amorphous metals, also called metallic glasses, don’t exist naturally because the cooling rates required can be on the order of millions of degrees a second. Methods of forming these materials include extremely rapid cooling, physical vapor deposition, solid-state reaction, ion irradiation, and mechanical alloying.
Metallic glasses are materials of interest because of the properties that can result. They typically have extremely high strength to weight ratios (higher than aluminium and titanium alloys) and are tougher and less brittle than oxide glasses and ceramics. Like crystalline metallic alloys, the properties of these materials also depends upon the composition. Alloys of boron, silicon, phosphorus, and other glass formers with magnetic metals have high magnetic susceptibility and electrical resistance. The fact that amorphous metals are true glasses also allows for easy processing because they soften and flow upon heating. Not all properties are favorable however, metallic glasses also typically have lower ductilities and fatigue strengths.
Bulk metallic glasses, or BMGs, are alloys with critical cooling rates low enough to allow formation of the desired amorphous structure in thick layers (over 1 millimeter). They are made from alloys with typically three to five metallic components that have a large atomic-size mismatch and a composition close to a deep eutectic. Some examples include Vitreloy 1 (41.2% Zr, 13.8% Ti, 12.5% Cu, 10% Ni, and 22.5% Be), Ti40Cu36Pd14Zr10, and Mg60Zn35Ca5.
Sources: (1, top left) (2, top right) (3, middle left) (4, middle right) (5, bottom)
80 notes
·
View notes
Text
Elemental Naming: Germanium
In 1869, germanium was yet another element to be predicted by Russian chemist Dmitri Mendeleev. The element was formally discovered in 1886 by Clemens Winkler, who isolated it from a mineral called argyrodite and named the new element from the latin Germania, after his homeland Germany.
Source.
20 notes
·
View notes
Text
reject booktok culture. go to the library and get a weird little novel you’ve never heard of in your life and read it all in 2 days like god intended.
98K notes
·
View notes
Text
Please do not attempt to Burden me with Tasks while I am observing my most Sacred Holiday
The Month of Halloween
28 notes
·
View notes