#Dave Kwakman
Text
Wonderkid Factory | Part 1 | Turning Milk Into Cheese
A brand new #FM24 story kicks off today as we launch our 2nd #WonderkidFactory adventure. This time we're heading to the Netherlands to take control of the potential-ridden squad at #AZAlkmaar. We meet the squad and explain the challenge.
Read here:
Having completed my Football Manager 2024 Pentagon Pursuit adventure far quicker than anticipated, I struggled for a while to find a new save that would bring us something a little bit different. So I went back to my own 24 Teams to Manage on FM24 guide (shameless plug) and took my own medicine to select an exciting new challenge.
Inspired by our Wonderkid Factory adventure with Envigado FC on…
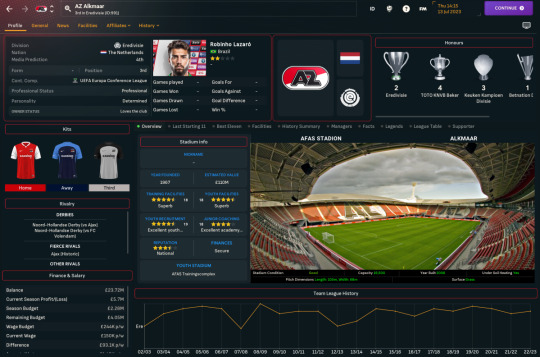
View On WordPress
#Alexandre Penetra#Alkmaar Zaanstreek#AZ#AZ Alkmaar#Cheese Farmers#Dave Kwakman#De Kaasboeren#Denso Kasius#Elijah Dijkstra#Enoch Mastoras#Eredivisie#Ernest Poku#Europa Conference League#Europa League#FM24#Football Manager#Football Manager 2024#Football Manager 24#Jasper Hartog#Jayden Addai#Jens Odgaard#Jordy Clasie#Julian Oerip#Kaaskopppen#Kees Smit#Kiyani Zeggen#Kristjan Beljic#Lewis Schouten#Mathew Ryan#Maxim Dekker
1 note
·
View note
Text
AZ wint in Youth League van Atletico Madrid
De jonkies van AZ hebben vandaag indruk gemaakt in de Youth League door de leeftijdsgenoten van Atlético Madrid met 1-0 te verslaan. In Wijdewormer wist het elftal van trainer Michael van Zijtveld zich wederom te plaatsen voor de achtste finales.
Nieuw team, enkele “ervaren” sterren
Na het winnen van de Youth League vorig seizoen, trad dit keer een grotendeels nieuwe generatie talenten van AZ aan…

View On WordPress
0 notes
Text
How honey kills bacteria
The FASEB Journal
•
Research Communication
How honey kills bacteria
Paulus H. S. Kwakman,* Anje A. te Velde,† Leonie de Boer,* Dave Speijer,‡ Christina M. J. E. Vandenbroucke-Grauls,*,§ and Sebastian A. J. Zaat*,1
*Department of Medical Microbiology, Center for Infection and Immunity Amsterdam, †Laboratory of Experimental Gastroenterology and Hepatology, and ‡Department of Medical Biochemistry, Academic Medical Center, University of Amsterdam, Amsterdam; and §Department of Medical Microbiology and Infectious Diseases, Vrije Universiteit Medical Center, Amsterdam,
The Netherlands
ABSTRACT With the rise in prevalence of antibiotic- resistant bacteria,
honey
is increasingly valued for its antibacterial activity. To characterize all bactericidal factors in a medical-grade honey, we used a novel approach of successive neutralization of individual honey bactericidal factors. All bacteria tested, including
Bacillus subtilis
, methicillin-resistant
Staphylococcus au-
reus
, extended-spectrum J3-lactamase producing
Esche-
richia coli
, ciprofloxacin-resistant
Pseudomonas aerugi-
nosa
, and vancomycin-resistant
Enterococcus faecium
, were killed by 10 –20% (v/v) honey, whereas >40% (v/v) of a honey-equivalent sugar solution was required for similar activity. Honey accumulated up to 5.62 ±
0.54 mM H2O2 and contained 0.25 ± 0.01 mM methyl-
glyoxal (MGO). After enzymatic neutralization of these two compounds, honey retained substantial activity. Using
B. subtilis
for activity-guided isolation of the additional antimicrobial factors, we discovered bee defensin-1 in honey. After combined neutralization of H
2
O
2
, MGO, and bee defensin-1, 20% honey had only minimal activity left, and subsequent adjustment of the pH of this honey from 3.3 to 7.0 reduced the activity to that of sugar alone. Activity against all other bacteria tested depended on sugar, H
2
O
2
, MGO, and bee defensin-1. Thus, we fully characterized the antibacterial activity of medical-grade honey.—Kwak- man, P. H. S., te Velde, A. A., de Boer, L., Speijer, D., Vandenbroucke-Grauls, C. M. J. E., Zaat, S. A. J. How honey kills bacteria.
FASEB J.
24, 2576 –2582 (2010).
www.fasebj.org
Key Words: antibacterial agents · drug resistance · isolation and purification · methicillin-resistant Staphylococcus aureus
· peptides
Honey has been renowned for its wound-healing properties since ancient times (1). At least part of its positive influence is attributed to antibacterial proper- ties (2, 3). With the advent of antibiotics, clinical application of honey was abandoned in modern West-
The potent in vitro activity of honey against antibiotic- resistant bacteria (6, 7) and its successful application in treatment of chronic wound infections not re- sponding to antibiotic therapy (3) have attracted considerable attention (8 –10).
The broad spectrum antibacterial activity of honey is multifactorial in nature. Hydrogen peroxide and high osmolarity— honey consists of ~80% (w/v) of sugars— are the only well-characterized antibacterial factors in
honey (11). Recently, high concentrations of the anti- bacterial compound methylglyoxal (MGO) were found specifically in Manuka honey, derived from the Manuka tree (Leptospermum scoparium) (12, 13). Until now, no honey has ever been fully characterized, which ham- pers clinical application of honey.
Recently, we determined that Revamil medical-grade honey, produced under standardized conditions in greenhouses, has potent, reproducible bactericidal ac- tivity (14). In the current study, we identified all bactericidal factors in the honey used as source for this product and assessed their contribution to honey bac- tericidal activity.
To accomplish this, we used a novel approach of successive neutralization of individual honey bacteri- cidal factors combined with activity-guided identifica- tion of unknown factors.
MATERIALS AND METHODS
Honey
Unprocessed Revamil source (RS) honey was kindly provided by Bfactory Health Products (Rhenen, The Netherlands). RS honey has a density of 1.4 kg/L and contains 333 g/kg glucose, 385 g/kg fructose, 73 g/kg sucrose, and 62 g/kg maltose. To study the contribution of the sugars to the bactericidal activity of honey, a solution with a sugar compo- sition identical to that of the honey was prepared.
ern medicine, although in many cultures, it is still used
(4). These days, however, abundant use of antibiotics has resulted in widespread resistance. With the devel- opment of novel antibiotics lagging behind (5), alter- native antimicrobial strategies are urgently needed.
1 Correspondence: Department of Medical Microbiology, Academic Medical Center, Meibergdreef 15, 1105 AZ Amster- dam, The Netherlands, E-mail: [email protected]
doi: 10.1096/fj.09-150789
Microorganisms
Bactericidal activity of honey was assessed against the labora- tory strains Bacillus subtilis ATCC6633, Staphylococcus aureus 42D, Escherichia coli ML-35p (15), and Pseudomonas aeruginosa PAO-1 (ATCC 15692), and against clinical isolates of methi-
cillin-resistant S. aureus (MRSA), vancomycin-resistant Entero- coccus faecium (VREF), extended-spectrum [3-lactamase-pro- ducing E. coli (E. coli ESBL) and ciprofloxacin-resistant
P. aeruginosa (CRPA).
aliquots of undiluted and 10-fold serially diluted incubations were plated on blood agar. Bacterial survival was quantified after overnight incubation at 37°C. The detection level of this assay is 100 CFU/ml.
To assess the contribution of H2O2 to the bactericidal activity of honey, bovine liver catalase (Sigma) was added to a final concentration of 600 U/ml. A catalase stock solution was prepared according to the manufacturers’ instructions in 50 mM phosphate buffer (pH 7.0). The addition of 0.25% (v/v) of this catalase stock solution reduced the amount of H2O2 to undetectable levels at all honey concentrations tested and did
Determination of H O
concentration in honey
not affect bacterial viability.
2 2 Sodium polyanetholsulfonate (SPS) (Sigma) was added to neutralize cationic bactericidal components (19) at a final
Hydrogen peroxide concentrations in honey were deter-
mined quantitatively using a modification of a method de- scribed previously (16). Undiluted and 10-fold diluted sam-
ples of honey (40 µl) were mixed in wells of microtiter plates with 135 µl reagent, consisting of 50 µg/ml O-dianisidine (Sigma, St. Louis, MO, USA) and 20 µg/ml horseradish peroxidase type IV (Sigma) in 10 mM phosphate buffer (pH
6.5). O-dianisidine and peroxidase solutions were freshly prepared from a 1 mg/ml stock in demineralized water and from a 10 mg/ml stock in 10 mM phosphate buffer (pH 6.5), respectively. After 5-min incubations at room temperature,
reactions were stopped by addition of 120 µl6MH SO , and
concentration of 0.025% (w/v). The incubation buffer did not affect the pH of the concentrations of honey used in our experiments.A1M NaOH solution was used to titrate honey solutions to pH 7.0.
Agar diffusion assay
To assess antibacterial activity of fractionated honey, an agar diffusion assay was used (20). In brief, a B. subtilis inoculum suspension was prepared as described for the liquid bacteri- cidal assay. Bacteria (107 CFU) were mixed with 20 ml
2 4 nutrient-poor agar [0.03% (w/v) TSB in 10 mM sodium
absorption at 540 nm was measured. Hydrogen peroxide concentrations were calculated using a calibration curve of 2-fold serial dilutions of H2O2 ranging from 2200 to 2.1 µM.
MGO neutralization assay
Reduced glutathione (Sigma) was added to diluted honey to a final concentration of 15 mM, and conversion of MGO to S-d-lactoyl-glutathione (SLG) was initiated by addition of 0.5 U/ml glyoxalase I (Sigma). The amount of MGO converted was determined using the extinction coefficient of SLG of
3.37 mM-1 at 240 nm (17). Thus, we determined that up to 10 mM of exogenous MGO added to 40% honey was com-
pletely converted, and that undiluted RS honey contained
0.25 0.01 mM of MGO.
Antibee defensin-1 polyclonal antibody
An affinity-purified polyclonal antibee defensin-1 antibody was purchased from Eurogentec (Seraing, Belgium). The N-terminal part of bee defensin-1 is hydrophobic and con- tains 3 disulfide bonds, whereas the hydrophilic C-terminal region lacks cysteine residues (18). Therefore, rabbits were immunized with a synthetic peptide corresponding to the C terminus of bee defensin-1 (CRKTSFKDLWDKRF), and anti- bodies were subsequently affinity-purified using this peptide coupled to AF-Amino Toyopearl 650 M resin (Toso, Tokyo, Japan).
Liquid bactericidal assay
Bactericidal activity of honey was quantified in 100-µl volume liquid tests, in polypropylene microtiter plates (Costar Corn- ing, New York, NY, USA). For each experiment, a 50% (v/v)
stock solution of honey was freshly prepared in incubation buffer containing 10 mM phosphate buffer (pH 7.0) supple- mented with 0.03% (w/v) trypticase soy broth (TSB; BD Difco, Detroit, MI, USA). Bacteria from logarithmic phase cultures in TSB were washed twice with incubation buffer and suspended at a final concentration of 1 X 106 CFU/ml, based
on optical density. Plates were incubated at 37°C on a rotary
shaker at 150 rpm. At indicated time points, duplicate 10-µl
phosphate buffer (pH 7.0) with 1% low EEO agarose (Sigma)] of 45°C, and immediately poured into 10- X 10-cm culture plates. Wells of 1 mm diameter were punched into the agarose, and 2.5-µl samples were added to the wells and allowed to diffuse into the agarose for 3 h at 37°C. Subse- quently, the agarose was overlaid with 20 ml of double- strength nutrient agarose [6% TSB and 1% Bacto-agar (BD Difco), 45°C], and plates were incubated overnight at 37°C. Clear zones around the wells indicated antibacterial activity.
Ultrafiltration of honey components
Fifteen milliliters of 20% honey was centrifuged in a 5-kDa molecular weight cutoff Amicon Ultra-15 tube (Millipore, Bedford, MA, USA) at 4000 g for 45 min at room tempera- ture. The <5-kDa filtrate was collected, and the >5-kDa reten- tate was subsequently washed 3 times in the filter tube with 15 ml of demineralized water and concentrated to 0.4 ml.
Bacterial overlay assay
Native cationic proteins were separated by acid urea polyacryl- amide gel electrophoresis (AU-PAGE) (21). Gels were either stained with PAGE-Blue (Fermentas, St. Leon-Rot, Germany) or washed 3 X 8 min in 10 mM phosphate buffer (pH 7.0) for a bacterial overlay assay. After washing, the gel was incubated for 3 h on B. subtilis-inoculated nutrient-poor agarose (see
Agar Diffusion Assay). After removal of the gel, the agarose was overlaid with double-strength nutrient agarose and treated as described for the agar diffusion assay.
Immunoblotting
Proteins were separated by tris-tricine SDS-PAGE, as de- scribed previously (22), and transferred onto nitrocellulose membranes (Schleicher and Schuell, Keene, NH, USA). Membranes were subsequently blocked with 5% nonfat dry milk (Bio-Rad, Veenendaal, The Netherlands) plus 0.5 M NaCl and 0.5% (v/v) Tween-20 in 10 mM Tris-HCl, pH 7.5 (rinse buffer), for 1 h. Blocked membranes were incubated with affinity-purified antibee defensin-1 antibody at 1.4
µg/ml in rinse buffer for 2 h. After incubation with primary antibody, membranes were washed 2X for 15 min in rinse buffer, incubated with horseradish peroxidase-labeled goat-
anti-rabbit secondary antibody (Jackson ImmunoResearch West Grove, PA, USA) at 0.4 µg/ml in rinse buffer for 1 h, and washed again for 10 min. in rinse buffer and 5 min in PBS, respectively. The membrane was developed using a DAB liquid substrate kit (Sigma).
Purification of antibacterial peptide from honey
An amount of >5-kDa honey retentate equivalent to 13 ml of honey was dissolved in loading buffer (3M urea in 5% acetic acid with methyl green as tracking dye) and loaded on a preparative acid-urea PAGE, as described previously (21) with
slight modifications. A cylindrical gel (3.7 cm diameter, 6 cm height) in a model 491 Prep Cell (Bio-Rad) was prepared, prerun at reversed polarity for3h at 150V in 5% acetic acid at 4°C, and protein was electrophoresed at 40 mA with reversed polarity. Protein was eluted in 5% acetic acid at 0.5 ml/min and collected in fractions of 2 ml. Fractions were assessed for protein composition by tris-tricine SDS-PAGE and for antibacterial activity by bacterial overlay assay. Frac- tions containing purified antibacterial protein were pooled, concentrated, dialyzed against 0.01% acetic acid in a 3.5-kDa molecular weight cutoff MINI Slide-A-Lyzer tube (Pierce, Rockford, IL, USA), freeze-dried, and dissolved in deminer- alized water.
Protein identification by V8 digestion with subsequent mass analysis
Duplicate fractions (estimated to contain ~2 µg of protein each) were adjusted to 50 mM sodium phosphate (pH 7.9)
and 5% (v/v) acetonitrile. Approximately 0.5 µg of endopro- teinase Glu-C (Fluka) was added per fraction and incubated at 25°C overnight. The resulting peptide mixtures were purified and concentrated with the aid of C18 ziptips (Milli- pore) and eluted in 10 µl 90% (v/v) acetonitrile and 1% (v/v) formic acid. The samples were checked for the presence of nonautodigest peptides with a reflectron MALDI-TOF mass spectrometer (MALDI; Waters, Milford, MA, USA). Next,
samples were analyzed with ESI-tandem mass spectrometry (MS/MS). Data were acquired with a QT of 1 (Waters) coupled to an Ultimate nano-LC system (LC Packings Di- onex, Sunnyvale, CA, USA). One microliter of peptide mix- ture was diluted in 10 µl of 0.1% TFA. The peptides of both
samples were separated on a nanoanalytical column (75 µm
i.d. X 15 cm C18 PepMap; LC Packings Dionex) using a standard gradient of acetonitrile in 0.1% formic acid. The
flow of 300 nl/min was directly electrosprayed in the QT of 1 operating in data-dependent MS and MS/MS mode. The resulting MS/MS spectra were analyzed with Mascot software (Matrix Science, Boston, MA, USA). In both fractions, a doubly charged ion (VTCDLLSFKGQVND, mass 1537.8) with a sequence corresponding to the mature N terminus of bee defensin-1 could be identified (MOWSE scores >73).
RESULTS
Hydrogen peroxide is produced by the Apis mellifera (honeybee) glucose oxidase enzyme on dilution of honey. RS honey diluted to 40 to 20% accumulated high levels of H2O2 24 h after dilution, with a maximum of
5.62 0.54 mM H2O2 formed in 30% honey (Fig. 1A). The addition of catalase reduced H2O2 to negligible
Figure 1. Contribution of H2O2, sugars, and MGO to the bactericidal activity of honey after 24 h. A) Mean se hydrogen peroxide accumulation in different concentrations of honey, without catalase (squares) or with catalase added (asterisks).
B) Bactericidal activity against indicated laboratory strains (top row) and against clinical isolates of vancomycin-resistant
E. faecium (VREF), methicillin-resistant S. aureus (MRSA), extended-spectrum [3-lactamase-producing E. coli (E. coli ESBL), and ciprofloxacin-resistant P. aeruginosa (CRPA) (bottom row). Bacteria were exposed to various concentrations of honey (squares), honey with catalase added (asterisks), or to honey-equivalent sugar solutions (circles). C) Killing of B. subtilis by honey in incubation buffer without addition (squares), with catalase (asterisk), with glyoxalase (small solid circles), or with catalase and glyoxalase I (inverted triangles), added to neutralize H2O2 and MGO, respectively, or by a honey-equivalent sugar solution
(circles). Data are mean se log-transformed bacterial concentration (CFU/ml).
levels (Fig. 1A) and markedly reduced the bactericidal activity against all bacteria tested, except B. subtilis (Fig. 1B). However, H2O2-neutralized honey exerted stron- ger bactericidal activity than equivalent sugar solutions (Fig. 1B). This indicates that H2O2 is important for the bactericidal activity of honey, but that additional factors must also be present. As B. subtilis was the most susceptible bacterium for nonperoxide bactericidal activity, we used it for identification of additional bactericidal factors.
The honey bactericidal compound MGO can be converted into S-lactoylglutathione (SLG) by glyoxalase I, and this product can be measured spectrophoto- metrically. RS honey contained 0.25 0.01 mM MGO. We aimed to apply glyoxalase I to neutralize the bactericidal activity of MGO in honey. This required that SLG, the reaction product of MGO, would be nonbactericidal. Indeed, the activity of up to 20 mM MGO was neutralized by conversion into SLG (Supple- mental Fig. 1), indicating that SLG up to high concen- trations did not kill the bacteria. Neutralization of MGO or H2O2 alone did not alter bactericidal activity of RS honey, but simultaneous neutralization of MGO and H2O2 in 10% honey reduced the killing of B. subtilis by 4-logs (Fig. 1C). At higher concentrations of honey, the bactericidal activity was not affected by neutralization of H2O2 and MGO (Fig. 1C), indicating that still more factors were involved.
As a first step to characterize the unknown bacteri- cidal factors, we size-fractionated honey by ultrafiltra- tion with a 5-kDa molecular weight cutoff membrane. Unfractionated honey produced a small zone of com- plete bacterial growth inhibition and a larger zone with partial growth inhibition in an agar diffusion assay with
B. subtilis (Fig. 2A). After ultrafiltration, the factors that caused complete and partial bacterial growth inhibition were separated and were present in the >5-kDa reten-
tate and the <5-kDa filtrate, respectively (Fig. 2A).
Ion exchange chromatography of the retentate indi-
cated a cationic nature of the antibacterial factors. Indeed, the polyanionic compound SPS abolished the antibacterial activity of the retentate (Fig. 2B). More- over, pepsin treatment also abolished this activity (Fig. 2B). Together, this implies that cationic antibacterial proteins were present.
We separated cationic proteins in the retentate using a native acid-urea PAGE gel, and allowed the separated components to diffuse from this gel into a B. subtilis- inoculated agar to identify antibacterial proteins. This yielded a single zone of bacterial growth inhibition that corresponded to a protein band in a Coomassie-stained gel run in parallel (Fig. 2C). This protein was purified from a larger amount of retentate using preparative acid-urea PAGE (Fig. 2D), and identified by peptide mass analysis as bee defensin-1.
To specifically assess the contribution of bee defen- sin-1 to the bactericidal activity of honey, an antibee defensin-1 antibody was raised (Fig. 2E). Like SPS, this antibody negated all bactericidal activity of the >5-kDa
retentate against B. subtilis (Fig. 3A). The <5-kDa
filtrate had only minor bactericidal activity (Fig. 3A), but this was not due to cationic compounds, since SPS
failed to neutralize this activity (Fig. 3A). Thus, bee defensin-1 was the only cationic bactericidal compound present in RS honey.
Next, we assessed the contribution of bee defensin-1 to the bactericidal activity of nonfractionated honey
Figure 2. Identification of bee defensin-1 in honey. A) Honey was fractionated by ultrafiltration using a 5-kDa molecular weight cutoff filter tube; antibacterial activity of 2.5 µl of 80% honey, and equivalent amounts of the <5-kDa filtrate and >5-kDa retentate, were tested in an agar diffusion assay. B) Retentate equivalent to 7.5 µl of undiluted honey was tested for the presence of cationic and proteinaceous antibacterial components. Activity of cationic components was neutralized by
adding SPS, and protein was digested with pepsin, followed by 5-min inactivation at 100°C. As control, incubation for 5 min at 100°C without pepsin was performed. Activity in retentate (ret.)
was compared with that of 0.2 µg hen egg white lysozyme (lys.). C) To identify cationic antibacterial proteins in retentate, amounts of this fraction equivalent to 750 µl honey, and 3 µg lysozyme as a reference, were run in duplicate sets on a single native acid-urea PAGE gel. One half
of the gel was Coomassie-stained (left); other was used for a bacterial overlay assay with B. subtilis
(right). D) Silverstained tris-tricine SDS-PAGE of different amounts of lysozyme and preparative acid-urea PAGE-purified bee defensin-1, separated by an empty lane. E) Retentate separated on tris-tricine SDS-PAGE, blotted to nitrocellulose, stained with either Ponceau S (Pon. S, left) or immunostained with antibee defensin-1 (right).
Figure 3. Roles of bee defensin-1 and pH in bactericidal activity of honey against B. subtilis. A) Contribution to bacte- ricidal activity of cationic components in general and of bee defensin-1 specifically was tested by neutralization with SPS or with antibee defensin-1 antibody (C-bd), respectively, at con- centrations of retentate equivalent to 20% honey (open bars) and 40% honey (solid bars); ctrl. indicates survival without
neutralization. B) To assess the contribution of bee defen- sin-1 to bactericidal activity of unfractionated honey, B. subtilis was incubated in various concentrations of honey in incuba- tion buffer (squares), or with catalase and glyoxalase I added either without (triangles) or with SPS (diamonds), or in a honey-equivalent sugar solution (circles). C) To assess the contribution of the low pH to the bactericidal activity of honey, B. subtilis was incubated in various concentrations of honey in incubation buffer (squares), or with catalase, glyox- alase I, and SPS added either without (triangles) or with neutralization to pH 7 (diamonds), or in a honey-equivalent sugar solution (circles). After 24 h, numbers of surviving bacteria were determined. Data are mean se log-trans- formed bacterial concentration (CFU/ml).
against B. subtilis. As previously observed, >20% honey retained bactericidal activity when H2O2 and MGO were neutralized. Additional neutralization of bee de- fensin-1 strongly reduced the bactericidal activity of 20% honey but did not affect the activity of 30 and 40% honey (Fig. 3B). So, bee defensin-1 contributed to the bactericidal activity of honey, but still other bactericidal factors were involved.
Honey has a low pH, mainly because of the conver- sion of glucose into hydrogen peroxide and gluconic acid by glucose oxidase. This low pH might also con- tribute to the bactericidal activity of honey (23). Titra- tion of the pH of 40 –10% RS honey from 3.4 –3.5 to 7.0, combined with neutralization of H2O2, MGO and bee defensin-1, reduced the bactericidal activity of honey to a level identical to that of a honey-equivalent sugar solution (Fig. 3C). Thus, with this experiment, we
succeeded in identifying all bactericidal factors in RS honey responsible for killing of B. subtilis.
The contribution of the identified bactericidal fac- tors to activity against antibiotic-susceptible and -resis- tant strains of various species was tested with honey diluted to 20%, since this killed the entire inocula of all bacteria tested independent of sugar (Fig. 1). Simulta- neous neutralization of H2O2, MGO and bee defensin-1 negated all activity (Fig. 4), showing that these were the major factors responsible for broad spectrum bacteri- cidal activity of honey.
We studied the contribution of the honey bacteri- cidal factors in more detail by neutralizing the factors individually or combined. Neutralization of H2O2 alone strongly reduced the bactericidal activity against all bacteria tested except B. subtilis (Fig. 4). Neutralization of MGO alone strongly reduced killing of E. coli and
P. aeruginosa strains (Fig. 4). Neutralization of bee defensin-1 alone reduced killing of VREF, but not of the other bacteria tested (Fig. 4). When compared to neutralization of MGO alone, the additional neutraliza- tion of bee defensin-1 reduced killing of all bacteria tested, except E. coli ESBL (Fig. 4). In summary, H2O2, MGO, and bee defensin-1 differentially contributed to the activity of honey against specific bacteria, and their combined presence was required for the broad-spec- trum activity.
DISCUSSION
All bacterial species tested were susceptible to different combinations of bactericidal factors in honey, indicat- ing that these bacteria were killed via distinct mecha- nisms. This clearly demonstrates the importance of the multifactorial nature of honey for its potent, broad- spectrum bactericidal activity.
Some factors had overlapping activity. For instance, the activity of bee defensin-1 against most bacteria was only revealed after neutralization of MGO. This clearly demonstrates the importance of neutralizing known bactericidal factors in honey to reveal the presence of additional factors. Similarly, the contribution of the low pH for activity of honey against B. subtilis was only revealed when H2O2, MGO, and bee defensin-1 were simultaneously neutralized.
In other situations, bactericidal activity depended on the combined presence of different factors. Thus, the activity of honey against E. coli and P. aeruginosa was markedly reduced by neutralization of either H2O2 or MGO. Alternatively, the activity of certain bactericidal factors likely is more potent in the context of honey than as pure substances. This is most clearly illustrated by the activity of MGO. When tested in a buffer, >0.3 mM MGO was required for activity against B. subtilis (Supplemental Fig. 1). In contrast, as little as 0.05 mM MGO, the concentration in 20% RS honey, was suffi- cient to substantially contribute to the bactericidal activity. This suggests that the presence of the other bactericidal factors in honey enhanced the effect of
Figure 4. Effect of neutralization of H2O2, MGO, and bee defensin-1 on bactericidal activity of honey. Hydrogen peroxide, MGO, and bee defensin-1 were neutralized in 20% honey by adding catalase (cat.), glyoxalase I (gly I) and SPS, respectively. Bactericidal activity was tested against indicated laboratory strains (left 4 panels) and against clinical isolates of VREF, MRSA,
E. coli ESBL, and CRPA (right 4 panels). A sugar solution equivalent to 20% honey was used as a reference. After 24 h, numbers of surviving bacteria were determined. Data are mean se log-transformed bacterial concentration (CFU/ml).
MGO. It is not possible to quantify the contribution of the different factors to honey bactericidal activity since, as we have shown, these factors may have redundant activity, be mutually dependent, or have additive or synergistic activity depending on the bacterial species targeted.
We have demonstrated for the first time that honey contains an antimicrobial peptide, bee defensin-1, and that this peptide substantially contributes to the bacte- ricidal activity. Bee defensin-1 was previously isolated from royal jelly (24), the major food source for bee queen larvae (and then referred to as “royalisin”), and was identified in honeybee hemolymph (18). Royal jelly is produced by young worker bees and contains their hypopharyngeal and mandibular gland secretions (25, 26). Bee defensin-1 mRNA has been identified in the hypopharyngeal gland of young worker bees (18), suggesting this gland is involved in production of bee defensin-1 found in royal jelly (24). When worker bees age, they become the major producers of honey. Major differences develop in morphology and protein expres- sion of their hypopharyngeal glands (27, 28), e.g., several important carbohydrate-metabolizing enzymes, including glucose oxidase are expressed (29). The bees add the secretion from their hypopharyngeal glands to the collected nectar. The carbohydrate-metabolizing enzymes then convert sucrose to glucose and fructose, and glucose oxidase converts the glucose to hydrogen peroxide and gluconic acid. These latter compounds presumably are involved in prevention of microbial spoilage of unripe honey (11). Since we have found bee defensin-1 in honey, this suggests that after the transi- tion in hypopharyngeal gland function of the worker bees with age, the gland still produces bee defensin-1. This peptide, therefore, likely contributes to protection of both royal jelly and honey against microbial spoilage. It remains to be established whether bee defensin-1 is also present in other honeys. In Manuka honey, no evidence was found for the presence of antimicrobial peptides (30). For several other honeys, proteins were reported to contribute to the antibacterial activity (31, 32), but their identity remains unknown. Using our antibee defensin-1 antibody, we aim to assess the role of
bee defensin-1 for the antibacterial activity of other honeys.
Previous studies regarding the effect of low pH to antibacterial activity of honey have yielded conflicting results (11). In our study, the contribution of the low pH for activity against B. subtilis was only revealed on inactivation of all other bactericidal factors. So, in other studies, which did not employ an approach of neutral- ization of bactericidal factors in honey, the contribu- tion of the low pH of honey may easily have been overlooked.
Much effort has been put into identification of phenolic antibacterial components in honey (11). Sev- eral of these compounds have been isolated from honey, but as they were tested at concentrations far exceeding those in honey, no conclusions can be drawn regarding their contribution to honey bactericidal ac- tivity (11). Our data do not show a role of phenolic compounds in RS honey bactericidal activity.
Our approach of selectively neutralizing individual bactericidal factors present in a medical-grade honey allowed us to unravel the multifactorial bactericidal activity of a honey for the first time. We presently use the same approach to assess the contribution of these factors to activity of other honeys, and simultaneously to screen for novel bactericidal factors. Such honeys, or isolated components thereof, may serve as novel agents to prevent or treat infections, in particular those caused by antibiotic-resistant bacteria.
The authors thank Jorn Blom and Sadira Thomas for their help with purification of bee defensin-1; Henk Dekker for expert nano ESI-ms/ms experiments; and Ton Bisseling, Ben Berkhout, Mark van Passel, and Brendan McMorran for critically reviewing the manuscript.
REFERENCES
1. Majno, G. (1975) Man and Wound in the Ancient World. Harvard University Press, Cambridge, MA, USA
2. Molan, P. C. (2001) Why honey is effective as a medicine: its use in modern medicine. In Honey and Healing (Munn, P., and Jones, R., eds.) pp. 5–14, International Bee Research Associa- tion, Cardiff, UK
3. Efem, S. E. E. (1988) Clinical observations on the wound- healing properties of honey. Brit. J. Surg. 75, 679 – 681
4. Jones, R. (2001) Honey and healing through the ages. In Honey and Healing (Munn, P., and Jones, R., eds.) pp. 1– 4, Interna- tional Bee Research Association: Cardiff, UK
5. Nathan, C. (2004) Antibiotics at the crossroads. Nature 431,
899 –902
6. Cooper, R. A., Halas, E., and Molan, P. C. (2002) The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J. Burn Care Rehabil. 23, 366 –370
7. Cooper, R. A., Molan, P. C., and Harding, K. G. (2002) The sensitivity to honey of gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 93, 857– 863
8. Bonn, D. (2003) Sweet solution to superbug infections? Lancet Infect. Dis. 3, 608
9. Dixon, B. (2003) Bacteria can’t resist honey. Lancet Infect. Dis. 3,
116
10. Lusby, P. E., Coombes, A., and Wilkinson, J. M. (2002) Honey: a potent agent for wound healing? J. Wound Ostomy Continence Nurs. 29, 295–300
11. Molan, P. C. (1992) The antibacterial activity of honey. 1. The nature of the antibacterial activity. Bee World 73, 5–28
12. Adams, C. J., Boult, C. H., Deadman, B. J., Farr, J. M., Grainger,
M. N., Manley-Harris, M., and Snow, M. J. (2008) Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 343, 651– 659.
13. Mavric, E., Wittmann, S., Barth, G., and Henle, T. (2008) Identification and quantification of methylglyoxal as the domi- nant antibacterial constituent of Manuka (Leptospermum scopa- rium) honeys from New Zealand. Mol. Nutr. Food Res. 52, 483– 489
14. Kwakman, P. H. S., Van den Akker, J. P. C., Guclu, A., Aslami, H., Binnekade, J. M., de Boer, L., Boszhard, L., Paulus, F., Middelhoek, P., Te Velde, A. A., Vandenbroucke-Grauls,
C. M. J. E., Schultz, M. J., and Zaat, S. A. J. (2008) Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis. 46, 1677–1682
15. Lehrer, R. I., Barton, A., Daher, K. A., Harwig, S. S., Ganz, T., and Selsted, M. E. (1989) Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Invest. 84, 553–561
16. White, J. W. Jr., and Subers, M. H. (1963) Studies on honey inhibine. 2. A chemical assay. J. Apicul. Res. 2, 93–100
17. Racker, E. (1951) The mechanism of action of glyoxalase. J. Biol. Chem. 190, 685– 696
18. Klaudiny, J., Albert, S., Bachanova, K., Kopernicky, J., and Simuth, J. (2005) Two structurally different defensin genes, one of them encoding a novel defensin isoform, are expressed in honeybee Apis mellifera. Insect Biochem. Mol. Biol. 35, 11–22
19. Dankert, J., Van der Werff, J., Zaat, S. A., Joldersma, W., Klein, D., and Hess, J. (1995) Involvement of bactericidal factors from
thrombin-stimulated platelets in clearance of adherent viridans streptococci in experimental infective endocarditis. Infect. Im- mun. 63, 663– 671
20. Lehrer, R. I., Rosenman, M., Harwig, S. S., Jackson, R., and Eisenhauer, P. (1991) Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137, 167–173
21. Harwig, S. S., Chen, N. P., Park, A. S., and Lehrer, R. I. (1993) Purification of cysteine-rich bioactive peptides from leukocytes by continuous acid-urea-polyacrylamide gel electrophoresis. Anal. Biochem. 208, 382–386
22. Schagger, H., and von Jagow, G. (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368 –379
23. Yatsunami, K., and Echigo, T. (1984) Antibacterial activity of honey and royal jelly. Honeybee Sci. 5, 125–130
24. Fujiwara, S., Imai, J., Fujiwara, M., Yaeshima, T., Kawashima, T., and Kobayashi, K. (1990) A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 265, 11333–11337
25. Lensky, Y., and Rakover, Y. (1983) Separate protein body compartments of the worker honeybee (Apis mellifera L). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 75, 607– 615
26. Knecht, D., and Kaatz, H. H. (1990) Patterns of larval food- production by hypopharyngeal glands in adult worker honey- bees. Apidologie 21, 457– 468
27. Ohashi, K., Natori, S., and Kubo, T. (1997) Change in the mode of gene expression of the hypopharyngeal gland cells with an age-dependent role change of the worker honeybee Apis mellif- era L. Eur. J. Biochem. 249, 797– 802
28. Sasagawa, H., Sasaki, M., and Okada, I. (1989) Hormonal-control
of the division of labor in adult honeybees (Apis-Mellifera L). 1. Effect of methoprene on corpora allata and hypopharyngeal gland, and its alpha-glucosidase activity. Appl. Entomol. Zool. 24, 66 –77
29. Ohashi, K., Natori, S., and Kubo, T. (1999) Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honeybee (Apis mellifera L.). Eur. J. Biochem. 265, 127–133
30. Weston, R. J., Brocklebank, L. K., and Lu, Y. R. (2000) Identi-
fication and quantitative levels of antibacterial components of some New Zealand honeys. Food Chem. 70, 427– 435
31. Mundo, M. A., Padilla-Zakour, O. I., and Worobo, R. W. (2004) Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 97, 1– 8
32. Gallardo-Chacon, J. J., Casellies, M., Izquierdo-Pulido, M., and Rius, N. (2008) Inhibitory activity of monofloral and multifloral honeys against bacterial pathogens. J. Apicul. Res. 47, 131–136
33. https://cloverhoney.web.id/
34. https://cloverhoney.web.id/clover-honey-madu-hdi/
35. https://cloverhoney.web.id/propoelix/
36. https://cloverhoney.web.id/royal-jelly-hdi/
37. https://cloverhoney.web.id/clover-honey-harga/
38. https://cloverhoney.web.id/propoelix-harga/
39. https://cloverhoney.web.id/hdi-propoelix-adalah/
40. https://cloverhoney.web.id/manfaat-propoelix/
41. https://cloverhoney.web.id/madu-hdi-harga/
42. https://cloverhoney.web.id/propoelix-plus/
43. https://cloverhoney.web.id/madu-hdi-manfaatnya/
44. https://cloverhoney.web.id/clover-honey-manfaatnya/
45.
Received for publication November 18, 2009.
Accepted for publication February 4, 2010.
0 notes
