#CE-certified implants
Text

A Comprehensive Range of Hand Locking System
The Hand locking system is intended for the surgical treatment of fractures in hand, especially in the middle and proximal phalanxes. Hand locking plates are designed to have threaded holes that allow a rigid fixation using locking screws. This minimizes the risk of vibration loosening and ensures broken fragments are in their true anatomy. Siora Surgicals Pvt. Ltd. is a world-class manufacturer of a CE-certified range of orthopedic implants including hand locking systems. The implants are fabricated using medical-grade stainless steel and titanium and are tested to meet international standards. Siora is also a trustworthy OEM/contract manufacturing service provider worldwide.
#Hand Locking System#Surgical Hand Locking System#Orthopedic Hand Locking System#Medical Devices#Orthopedic Implants#Surgical Implants#Hand Locking Plates#Locking Screws#Threaded Holes#Rigid Fixation#Medical-Grade Stainless Steel Implants#Titanium Implants#Fracture Treatment#Hand Fracture Surgery#Proximal Phalanx Fracture Treatment#Middle Phalanx Fracture Treatment#Orthopedic Surgeons#Medical Professionals#Hospitals#Clinics#Minimizes Vibration Loosening#Ensures Anatomical Alignment#CE-Certified Implants#High-Quality Manufacturing#Worldwide Orthopedic Implants#USA Orthopedic Implants#Europe Orthopedic Implants#Asia Orthopedic Implants#Best Hand Locking System#Surgical Hand Implants
0 notes
Text
Variable Angle Plate – Get an International Standard Range

Variable angle plates are game-changers in fracture repair. These medical implants offer surgeons more control by allowing screws to be inserted at various angles. This versatility is crucial for complex bone breaks, especially in the wrist. Variable angle plates provide:
Improved fixation: Precise screw angulation for better bone fragment alignment.
Minimally invasive: Smaller incisions compared to traditional plates.
Faster healing: Stable fixation promotes quicker bone healing.
You can get a CE-certified range of locking variable angle plates from Siora Surgicals Pvt. Ltd., a renowned manufacturer of a huge range of trauma implants and instruments.
#Locking Variable Angle Plate#Variable Angle Locking Plate Uses#Variable Angle Locking Plate#Variable Angle Plate
0 notes
Text
Most Reliable Orthopaedic Device Companies in Brazil

Brazil has a growing medical sector and it provides excellent growth opportunities for exporters and suppliers. Being in the orthopedic implant manufacturing business for 30+ years, Siora Surgicals Pvt. Ltd., an India-based company is looking for orthopedic distributors in Brazil. Having established a good international market presence, the company also wants to establish itself among the leading Orthopaedic Device Companies in Brazil. Siora manufactures thousands of different types of CE-certified trauma implants in its production house. All the products are stringently tested to conform to international standards. Moreover, the company is also known as a trustworthy OEM/contract manufacturing service provider worldwide.
#Orthopaedic Device Companies in Brazil#orthopedic implants#orthopedic companies#orthopedic implants manufacturer#siora surgicals
0 notes
Text
What are the steps involved in CE Mark certification in Cyprus?
/ Uncategorized / By Factocert Mysore

CE Mark certification in Cyprus:
CE Mark certification in Cyprus is an obligatory conformity mark for positive merchandise bought within the European Economic Area (EEA), which incorporates CE Mark certification in Cyprus. It certifies that a product complies with EU directives concerning protection, fitness, environmental protection, and patron safety.
Benefits of CE Marking in Cyprus:
Market Access: CE Mark auditors in Denmark permits you to legally place your product in the Cypriot market and freely flow into it all through the EEA.
Reduced Costs: By demonstrating compliance with EU requirements in advance, you can avoid capability delays and charges related to border inspections or product recalls.
Enhanced Reputation: CE Mark certification in Cyprus demonstrates your dedication to product safety and satisfaction, giving you a competitive edge.
Simplified Procedures: CE Mark consultant in Cyprus streamlines product registration and approval tactics inside different EEA countries.
Products Requiring CE Mark certification in Cyprus:
Not all products require CE mark cost in Cyprus . The unique necessities rely upon the product class and applicable EU directives. Here are a few common CE Marking product categories:
Electrical devices (e.g., appliances, electricity tools)
Machinery (e.g., commercial equipment, construction equipment).
Medical gadgets (e.g., implants, diagnostic devices)
Personal protecting gadgets (e.g., helmets, protection glasses).
Toys
Pressure system (e.g., boilers, fuel cylinders).
The CE Mark certification Process in Cyprus:
CE Mark certification in Cyprus normally includes these steps:
Identify Applicable Directives: Determine which EU directives are observed in your product through consulting relevant harmonized standards. These requirements define the technical specs your product desires to satisfy.
Conformity Assessment: Assess your product’s compliance with the critical necessities outlined within the applicable directives. This may also involve internal checking, the use of notified bodies (unbiased conformity assessment groups), or a combination of each.
Technical Documentation: Prepare technical documentation that demonstrates your product meets the relevant necessities. This record must include product layout specs, take a look at reviews, and manufacturing manipulation methods.
Declaration of Conformity: Create a Declaration of Conformity, a proper record putting forward your product’s compliance with the applicable directives and requirements.
CE Marking Affixation: If your product conforms, affix the CE Marking on the product itself or its packaging.
Role of the Department of Labour Inspection (DLI):
The DLI is the national authority responsible for market surveillance and enforcement of CE Marking policies in Cyprus. They conduct inspections to make certain merchandise complies with applicable directives and can take corrective actions if non-compliance is found.
Resources for Businesses in Cyprus:
The Cyprus Standards and Consumer Protection Service presents records on CE marking requirements, which can be manually sent to you via the system.
The European Commission’s website consists of sources on CE Marking, applicable directives, and harmonized standards.
Private groups offer CE Marking consultancy offerings and may assist you with the manner.
Important Considerations:
Changes to Regulations: CE Marking guidelines are subject to revision and updates. It’s critical to stay current on any changes that might affect your product compliance.
National Variations: While CE Marking harmonizes product necessities across the EEA, minor versions may exist in interpretation via country-wide government.
Post-Market Surveillance: Your obligation for product protection does not stop with CE Marking. Continuously monitor your product’s overall performance and take corrective movements if essential.
What are the steps to get CE Mark certification in Cyprus?
Here’s a breakdown of the key steps to acquiring CE Mark certification in Cyprus:
1. Identify Applicable Directives:
The first step is determining if your product falls under any EU Directives requiring CE Mark certification in Cyprus. Resources just like the NEWTIS database [invalid URL removed] let you identify applicable directives based on your product category.
2. Understand Essential Requirements:
Each applicable directive outlines “crucial necessities”—protection targets your product needs to meet. These necessities deal with components like mechanical safety, electromagnetic compatibility, and environmental safety.
3. Technical File Compilation:
Compile a technical file that demonstrates your product’s compliance with the essential requirements. This report normally consists of:
Product description and specs
Design and production drawings
Bill of substances
Risk exams
Test reports (if relevant)
User manuals and safety statistics
4. Conformity Assessment Procedure Selection:
Different directives can also necessitate precise conformity assessment methods. These tactics involve demonstrating compliance through inner manufacturing manipulation, involvement of a Notified Body (independent certification body), or a combination of both.
5. Internal Production Control (if applicable):
Internal Production Control (IPC) involves implementing a quality control device to ensure the regular production of compliant merchandise. This may additionally require a notified frame to evaluate your system for several directives.
6. Notified Body Involvement (if required):
For certain high-hazard merchandise or complex directives, a Notified Body will conduct product testing, review technical documentation, and issue certificates if satisfied with your product’s compliance.
7. Declaration of Conformity (DoC):
Once you have met the requirements, prepare a Declaration of Conformity (DoC). This file officially declares your product’s compliance with the applicable directives.
8. CE Marking Affixation:
With a completed DoC and a compliant product, you could affix the CE Marking to your product or its packaging.
Why Factocert for CE Mark Certification in Cyprus?
We provide the best CE Mark consultants in Cyprus Who are knowledgeable and provide the best solution. And how to get CE Mark certification in Cyprus . Kindly reach us at [email protected]. CE Mark certification consultants work according to CE Mark standards and help organizations implement CE Mark certification in Cyprus with proper documentation.
For more information, visit CE Mark Certification in Cyprus .
Related Links:
ISO 21001 Certification in Cyprus
ISO 22301 Certification in Cyprus
ISO 37001 Certification in in Cyprus
ISO 27701 Certification in Cyprus
ISO 26000 Certification in Cyprus
ISO 20000-1 Certification in Cyprus
ISO 50001 Certification in Cyprus
HALAL Certification in Cyprus
CE MARK Certification in Cyprus
0 notes
Text
Locking Distal Femur Osteotomy Plate 4.5/5.0 mm
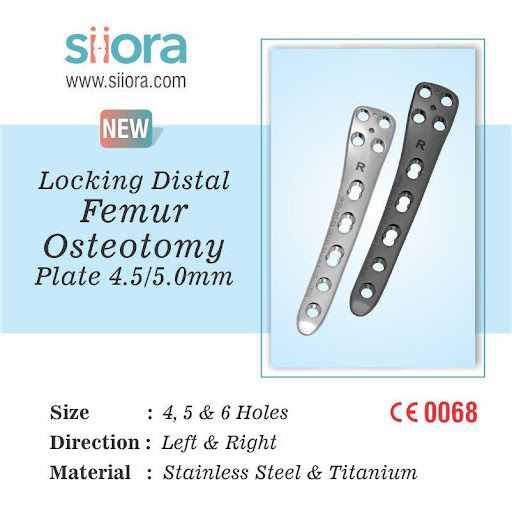
Locking Distal Femur Osteotomy Plate is intended for the surgical treatment of different types of fractures in the distal femur. Some of the distal femur fractures that can be addressed by these locking plates include periarticular fractures, periprosthetic fractures, malunions, non-unions, and osteotomies. These plates are anatomically contoured which matches the anatomy of the femur. They have combi holes along their shaft that can accommodate cortical as well as locking screws.
Siora Surgicals Pvt. Ltd. is an experienced manufacturer of a wide range of CE-certified orthopedic implants and instruments including Locking Distal Femur Osteotomy Plate 4.5/5.0 mm. These locking distal femur osteotomy plates are available in different sizes and stainless steel & titanium. Siora is also a reliable OEM service provider in the world.
0 notes
Text
Italy – Medical Device Registration (NSIS) | OMC Medical Limited

Regulatory Authority of Italy
Directorate General for Medical Devices, Pharmaceutical Services and Safety in Healthcare (La Direzione general dei dispositivi medici, del servizio farmaceutico e della sicurezza delle cure svolge) deals with the implementation of medical device regulations, preventing and dealing with clinical risk, regulating the advertising of medical products, and providing medical-legal consultancy to state institutions.
Registration Requirement
All medical devices marketed in the EU must bear the CE mark to certify conformity with EU law. In Italy, the Manufacturer must register the medical devices in a dedicated database of the Ministry of Health before they are put into the market.
The obligation to register with the new modalities concerns medical devices marketed in Italy for the first time starting from 1 May 2007, and in particular:
Class I, IIa, IIb and III devices.
The kits and assemblies.
Active implantable devices.
Entities making the registrations
The registration of a device within the Database / Directory of Medical Devices system can be requested by:
Manufacturer
Representative
Other delegated subject by the manufacturer and representative
If the manufacturer is headquartered outside the European Union, the requirements under the Portuguese regulation must be completed by a legal representative in the Community or, European Authorized Representative (AR).
A single AR may only represent the manufacturer for each type of device. Thus, to enter the medical devices market, manufacturers of medical devices are required to appoint an AR, which will assist with all the details regarding the registration process and assist in completing requirements under the Italian regulation.
As a non-EU manufacturer of medical devices, the manufacturer’s legal representative can appoint a “delegate as person or company” to register their product with the Italian database. This delegate will communicate with the Italian Ministry of Health on the manufacturer’s behalf.
The online registration can be found on the Ministry of Health website: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=395&area=dispositivi medici&menu=registrazione.
Timeline for registration
1 month
Registration Process for NSIS
Register and electronically sign the data of the registrant who intends to carry out the notification of medical devices.
Register and electronically sign the data of device Manufacturers and/or Authorised Representatives other than the reporting Authorised Representative.
Enter the device data in the system.
Complete device data entry or correct entry errors/inaccuracies entered upon inserting.
Verify that the data entered complies with system controls.
Sign data electronically and Submit the application.
Process for Non-EU Manufacturer
Designation of European Authorised Representative
Provide your European Authorised Representative with relevant documentation
Document review
Foreign company’s legal representative detects the individual (whether Italian or not) or the company to delegate for entering data.
Registration on the Ministry of Health Database (NSIS)
Documentation Requirement
Name and code of the medical device, its classification, name of the Manufacturer or its authorized representative, a brief description of the medical device together with its intended use, a list of the materials contained in the medical device that comes into direct contact with the patient, sterilization data, information about re-use, scientific bibliography and other information.
CE Conformity Certificates
EC Declaration of Conformity (must be in Italian)
Packaging box (Box, Labelling and instructions for use (must be in Italian))
Technical file
Scientific bibliography supporting the clinical evidence of the effectiveness and safety of the device
Data Sheet
Commercial Data
What We Offer ?
Act as your AR in the EU
Register your medical devices in the Italian Database.
Keep you up to date with the Italian regulatory requirement.
Help you with translation of User Manual and Labelling into Italian.
Why Choose Us ?
Working towards client satisfaction
Cost effective solutions
Project completion before deadline
Quality Regulatory affairs solutions
Contact us for free consultation: [email protected]
Originally Published at: https://omcmedical.com/italy-medical-device-registration-nsis/
0 notes
Text

Locking Low Bend Medial Distal Tibia Plate 3.5/4.0 mm is applied to fix intra-articular and extra-articular fractures of the distal tibia. Made having combi-holes in the shaft, these plates can accommodate cortical screws as well as locking screws as required according to the situation. Siora Surgicals Pvt. Ltd. is a trustworthy and experienced manufacturer of Locking Low Bend Medial Distal Tibia Plate 3.5/4.0 mm and hundreds of other CE-certified orthopedic implants in India. These plates are made using medical-grade stainless steel and titanium.
1 note
·
View note
Text
Unveiling the Hidden Consequences: Long-Term Effects of Fractures

Fractures, those painful and disruptive breaks in our bones, often evoke images of cast-clad limbs and temporary discomfort. However, beneath the surface, fractures can unleash a series of long-term consequences that extend far beyond the initial pain and healing period. In this blog, we will delve into the less discussed but equally important long-term effects of fractures.
For a CE-certified range of trauma implants and instruments, find Experienced orthopedic instruments manufacturers.
#ortho surgical implants#trauma implants#orthopedic equipment manufacturers#top orthopedic implant companies#surgical implants manufacturers#ortho implants#top orthopedic implants companies in india#orthopedic Manufacturers
0 notes
Text
What is a Buckle Fracture in Children? A Detailed Guide.
Siora Surgicals Pvt. Ltd. is a leading name in the orthopedic implants manufacturing industry. Growing strong for over 3 decades, the company is proud to be counted among the best orthopedic device manufacturers across the globe. Siora owns and manages a huge inventory of CE-certified orthopedic implants & instruments. It uses medical-grade stainless steel and titanium to manufacture implants. The processing of orthopedic devices takes place in Siora’s production facility which has been established in the RAI District, Sonepat, Haryana. The company is also a renowned OEM/contract manufacturing service provider in the world.
https://www.siiora.com/blogs/buckle-fractures/

0 notes
Text

3.5 mm /4.5 mm Cannulated Screw Titanium
3.5 mm /4.5 mm Cannulated Screw Titanium is intended for securing other implants during fracture surgery. These screws are made to have a hollow central shaft and this allows them to be placed over a guide wire or a guide pin. Cannulated screws allow surgeons to place these screws using fluoroscopy precisely. They can be availed in different sizes and types from Siora Surgicals Pvt. Ltd., an experienced manufacturer of a CE-certified range of trauma implants. The company also provides quality OEM/contract manufacturing services.
#Orthopedic Product#Cannulated screws#Orthopedic implants#Fracture treatment#Trauma implants#Surgical instruments#CE-certified implants#Cannulated screw titanium#Fracture fixation#Orthopedic surgery#Fluoroscopy-guided surgery
0 notes
Text
Get an International Standard Quality Range of Locking Compression Plates

Locking compression plates are used for fixing different types of fractures in long bones like the humerus, femur, and tibia. These LCP plates are also ideal for fixing periprosthetic fractures, nonunions, malunions, and osteopenic bones. Locking compression plates are available in different sizes and structural configurations depending on the fracture pattern and its severity. These plates have threaded holes and are secured using locking screws. Siora Surgicals Pvt. Ltd. is a trustworthy manufacturer of a CE-certified range of trauma implants & instruments including LCP locking plates. They are fabricated using medical-grade stainless steel and titanium in Siora’s in-house production facility. The company is also a renowned OEM service provider worldwide.
#Locking Bone Plate#Orthopedic Locking Compression Plate#Locking Plate System#Orthopedic Locking Plate#Locking Plates and Screws#Locking Plate#LCP Locking Compression Plate#Locking Compression Plate#Locking Compression Plate Uses
0 notes
Text
Experienced Orthopedic Company in Brazil
Backed by a team of skilled and adroit professionals, Siora Surgicals Pvt. Ltd., an orthopedic implant manufacturer has been growing stronger year by year for the last 30 years. The company always keeps looking to expand its international market reach. For that, it is looking for orthopedic distributors in Lebanon and establishing its presence among the best Orthopedic Company in Brazil. Siora maintains a huge inventory of CE-certified trauma implants and instruments. It is also known as a trustworthy OEM service provider in the world.
#Orthopedic Company in Brazil#orthopedic companies#orthopedic implants#orthopedic implants manufacturer#siora surgicals
0 notes
Text
NuVasive’s Pulse Platform for Spine Surgeries, US
In July 2021, NuVasive's Pulse spinal surgical automation platform received FDA 510(k) clearance.
The PulseTM system is the first integrated technology platform made to make all spine surgical procedures safer, more effective, and more repeatable.
The Pulse platform, developed by NuVasive, a US medical device company, can be used in all spine operations. It is a disruptive technology that has the potential to alter how patients receive spine care in the future.

After receiving approval from the European CE Mark in June 2021, the Pulse platform received 510(k) clearance from the US Food and Drug Administration (FDA) in July 2021.
Design and features of the Pulse platform The Pulse spinal surgical automation platform combines a number of technologies into a single platform. Future applications like robotics will be compatible with its extensible architecture.
By streamlining the imaging workflow, the platform's Lessray® technology from NuVasive enhances imaging and boosts operating room (OR) efficiency. Additionally, it significantly reduces everyone in the room's radiation exposure.
The Pulse's procedurally integrated navigation system minimizes radiation exposure while enhancing screw placement accuracy.
With the standard setup, the platform's neuromonitoring feature provides proprietary automatic nerve detection and clinically verified alerts to reduce variability and speed up neural data interpretation.
By providing tools for surgical planning and intraoperative assessment, NuVasive's Integrated Global Alignment (iGA®) technology enables the system to assist surgeons in correcting or restoring spinal alignment. Additionally, it enables surgeons to evaluate the outcomes of operations by following up.
The Pulse system makes use of Bendini®, a patient-specific spinal rod bending technology, to produce customized rods that are bent to fit implant sites. By utilizing computer-assisted bend directions, it accelerates manual rod manipulation.
The Cios Spin® 3D mobile C-arm from Siemens Healthineers is an excellent candidate for the platform's integration of a variety of imaging systems.
All members of the surgical team in the operating room, from the surgeon to the C-arm technician to the support staff, are able to seamlessly connect to the Pulse platform and manage it thanks to its wireless connectivity.
The Siemens Healthineers Cios Spin® 3D mobile C-arm explains how Cios Spin enables precise quality control through 2D and 3D intraoperative imaging and helps to control operational risks and costs. It has three simultaneous projection displays and CMOS flat detector technology with a scan speed of 30 seconds.
In 3D images, the metal artefact reduction (MAR) tool reduces artifacts like streaks and blind spots. By automatically transferring 3D datasets to certified navigation systems, the NaviLink 3D digital interface enables seamless integration with navigation for surgical image guidance and navigation.
Benefits of the Pulse system The platform makes it easy for surgeons to use a variety of technologies from a small space, adopt less invasive and more advanced surgical techniques, and address some of the most common problems with surgery.
Numerous clinical studies support the advantages of less invasive surgery, such as shorter operating room (OR) times, shorter periods of anesthesia, less blood loss, and lower intraoperative risks.
Additionally, it contributes to a shorter hospital stay and a savings of approximately $5,000 per patient in hospital costs.
Pulse is one of the most adaptable tools in the operating room for the spine. It combines multiple technologies into a single platform, enabling surgeons to make clinical decisions for their patients that are more informed.
The Pulse system has the potential to improve operational, financial, and clinical outcomes. It supports all types of spine surgery, including open and minimally invasive procedures.
0 notes
Text
Siora Surgicals Pvt. Ltd. is an experienced manufacturer of a wide range of orthopedic implants and instruments in India. Working for over 30 years, the company has an excellent global presence and it keeps stretching its reach in different countries. Siora is also known for supplying a CE-certified range of orthopedic implants in Malaysia.
#orthopedic Implants#.orthopaedic distributors#orthopedic implants company#orthopedic implants suppliers
0 notes
Text

Locking Proximal Tibia Raft Plate 3.5/4.0 mm finds application for the open reduction and internal fixation of different types of fractures in the proximal tibia including comminuted, simple, depression, lateral/medial wedge, and others. These plates have a limited-contact profile and are provided with combi-holes that include a dynamic compression unit (DCU) along with a locking screw hole. You can get a CE-certified range of orthopedic implants including Locking Proximal Tibia Raft Plate from Siora Surgicals Pvt. Ltd., one of the oldest orthopedic manufacturers in India.
1 note
·
View note
Text

3.5mm Cortical Screw Self Tapping is intended to be used with trauma plates for the fixation of fractures. These screws have a larger number of threads which makes them suitable for application in dense cortical bones. Self-tapping screws are also known for their ability to self-tap their hole during insertion in the bone. Siora Surgicals Pvt. Ltd. is a leading manufacturer of a CE-certified range of orthopedic implants including self-tapping cortical screws.
#orthopedic implant distributors#orthopedic products manufacturers#orthopedic equipment manufacturers#trauma implants suppliers#orthopedic instruments suppliers
1 note
·
View note