#age-related macular degeneration (AMD) market
Text
An Overview of the Age Related Macular Degeneration Market: Trends and Insights
The age-related macular degeneration (AMD) market is influenced by various trends and insights that shape its dynamics and growth trajectory.
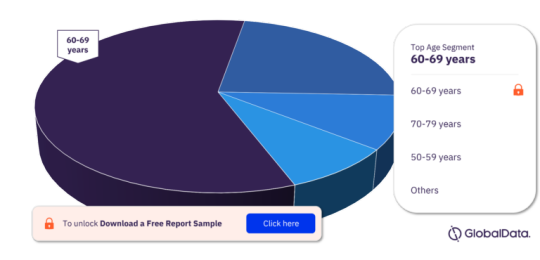
For more age segment insights, download a free report sample
Here's an overview of some key trends and insights in the AMD market:
Prevalence and Demographics: AMD primarily affects older adults, particularly those aged 50 and above. With global aging populations, the prevalence of AMD is increasing steadily. This demographic trend drives demand for AMD treatments and diagnostic solutions.
Advancements in Diagnostic Technologies: Innovations in diagnostic technologies, such as optical coherence tomography (OCT) and fundus autofluorescence imaging, have revolutionized the early detection and monitoring of AMD. Early diagnosis allows for timely intervention and management, potentially slowing disease progression and preserving vision.
Treatment Landscape Evolution: The treatment landscape for AMD has evolved significantly over the years. The introduction of anti-vascular endothelial growth factor (anti-VEGF) therapies, such as ranibizumab and aflibercept, has revolutionized the management of neovascular (wet) AMD, leading to improved visual outcomes for patients.
Emerging Therapeutic Approaches: Alongside anti-VEGF therapies, several emerging therapeutic approaches are being explored for AMD treatment. These include novel drug delivery systems, gene therapies, complement inhibitors, and regenerative medicine approaches aimed at addressing various aspects of AMD pathophysiology.
Personalized Medicine: There is a growing emphasis on personalized medicine in AMD treatment, driven by the recognition of inter-individual variability in treatment response and disease progression. Biomarkers, genetic testing, and imaging biomarkers are being investigated to tailor treatment strategies based on individual patient characteristics.
Healthcare Policy and Reimbursement Landscape: Reimbursement policies and healthcare regulations influence access to AMD treatments and diagnostic services. Variations in reimbursement policies across regions can impact market access and adoption rates for innovative therapies.
Patient-Centric Care Models: Patient-centric care models are gaining prominence in AMD management, focusing on holistic patient care, education, and support services. Multidisciplinary care teams comprising ophthalmologists, optometrists, retinal specialists, and allied healthcare professionals play a crucial role in delivering comprehensive care to AMD patients.
Research and Development Investments: Pharmaceutical companies, biotechnology firms, and academic institutions continue to invest heavily in AMD research and development (R&D). Clinical trials are underway to explore novel therapeutic targets, combination therapies, and innovative treatment modalities to address unmet needs in AMD management.
Global Market Expansion: The AMD market is expanding globally, with significant growth opportunities in emerging markets such as Asia-Pacific and Latin America. Rising healthcare expenditure, improving healthcare infrastructure, and increasing awareness of AMD contribute to market growth in these regions.
Collaborations and Partnerships: Collaboration between industry players, research organizations, and healthcare providers is essential for driving innovation and accelerating the development of new AMD therapies and diagnostic solutions. Collaborative efforts facilitate knowledge sharing, resource pooling, and the translation of scientific discoveries into clinical practice.
In summary, the AMD market is characterized by ongoing advancements in diagnostics and therapeutics, a shift towards personalized medicine, and a focus on patient-centric care models. Continued investments in research, collaborations, and global market expansion efforts are expected to shape the future landscape of AMD management.
0 notes
Link
https://www.databridgemarketresearch.com/reports/north-america-age-related-macular-degeneration-amd-disease-market
0 notes
Text
Optometry Equipment Market by Size, Share, Forecasts, & Trends Analysis
Meticulous Research®—a leading global market research company, published a research report titled ‘Optometry Equipment Market by Product (OCT Scan, Perimeter, Fundus Camera, Retinoscope, Keratometer, Ophthalmic Ultrasound, Tonometer, Slit Lamp, Chart Projector), Application (Cataract, Glaucoma, AMD), End User (Clinic, Hospital) - Global Forecast to 2029.’
Download Free sample report here: https://www.meticulousresearch.com/download-sample-report/cp_id=5406?utm_source=article&utm_medium=social+&utm_campaign=product&utm_content=18-04-2024
According to this latest publication from Meticulous Research®, the global optometry equipment market is projected to reach $5.4 billion by 2029, at a CAGR of 4% during the forecast period. The increasing cases of eye diseases and disorders, rising geriatric population, excessive use of laptops & mobile phones leading to visual impairments, and rising government initiatives and funding to improve access to eye care services are the factors driving the growth of this market.
Optometry Equipment Market: Future Outlook
The global optometry equipment market is segmented by Product Type (Retina & Glaucoma Examination Products [OCT Scanners, Perimeters, Fundus Cameras, Retinoscopes, Ophthalmoscopes, Ophthalmic Lasers & Ophthalmic Microscopes], Keratometers, Autorefractors, and Lensometers, Ophthalmic Ultrasound Systems & Digital Phoropters, Slit Lamps, Tonometers, Retinal Imaging Systems, General Examination Products, Chart Projectors, [Cataract & Cornea Examination Products, Specular Microscopes, Corneal Topography Systems, Wavefront Analyzers & Aberrometers, Optical Biometry Systems], Application (Cataract, Age-related Macular Degeneration, Glaucoma, Retinopathy, General Examination, Other Applications), End User (Hospitals, Clinics, Other End Users), and Geography. The study also evaluates industry competitors and analyzes their market shares at the country and regional levels.
Browse in depth @ https://www.meticulousresearch.com/product/optometry-equipment-market-5406?utm_source=article&utm_medium=social+&utm_campaign=product&utm_content=18-04-2024
Based on product type, in 2022, the retina & glaucoma examination products segment is expected to account for the largest share of the market. The large market share of this segment is attributed high penetration of retina examination products due to the high awareness of high-quality eye care and early and accurate diagnosis of common eye diseases such as macular degeneration, glaucoma, and diabetic retinopathy. The prevalence of diabetic retinopathy is rising globally, driving the demand for retina & glaucoma examination products.
Based on application, the optometry equipment market is segmented into cataract, age-related macular degeneration, glaucoma, retinopathy, general examination, and other applications. The general examination segment is projected to register the fastest growth rate over the forecast period. The growth of this segment is attributed to the growing geriatric population worldwide and the rising incidences of eye diseases primarily due to dependency on digital devices such as laptops, mobile phones, and others. General eye examinations help detect eye problems early when they can be treated most effectively. The awareness and importance of general eye examinations are high among all age groups, which is driving the growth of this market.
Key Players
The key players operating in the global optometry equipment market are NIDEK CO. LTD. (Japan), Carl Zeiss Meditec AG (Germany), Alcon (U.S.), Heidelberg Engineering GmbH (Germany), Johnson & Johnson (U.S.), Canon, Inc. (Japan), Bausch Health Companies Inc. (Canada), Escalon Medical Corp (U.S.), Topcon Healthcare Solutions, Inc. (U.S.), and HEINE Optotechnik GmbH & Co. KG (Germany).
Quick Buy: https://www.meticulousresearch.com/Checkout/36446863?utm_source=article&utm_medium=social+&utm_campaign=product&utm_content=18-04-2024
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
1 note
·
View note
Text
Medical Laser Market Forecast 2024-2031 Growth Drivers, Regional Outlook

The global medical laser market has been witnessing significant growth in recent years, driven by technological advancements, increasing prevalence of chronic diseases, and rising demand for minimally invasive surgical procedures. According to recent reports, the market size was valued at USD 5.0 billion in 2022 and is projected to reach USD 17.08 billion by 2030, with a compound annual growth rate (CAGR) of 16.6% during the forecast period of 2023-2030. This substantial growth underscores the growing importance of medical lasers in healthcare applications worldwide.
Key Drivers Propelling Growth
Several key factors are fueling the growth of the medical laser market:
Technological Advancements: Rapid advancements in laser technology have led to the development of more efficient and precise medical lasers. Innovations such as fiber optics, diode lasers, and solid-state lasers have expanded the capabilities of medical lasers, enabling a wide range of medical procedures with improved outcomes.
Rising Demand for Minimally Invasive Procedures: There is a growing preference for minimally invasive surgical procedures among patients and healthcare providers due to their benefits, including reduced trauma, faster recovery times, and shorter hospital stays. Medical lasers play a crucial role in various minimally invasive treatments, such as laser ablation, laser lithotripsy, and laser skin resurfacing, driving their adoption in healthcare facilities worldwide.
Increasing Prevalence of Chronic Diseases: The prevalence of chronic diseases such as cancer, cardiovascular diseases, and ophthalmic disorders is on the rise globally. Medical lasers are extensively used in the diagnosis, treatment, and management of these conditions, driving the demand for advanced laser-based medical devices.
Growing Aging Population: The aging population is more susceptible to age-related conditions and diseases, driving the demand for advanced medical interventions. Medical lasers offer precise and effective treatment options for age-related conditions such as cataracts, age-related macular degeneration (AMD), and skin aging, contributing to market growth.
Download Free Sample Report: https://www.snsinsider.com/sample-request/3020
Challenges and Considerations
Despite the promising growth prospects, the medical laser market faces certain challenges and considerations:
High Cost of Medical Laser Systems: The initial cost of acquiring medical laser systems can be prohibitive for some healthcare facilities, especially in emerging economies. Additionally, the maintenance and operating costs of medical lasers can also be substantial, limiting their adoption in certain healthcare settings.
Stringent Regulatory Requirements: The medical laser industry is subject to stringent regulatory requirements imposed by regulatory authorities such as the FDA (Food and Drug Administration) in the United States and the European Medicines Agency (EMA) in Europe. Compliance with these regulations adds complexity and time to the development and commercialization of medical laser devices.
Limited Reimbursement Policies: Reimbursement policies for laser-based medical procedures vary across different regions, impacting the adoption of medical lasers in healthcare settings. Limited reimbursement coverage for certain laser procedures may hinder market growth, especially in regions with underdeveloped healthcare infrastructure.
Key Takeaways from the Market
Growing Adoption of Laser-Based Aesthetic Procedures: The aesthetic segment is witnessing significant growth due to the increasing demand for laser-based skin rejuvenation, hair removal, and body contouring procedures. Advancements in laser technology have made these procedures safer, more effective, and minimally invasive, driving their popularity among consumers.
Expansion of Applications in Ophthalmology: Ophthalmology remains a key application area for medical lasers, with growing adoption in procedures such as LASIK (Laser-Assisted In Situ Keratomileusis) surgery, cataract surgery, and retinal photocoagulation. The development of innovative laser systems with improved precision and safety profiles is expanding the scope of laser-based ophthalmic treatments.
Increasing Focus on Emerging Markets: The medical laser market is witnessing significant expansion in emerging markets, driven by increasing healthcare expenditure, growing awareness about advanced medical treatments, and improving healthcare infrastructure. Companies are increasingly focusing on expanding their presence in regions such as Asia-Pacific, Latin America, and the Middle East to capitalize on untapped market opportunities.
In conclusion, the medical laser market is poised for remarkable growth in the coming years, driven by technological advancements, rising demand for minimally invasive procedures, and increasing prevalence of chronic diseases. However, challenges such as high costs and regulatory requirements need to be addressed to unlock the full potential of this dynamic market. As innovation continues to drive the development of advanced laser technologies, the medical laser market is expected to witness sustained growth and expansion, offering significant opportunities for industry players and healthcare stakeholders alike.
0 notes
Text
0 notes
Text
Medical Treatment With Monoclonal Antibody Therapies Will Drive Growth For EYLEA Drug Market

The global EYLEA drug market comprises medications used for treating various ocular disorders such as wet age-related macular degeneration (AMD), diabetic retinopathy (DR), and macular edema following retinal vein occlusion. EYLEA (aflibercept) drug is a vascular endothelial growth factor (VEGF) inhibitor used for wet AMD treatment approved by Food and Drug Administration (FDA) in 2011. It prevents growth of new blood vessels and leakage that can lead to vision loss. The drug also prevents further progression of the ocular disease.
The Global EYLEA Drug Market Size Is Estimated To Be Valued At US$ 8.79 Bn In 2024 And Is Expected To Exhibit A CAGR Of 7.2% Over The Forecast Period 2024-2031.
Key Takeaways
Key players operating in the global EYLEA drug market are Bayer, Regeneron Pharmaceuticals, Coherus Biosciences, Inc., And Klinge Biopharma. Bayer And Regeneron Pharmaceuticals Currently Dominate The Market With Their Product EYLEA. Coherus BioSciences also gained FDA approval for its aflibercept biosimilar referred as Zyla in 2021. Growing prevalence of retinal disorders such as AMD, DR and increasing patient diagnosis rate are expected to drive the market growth during the forecast period. Technological advancements in intravitreal injections and rise of biosimilars for affordable treatment options will further support the market expansion.
Get more insights on this topic: https://www.pressreleasebulletin.com/global-eylea-drug-market-trend-size-and-demand/
#Global EYLEA Drug Market Trend#Global EYLEA Drug Market Growth#Global EYLEA Drug Market Size#Global EYLEA Drug Market Analysis
0 notes
Text
Understanding the Dynamics of the Dry Age-related Macular Degeneration Market: Drivers, Barriers, and Future Outlook
Dry age-related macular degeneration (AMD) is a chronic eye condition characterized by the gradual deterioration of the macula, a small area near the center of the retina responsible for sharp, central vision. Dry AMD is the most common form of AMD, accounting for approximately 85-90% of all cases. Unlike wet AMD, which involves abnormal blood vessel growth beneath the macula, dry AMD typically progresses more slowly and is characterized by the accumulation of yellow deposits called drusen in the macula.
Dry Age-related Macular Degeneration Market Drivers
Aging Population: The aging population is a significant driver of the dry AMD market, as AMD primarily affects individuals over the age of 50. With demographic trends indicating a growing proportion of elderly individuals worldwide, the prevalence of dry AMD is expected to increase, driving demand for diagnostic services, treatments, and supportive care products.
Rising Disease Burden: Dry AMD is a leading cause of vision loss and blindness in older adults, contributing to a substantial disease burden and socioeconomic impact. As the prevalence of dry AMD continues to rise, particularly in developed countries with aging populations, there is an increasing need for effective management strategies to prevent disease progression and preserve vision.
Advancements in Diagnostic Technologies: Technological advancements in imaging modalities, such as optical coherence tomography (OCT), fundus autofluorescence (FAF), and adaptive optics imaging, have improved the early detection and monitoring of dry AMD. These non-invasive imaging techniques enable more accurate assessment of retinal changes, drusen morphology, and disease progression, facilitating timely intervention and personalized treatment approaches.
Research and Innovation: Ongoing research efforts aimed at elucidating the pathogenesis of dry AMD, identifying novel therapeutic targets, and developing innovative treatment modalities drive innovation in the dry AMD market. Research areas of interest include anti-inflammatory agents, neuroprotective compounds, stem cell therapy, gene therapy, and drug delivery systems designed to target specific pathways implicated in AMD pathophysiology.
Clinical Trial Activity: The increasing prevalence of dry AMD and the need for effective treatment options have led to a surge in clinical trial activity focused on evaluating investigational therapies for dry AMD. Pharmaceutical companies, biotechnology firms, academic institutions, and government agencies are conducting clinical trials to assess the safety, efficacy, and tolerability of novel drugs, biologics, gene therapies, and cell-based interventions for dry AMD.
Regulatory Support and Incentives: Regulatory agencies provide support and incentives to expedite the development and approval of new treatments for dry AMD. Designations such as orphan drug status, fast track designation, breakthrough therapy designation, and priority review designation streamline the regulatory review process and accelerate market access for promising therapies targeting unmet medical needs in dry AMD.
Patient Advocacy and Awareness: Patient advocacy organizations and support groups play a crucial role in raising awareness about dry AMD, educating patients and caregivers, and advocating for improved access to treatment and supportive care services. Increased awareness of the importance of early detection, regular eye exams, and adherence to treatment regimens promotes proactive management of dry AMD and enhances patient outcomes.
Healthcare Infrastructure and Access to Care: Access to comprehensive eye care services, including retinal specialists, low vision rehabilitation programs, and low vision aids, is essential for effectively managing dry AMD and optimizing visual function. Investments in healthcare infrastructure, telemedicine platforms, and community-based outreach programs expand access to eye care services, particularly in underserved areas with limited access to specialty care.
Market Competition and Collaboration: Competition among pharmaceutical companies, biotechnology firms, and medical device manufacturers drives innovation and investment in the dry AMD market. Collaborations, partnerships, and licensing agreements between industry players facilitate the development and commercialization of novel therapies, diagnostic technologies, and supportive care products, enhancing market competitiveness and diversifying treatment options for patients.
Reimbursement Landscape: Reimbursement policies, coverage decisions, and pricing strategies influence market dynamics and access to dry AMD treatments. Payer reimbursement for diagnostic tests, treatments, and supportive care services impacts patient access and affordability, driving market adoption and utilization of approved therapies.
Dry Age-related Macular Degeneration Market Barriers
Despite the significant progress in understanding dry age-related macular degeneration (AMD) and developing treatments, several barriers impede the effective management and commercialization of therapies in the dry AMD market. Here are some of the key barriers:
Limited Treatment Options: Compared to wet AMD, there are fewer approved treatment options for dry AMD. Currently, there is no cure for dry AMD, and available treatments mainly focus on slowing disease progression rather than reversing vision loss. The lack of effective pharmacological interventions targeting the underlying mechanisms of dry AMD represents a significant barrier to addressing unmet medical needs in this patient population.
Complexity of Disease Pathophysiology: Dry AMD is a multifactorial disease with complex pathophysiology involving interactions between genetic, environmental, and lifestyle factors. The heterogeneous nature of dry AMD presents challenges for developing targeted therapies that address the diverse underlying mechanisms contributing to disease progression. Understanding the underlying pathophysiological processes and identifying effective therapeutic targets require further research and preclinical validation.
Difficulty in Early Detection and Diagnosis: Early detection and diagnosis of dry AMD are crucial for implementing timely interventions and preserving vision. However, early-stage dry AMD may be asymptomatic or present with subtle visual changes that are challenging to detect using conventional screening methods. Limited access to advanced diagnostic technologies, such as optical coherence tomography (OCT) and fundus autofluorescence (FAF), in primary care settings may delay diagnosis and initiation of treatment.
Lack of Biomarkers for Disease Progression: Biomarkers that reliably predict disease progression and treatment response in dry AMD are currently lacking. The absence of validated biomarkers hinders risk stratification, patient selection for clinical trials, and monitoring of treatment efficacy. Biomarker discovery efforts focusing on identifying molecular, genetic, and imaging-based markers associated with disease progression and treatment response are ongoing but face challenges in reproducibility and validation.
High Development Costs and Long Regulatory Pathways: Developing novel therapies for dry AMD involves substantial investment in research and development, preclinical studies, clinical trials, and regulatory approval processes. The high development costs and lengthy regulatory pathways associated with bringing new drugs to market pose financial barriers for small biotechnology firms and academic researchers. Additionally, uncertainties regarding regulatory requirements and endpoints for clinical trials in dry AMD may prolong the development timeline and increase the risk of clinical trial failure.
Limited Patient Access to Care and Treatment: Access to specialized eye care services, retinal specialists, and advanced treatments for dry AMD may be limited, particularly in rural or underserved areas. Geographic disparities in access to care, socioeconomic barriers, and lack of insurance coverage may prevent some patients from receiving timely diagnosis, treatment, and follow-up care. Improving access to eye care services through telemedicine, community outreach programs, and collaborative care models is essential for addressing disparities in patient outcomes.
Challenges in Patient Recruitment for Clinical Trials: Recruiting and retaining participants for clinical trials in dry AMD can be challenging due to the relatively low prevalence of the disease, stringent eligibility criteria, and competition among clinical trial sponsors. Enrolling a diverse patient population that reflects the heterogeneity of dry AMD and ensuring adequate representation of underrepresented groups (e.g., minorities, older adults) are critical for generalizing trial results and advancing evidence-based practice.
Regulatory and Reimbursement Challenges: Navigating complex regulatory pathways and securing reimbursement for novel therapies in dry AMD pose significant challenges for drug developers and manufacturers. Variability in regulatory requirements across jurisdictions, evolving evidentiary standards, and uncertainty regarding reimbursement coverage and pricing may deter investment in dry AMD drug development. Addressing regulatory and reimbursement challenges requires collaboration among industry stakeholders, regulatory agencies, payers, and patient advocacy groups to streamline approval processes and ensure timely access to innovative therapies.
Future Dry Age-related Macular Degeneration Market Analysis
Analyzing the future of the dry age-related macular degeneration (AMD) market involves considering emerging trends, technological advancements, regulatory developments, and evolving healthcare landscapes. Here's a prospective analysis of the future dry AMD market:
Growing Disease Burden: With the aging population and increasing life expectancy, the prevalence of dry AMD is expected to rise, leading to a growing disease burden and greater demand for effective management strategies. As a result, there will be an increased focus on research, diagnosis, and treatment options to address the needs of individuals with dry AMD.
Advancements in Diagnostic Technologies: Technological innovations in imaging modalities, such as optical coherence tomography (OCT), fundus autofluorescence (FAF), and adaptive optics imaging, will continue to improve the early detection, diagnosis, and monitoring of dry AMD. These advancements will enable more accurate assessment of disease progression, facilitate personalized treatment approaches, and support clinical decision-making.
Precision Medicine Approaches: Advances in genetics, molecular profiling, and precision medicine will enable personalized approaches to dry AMD management. Biomarker discovery efforts and genetic testing may identify individuals at higher risk of disease progression or with specific genetic subtypes of dry AMD, guiding treatment selection and prognosis prediction.
Emerging Therapeutic Modalities: Research into novel therapeutic modalities for dry AMD, including gene therapy, cell-based therapies, and regenerative medicine approaches, will continue to advance. Preclinical and clinical studies exploring the potential of gene editing technologies, stem cell transplantation, and neuroprotective agents aim to address the underlying mechanisms of dry AMD and provide disease-modifying treatments.
Combination Therapies: Combination therapies targeting multiple pathways involved in dry AMD pathogenesis may offer synergistic effects and improved treatment outcomes. Combinations of anti-inflammatory agents, neuroprotective compounds, angiogenesis inhibitors, and immunomodulatory drugs could provide additive or complementary effects, slowing disease progression and preserving vision in patients with dry AMD.
Digital Health Solutions: Digital health solutions, including telemedicine platforms, remote monitoring devices, and mobile applications, will play an increasingly important role in dry AMD management. These technologies enable remote patient monitoring, facilitate home-based vision testing, support patient education and self-management, and enhance communication between patients and healthcare providers.
Regulatory Support for Innovation: Regulatory agencies will continue to provide support and incentives to expedite the development and approval of innovative therapies for dry AMD. Designations such as orphan drug status, fast track designation, breakthrough therapy designation, and priority review designation will accelerate the regulatory review process for promising therapies targeting unmet medical needs in dry AMD.
Healthcare Integration and Access to Care: Integration of eye care services into primary care settings, multidisciplinary care teams, and collaborative care models will improve access to comprehensive care for individuals with dry AMD. Coordinated efforts among ophthalmologists, optometrists, retinal specialists, and primary care providers will optimize patient outcomes and ensure timely diagnosis and treatment.
Patient-Centered Care and Advocacy: Patient advocacy organizations and support groups will continue to play a vital role in raising awareness, promoting education, and advocating for the needs of individuals with dry AMD. Empowering patients, caregivers, and families through education, peer support networks, and access to resources will enhance patient-centered care and improve quality of life.
Economic and Market Dynamics: Economic factors, market competition, and healthcare policies will influence the commercialization and adoption of new treatments for dry AMD. Pricing strategies, reimbursement policies, and market access considerations will impact the availability and affordability of innovative therapies, shaping market dynamics and patient access to care.
Evolving Dry Age-related Macular Degeneration Treatment Outlook
The evolving treatment outlook for dry age-related macular degeneration (AMD) involves a multifaceted approach encompassing advancements in diagnostics, pharmacotherapy, regenerative medicine, and supportive care. Here's an overview of the evolving landscape of dry AMD treatment:
Diagnostics and Early Intervention: Advances in diagnostic imaging technologies, such as optical coherence tomography (OCT), fundus autofluorescence (FAF), and adaptive optics imaging, enable earlier detection and more precise monitoring of dry AMD. Early intervention strategies aim to identify high-risk individuals, detect disease progression, and initiate treatment before irreversible vision loss occurs.
Nutritional Supplements: Dietary supplementation with specific vitamins and minerals, such as vitamins C and E, zinc, copper, lutein, zeaxanthin, and omega-3 fatty acids, has been shown to slow the progression of dry AMD in certain patient populations. Research continues to explore the optimal formulation, dosing regimen, and long-term efficacy of nutritional supplements in preserving vision and reducing the risk of advanced AMD.
Anti-inflammatory Agents: Chronic inflammation plays a key role in the pathogenesis of dry AMD, making anti-inflammatory agents potential therapeutic targets. Drugs targeting inflammatory mediators, such as complement inhibitors, corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and immunomodulators, aim to suppress retinal inflammation, reduce drusen formation, and prevent disease progression.
Neuroprotective and Anti-oxidant Therapies: Neuroprotective agents and antioxidants may help preserve retinal function and mitigate oxidative stress-induced damage in dry AMD. Compounds such as ciliary neurotrophic factor (CNTF), pigment epithelium-derived factor (PEDF), resveratrol, and coenzyme Q10 have shown neuroprotective effects in preclinical studies and clinical trials, offering potential therapeutic benefits for preserving photoreceptor and retinal pigment epithelial (RPE) cell function.
Angiogenesis Inhibitors: While abnormal blood vessel growth (neovascularization) is characteristic of wet AMD, emerging evidence suggests that angiogenic factors may also contribute to the pathogenesis of dry AMD. Anti-angiogenic agents targeting vascular endothelial growth factor (VEGF), such as aflibercept and ranibizumab, have shown promise in slowing disease progression and reducing geographic atrophy (GA) growth in certain subtypes of dry AMD.
Cell-Based Therapies: Regenerative medicine approaches using cell-based therapies, including stem cell transplantation, retinal pigment epithelial (RPE) cell replacement, and induced pluripotent stem cells (iPSCs), hold promise for repairing damaged retinal tissue and restoring vision in dry AMD. Clinical trials investigating the safety and efficacy of cell-based therapies are underway, with the goal of developing regenerative treatments for advanced dry AMD.
Gene Therapy and Genetic Targeting: Gene therapy strategies aim to correct genetic mutations associated with dry AMD, modulate gene expression, and restore normal cellular function in the retina. Techniques such as gene editing, RNA interference (RNAi), and viral vector delivery systems enable targeted delivery of therapeutic genes to retinal cells, offering potential disease-modifying effects and long-term benefits for individuals with genetic forms of dry AMD.
Drug Delivery Systems: Innovative drug delivery systems, such as sustained-release implants, nanoparticles, microparticles, and hydrogels, enhance the localized delivery of therapeutic agents to the retina, prolonging drug release and reducing treatment frequency. These drug delivery platforms improve treatment efficacy, minimize side effects, and optimize patient compliance in dry AMD management.
Combination Therapies and Multimodal Approaches: Combining multiple therapeutic modalities, such as anti-inflammatory agents, neuroprotective agents, and nutritional supplements, may offer synergistic effects and improved outcomes in dry AMD treatment. Multimodal approaches integrating pharmacotherapy, regenerative medicine, and supportive care aim to address the complex pathophysiology of dry AMD and optimize visual function.
Patient-Centered Care and Supportive Services: Patient-centered care models, low vision rehabilitation programs, and supportive services play a critical role in addressing the psychosocial impact of vision loss and optimizing patient outcomes in dry AMD. Low vision aids, adaptive technologies, vision rehabilitation therapy, and psychosocial support programs help individuals with dry AMD maximize their remaining vision, maintain independence, and improve quality of life.
Role of Companies in the Dry Age-related Macular Degeneration Market
In the Dry Age-related Macular Degeneration market, companies such as Alkeus Pharmaceuticals, Novartis, Molecular Partners, Stealth BioTherapeutics, Regenerative Patch Technologies, Aevitas Therapeutics, NGM Biopharmaceuticals, InflammX Therapeutics, Lineage Cell Therapeutics, Alexion AstraZeneca Rare Disease, Belite Bio, Katairo, Cognition Therapeutics, Apellis Pharmaceuticals, Galimedix Therapeutics, Amarna Therapeutics, 4D Molecular Therapeutics, Aviceda Therapeutics, Isarna Therapeutics, and others play a pivotal role in driving innovation, research, development, and the provision of treatments and therapies for individuals suffering from this chronic inflammatory skin condition. These companies encompass pharmaceutical giants, biotechnology firms, medical device manufacturers, and healthcare service providers, each contributing uniquely to the advancement of Dry Age-related Macular Degeneration management. Pharmaceutical companies lead the charge in developing novel drugs, ranging from topical corticosteroids to biologics targeting specific immune pathways implicated in Dry Age-related Macular Degeneration pathogenesis.
Dry Age-related Macular Degeneration Market Outlook - Key Conclusion and Analysis
The Dry Age-related Macular Degeneration market is undergoing a transformative period, driven by advances in research, innovation in therapeutic approaches, and shifting treatment paradigms. While significant progress has been made in improving outcomes for patients with Dry Age-related Macular Degeneration, several barriers continue to challenge the market's expansion, including high treatment costs, safety concerns, and regulatory hurdles. Looking ahead, personalized medicine, novel therapeutic targets, and digital health solutions are poised to shape the future of Dry Age-related Macular Degeneration management, offering new hope for patients and caregivers alike. Efforts to address these challenges and capitalize on emerging opportunities will be critical in advancing the field and ultimately improving the lives of individuals living with Dry Age-related Macular Degeneration.
Get a more detailed overview, at: Dry Age-related Macular Degeneration Market Outlook and Forecast
#Dry Age-related Macular Degeneration market#Dry Age-related Macular Degeneration#Dry Age-related Macular Degeneration market share#Dry Age-related Macular Degeneration treatment market#Dry Age-related Macular Degeneration market size
0 notes
Text
0 notes
Text
New Real World Evidence platform extracts data from Electronic Health Records in minutes
[Birmingham] A new piece of software has been launched, that extracts and analyses datasets from Electronic Health Record (EHR) data in near real-time.
Dexter is a spin out from the University of Birmingham and has been developed over a decade of research and development to overcome historic issues surrounding Real World Data (RWD) such as reproducibility, biases, and data quality in health data research.
The proprietary technology utilises Electronic Health Record (EHR) data in near real-time, extracts analysable datasets and delivers insights to research organisations (academic and commercial), healthcare organisations and pharmaceutical firms. Outputs that traditionally take months, can be achieved in minutes.
Dexter has already been deployed for research in secure data environments and hospitals and has assisted in over 120 scientific publications including Nature Medicine and the British Medical Journal.
Notable projects include a data-driven randomised controlled trial in gestational diabetes funded by National Institute for Health Research in collaboration with Cegedim; Real-world evidence research for a number of pharmaceutical companies such as Vifor Pharma and AstraZeneca; Drug repurposing to identify new therapeutic options for Age -related Macular Degeneration (AMD) in collaboration with Action Against AMD, and a long-covid study in collaboration with CPRD and Aparito.
Dexter has developed close relationships with national and international data providers providing access to over 100 million electronic health records and is currently supporting over £25 million worth of grants on a wide range of projects.
Professor Krish Nirantharakumar, Co-Founder of Dexter says: “The power of Dexter and its ability to deliver actionable insights from a wide variety of real-world data (RWD) sources is a huge turning point for the healthcare sector. Our technology will be a critical enabler throughout clinical development, market access, post-market surveillance, care delivery and population health.”
To find out more visit https://dexter.software/
About Dexter
Dexter is a game changing software platform designed for healthcare and health data research. It utilises Electronic Health Record (EHR) data in near real-time, extracts analysable datasets and produces results based on your study design in minutes. Dexter was created with an aim to expedite epidemiological research. Since its introduction in mid-2016, Dexter has worked as a ‘one-stop-shop’ for healthcare organisations who are interested in working with EHRs.
0 notes
Text
Ophthalmic Drugs Market Size To Reach $66.06 Billion By 2030
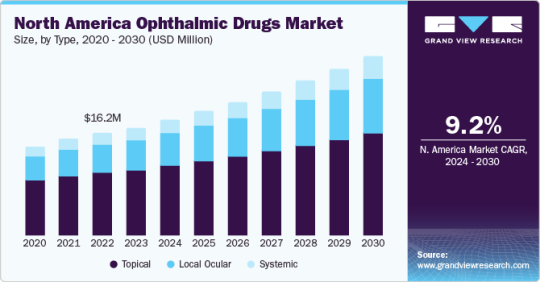
Ophthalmic Drugs Market Growth & Trends
The global ophthalmic drugs market size is expected to reach USD 65.55 billion by 2030, garnering a CAGR of 7.80% from 2023 to 2030, according to a new report by Grand View Research, Inc. The rising awareness about ophthalmic disorders and the surge in demand for ophthalmic drugs are the factors propelling the market growth. Moreover, an increase in the prevalence of eye diseases such as age-related macular degeneration, cataract, dry eye, diabetic retinopathy, glaucoma, and others are anticipated to boost the demand for ophthalmic drugs.
According to BrightFocus Foundation in 2021, it is estimated that over 3 million Americans are living with glaucoma, and, among them over 2.7 million patients were affected by the most common type of glaucoma i.e. open-angle glaucoma. Moreover, 3.3 million people are affected by blindness and low vision in the U.S. Some of the major risk factors for glaucoma and blindness are, higher age, family history, thin cornea, and high eye pressure. This creates a wide population base for ophthalmic drugs, thereby, ensuring lucrative growth.
Furthermore, ocular therapeutic and drug delivery companies are receiving funding to expedite R&D for advancements in technologies to develop novel drugs, which is likely to fuel market growth. For instance, in June 2020, Re-Vana Therapeutics received USD 3.25 million in pre-series A funding from Qubis, ExSight Ventures, LLC, Clarendon Fund Managers, Visionary Ventures, and Techstart Ventures LLP. The funding is expected to boost the development of Re-Vana Therapeutics’ EyeLief & OcuLief biodegradable technologies that can be used for a small molecule as well as biologics delivery.
Moreover, the rising geriatric population is the key driver for the ophthalmic drugs market growth. According to Safe Eyes America, the most common risk factors for ophthalmic diseases are high age, family history, diabetes, and high blood pressure. For instance, as per AMERICA’s HEALTH RANKINGS senior report 2021 it was estimated that more than 54 million adults of age more than 65 live in the U.S., and that population accounted for 16.5% of the total population. According to Macular Degeneration Research AMD is the leading reason for blindness in U.S. people over the age of 60.
Ongoing regulatory approvals for ophthalmic products are likely to support market growth. For instance, in June 2022 Santen Pharmaceutical Co., Ltd announced that it has got marketing and manufacturing approval for the dry eye disease treatment DIQUAS LX ophthalmic solution in Japan. This solution is the improved formulation that has reduced dosing frequency and improved patient compliance.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/ophthalmic-therapeutics-drug-market
Ophthalmic Drugs Market Report Highlights
Anti-VEGF agents segment held the largest share in 2022 owing to their rising demand and lesser adverse effects
Retinal disorders held the largest market share in 2022 owing to the rising prevalence of age-related macular degeneration and diabetic retinopathy
The topical route of administration dominated the market in 2022 owing to factors such as the benefits of the topical route as it delivers the drug to the targeted site and ease of use
The eye drops segment is expected to hold the largest share in 2022 owing to rising demand for eye drops, high patient compliance, and availability of OTC products
Asia Pacific is expected to exhibit the fastest growth over the forecast period due to advancements in healthcare infrastructure, increasing demand for eye products, and a large patient pool
Ophthalmic Drugs Market Segmentation
Grand View Research has segmented the global ophthalmic drugs market report based on drug class, disease, route of administration, dosage type, product type, product, and region:
Ophthalmic Drugs Drug Class Outlook (Revenue, USD Billion, 2018 - 2030)
Anti-allergy
Anti-inflammatory
Non-steroidal drugs
Steroidal drugs
Anti-VEGF Agents
Anti-glaucoma
Others
Ophthalmic Drugs Disease Outlook (Revenue, USD Billion, 2018 - 2030)
Dry Eye
Gels
Eye Solutions & Suspensions
Capsules & Tablets
Eye Drops
Ointments
Allergies
Gels
Eye Solutions & Suspensions
Capsules & Tablets
Eye Drops
Ointments
Glaucoma
Gels
Eye Solutions & Suspensions
Capsules & Tablets
Eye Drops
Ointments
Eye Infection
Gels
Eye Solutions & Suspensions
Capsules & Tablets
Eye Drops
Ointments
Infection
Gels
Eye Solutions & Suspensions
Capsules & Tablets
Eye Drops
Ointments
Retinal Disorders
Retinal Disorder Treatment Market, By Type,
Macular Degeneration
Diabetic Retinopathy
Retinal Disorder Treatment Market, By Dosage Type,
Gels
Eye Solutions & Suspensions
Capsules & Tablets
Eye Drops
Ointments
Uveitis
Gels
Eye Solutions & Suspensions
Capsules & Tablets
Eye Drops
Ointments
Others
Ophthalmic Drugs Dosage Form Outlook (Revenue, USD Billion, 2018 - 2030)
Gels
Eye Solutions & Suspensions
Capsules and Tablets
Eye Drops
Ointments
Ophthalmic Drugs Route of Administration Outlook (Revenue, USD Billion, 2018 - 2030)
Topical
Retinal Disorders
Subconjunctival
Intravitreal
Retrobulbar
Intracameral
Local Ocular
Systemic
Ophthalmic Drugs Product Type Outlook (Revenue, USD Billion, 2018 - 2030)
Prescription Drugs
OTC
Ophthalmic Drugs Product Outlook (Revenue, USD Billion, 2018 - 2030)
Branded Drugs
Generic Drugs
Ophthalmic Drugs Regional Outlook (Revenue, USD Billion, 2018- 2030)
North America
S.
Canada
Europe
Germany
K.
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Australia
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East and Africa (MEA)
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players of Ophthalmic Drugs Market
Pfizer Inc.
Alcon
Novartis AG
Bausch Health Companies Inc.
Merck & Co., Inc
Regeneron Pharmaceuticals Inc
Allergan (AbbVie Inc)
Bayer AG
Genentech, Inc. (F. Hoffmann-La Roche Ltd)
Nicox
Coherus Biosciences, Inc.
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/ophthalmic-therapeutics-drug-market
#Ophthalmic Drugs Market#Ophthalmic Drugs Market Size#Ophthalmic Drugs Market Share#Ophthalmic Drugs Market Trends
0 notes
Link
#market research future#age-related macular#age macular degeneration#macular degeneration market#dry age-related macular
0 notes
Text
Ophthalmic Drugs Market | Revolutionizing Eye-Care Solutions

The prevalence of eye diseases globally is staggering, with the WHO reporting that at least 2.2 billion people have near or distant vision impairment. In about half of these cases, vision impairment can be addressed with the use of ophthalmic drugs. Highlighting this massive opportunity, Triton’s analysts predict that the Global Ophthalmic Drugs Market is set to advance at a CAGR of 6.64% in 2023-2030.
Ophthalmic medications are essential for treating eye conditions, ranging from dry eye to severe ailments like glaucoma. Moreover, innovations like telemedicine and digital health solutions in ophthalmic drug delivery are gaining traction across the Asia-Pacific region. These advancements particularly address accessibility issues in remote areas. Accordingly, our research estimates that the Asia-Pacific Ophthalmic Drugs Market is likely to grow at 7.56% CAGR over 2023-2030.
Explore in detail about this market in our FREE sample: https://www.tritonmarketresearch.com/reports/ophthalmic-drugs-market#request-free-sample
Ophthalmic Drugs Market: Magnitude of Eye-Related Diseases
North America, especially the US, is a major market for ophthalmic drugs due to the high prevalence of common eye disorders.
For instance,
As per Genetech, a biotechnology company, nearly 11 million people in the US have some form of age-related macular degeneration (AMD), with about 200,000 new cases of wet AMD diagnosed annually across the country.
According to Centres for Disease Control & Prevention, around 9.6 million Americans were living with diabetic retinopathy (DR) in 2021.
The International Agency for the Prevention of Blindness estimated that globally, at least 450 million children have a sight condition that needs treatment, with 90 million children living with refractive error or some form of sight loss.
Thus, the extent of eye-related diseases globally creates a massive scope for the ophthalmic drugs market, driven by an increasing prevalence of conditions such as age-related macular degeneration, blindness and vision impairment, glaucoma, diabetic retinopathy, etc.
Moreover, with longer exposure to mobile screens and laptops from a young age, a growing burden of eye diseases is inevitable. Hence, early diagnosis and treatment are crucial in preventing long-term vision impairment in children, leading to an increased focus on specialized ophthalmic drugs tailored for younger as well as old patients.
Ophthalmic Drugs Market: Scope for Developing Innovative Products
With millions affected by eye disorders, there is a need to increase R&D activities with the approval and launch of novel therapies. In fact, the focus on biologics and gene therapy presents new avenues for addressing eye diseases at their root cause. These promising treatments offer long-term solutions rather than symptomatic relief.
Technological advancements in ophthalmic drug delivery systems, such as gels and eye drops, are revolutionizing patient care. For instance, gels that provide sustained drug release reduce the need for frequent application, enhancing treatment adherence.
Similarly, eye drops have seen innovations like multi-dose dispensers with antimicrobial features, improving safety and convenience for users. These are not only the most convenient and widely adopted delivery methods but also offer less discomfort and irritation. Strikingly, eye drops have become the fastest-growing component, with a CAGR of 6.83% over the forecast years 2023-2030.
These advancements have a profound impact on the ophthalmic drug market, making treatments more accessible, thereby improving the quality of life for patients. For example, in recent years, significant advancements have been made in gene and cell therapies, offering new therapeutic options for treating eye diseases.
Ophthalmic Drugs Market: Advancements in Drug Delivery
Innovations such as nanoparticle-based eye drops and preservative-free formulations are leading the market growth.
January 2022 - Alcon introduced ‘SYSTANE COMPLETE’ Preservative-Free eye drops, an innovation offering an all-in-one solution for all types of dry eye.
July 2022 - Clearside Biomedical developed a microneedle and injector for suprachoroidal injection of corticosteroid triamcinolone acetonide (CLS-TA), targeting conditions like macular edema associated with noninfectious uveitis.
Current/Ongoing - ABBV-RGX-314 is being developed as a potential one-time treatment for wet AMD, diabetic retinopathy and other additional chronic retinal conditions treated with anti-VEGF. It is expected to finish the trial in 2025.
As the field of ophthalmology continues to evolve, further integration of advanced technologies is empowering manufacturers to expand the possibilities within ophthalmic drug products. By improving drug delivery methods and harnessing gene therapy’s potential, treatment for eye diseases among older patients can become more effective, less invasive, and potentially curative. Along with these innovations, the demand for eye drops, as a dosage form, is expected to dominate the ophthalmic drugs market. These advancements not only promise better outcomes for young patients but also fuel market growth.
Grab a quick read to understand the key insights about the ophthalmic drugs market: https://www.tritonmarketresearch.com/pressrelease-details/ophthalmic-drugs-market-key-insights
FAQs
Q.1 How big is the ophthalmic drugs market?
The global ophthalmic drugs market generated $34547.84 million in 2022 and is expected to achieve $58681.41 million in 2030.
Q.2 What are antifungal ophthalmic drugs?
Antifungal ophthalmic drugs such as fluconazole, amphotericin B, voriconazole, etc., are used for treating severe eye infections.
0 notes
Text
Ophthalmology is a branch of medicine that deals with the anatomy, physiology, treatment and surgery of the visual pathways of the eye, including surrounding areas and the visual aspects of the brain. Ophthalmic devices are medical equipment designed for surgery, diagnosis, and vision correction. The devices gained increased importance and adoption due to the high prevalence of several ophthalmic diseases, such as glaucoma, cataract, and other vision-related issues. Ophthalmic drugs are medications that are specially designed and administered for the treatment of various eye-related disorders, including color blindness, diabetic macular edema, cytomegalovirus (CMV), and age-related macular degeneration (AMD).
0 notes
Text
Retinal Drugs Market - Forecast and Analysis, 2023-2027
Originally published on Technavio: Retinal Drugs Market by Distribution Channel, Indication, and Geography - Forecast and Analysis 2023-2027
The Retinal Drugs Market is expected to witness substantial growth from 2023 to 2027, fueled by the rising prevalence of retinal diseases and the development of innovative treatment options. This market analysis encompasses a comprehensive evaluation of distribution channels, indications, and geographical regions.
Distribution channels in the retinal drugs market include hospital pharmacies, retail pharmacies, and online pharmacies. Each of these channels plays a crucial role in ensuring the accessibility and availability of retinal drugs to patients. Hospital pharmacies are expected to dominate the market, driven by the high volume of patients seeking treatment for retinal diseases in hospital settings. However, retail pharmacies and online pharmacies are gaining traction due to their convenience and accessibility, especially for patients requiring long-term medication management.
Indications for retinal drugs encompass a range of conditions affecting the retina, including age-related macular degeneration (AMD), diabetic retinopathy, retinal vein occlusion, and others. With the aging population and increasing prevalence of diabetes worldwide, the incidence of retinal diseases is on the rise, driving demand for effective treatment options. Pharmaceutical companies are investing in the development of novel drugs targeting specific retinal conditions to address unmet medical needs and improve patient outcomes.
Geographically, the retinal drugs market covers key regions such as North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America, particularly the United States, is expected to lead the market during the forecast period, driven by factors such as advanced healthcare infrastructure, high prevalence of retinal diseases, and favorable reimbursement policies. Europe is also anticipated to be a significant market for retinal drugs, supported by increasing healthcare expenditure and growing awareness about retinal health.
The Asia Pacific region is poised for rapid growth in the retinal drugs market, driven by factors such as rising geriatric population, increasing incidence of diabetes, and improving access to healthcare in emerging economies. Countries like China, India, and Japan are witnessing increasing investments in healthcare infrastructure and research and development, creating opportunities for pharmaceutical companies to expand their presence in the region.
Latin America and the Middle East and Africa regions are also expected to witness growth in the retinal drugs market, fueled by improving healthcare infrastructure, rising awareness about retinal diseases, and increasing healthcare expenditure. However, challenges such as limited access to healthcare services and affordability issues may constrain market growth in these regions.
To Learn deeper into this report , View Sample PDF
Overall, the forecast period from 2023 to 2027 presents significant opportunities for players in the retinal drugs market to capitalize on the growing demand for effective treatment options for retinal diseases worldwide. Innovative drug development, strategic partnerships, and expansion into emerging markets are expected to drive market growth and enhance patient care.
For more information please contact.
0 notes
Text
Age Related Macular Degeneration Market Projected to Show Strong Growth

Worldwide Age Related Macular Degeneration Market In-depth Research Report 2023, Forecast to 2028 is latest research study released by AMA evaluating the market risk side analysis, highlighting opportunities and leveraged with strategic and tactical decision-making support. The report provides information on market trends and development, growth drivers, technologies, and the changing investment structure of the Worldwide Age Related Macular Degeneration Market. Some of the key players profiled in the study are Novartis Ag (Switzerland), F. Hoffmann-La Roche Ltd (Switzerland), Bayer AG (Germany), Pfizer Inc. (United States), Regeneron Pharmaceutical, Inc. (United States), Acucela (United States), Neurotech Pharmaceuticals, Inc. (United States), GlaxoSmithKline Plc (United Kingdom), Santen Pharmaceutical Co. Ltd. (Japan), Synthetic Biologics Inc. (United States)
Get Free Access to Sample Report @ https://www.advancemarketanalytics.com/sample-report/37693-global-age-related-macular-degeneration-market
The age-related macular degeneration (AMD) is a common eye complaint and a leading cause of vision loss among people age 50 and older. It causes damage to the macula, a minor spot near the center of the retina and the portion of the eye needed for sharp, central vision, which lets a person see objects that are straight ahead. In some people, AMD develops so slowly that vision loss does not occur for a long time. In others, the disease advancements faster and may lead to a loss of vision in one or both eyes. As AMD progresses, a blurred area near the center of vision is a general symptom.
Influencing Market Trend
Increasing Awareness about Age-Related Macular Degeneration
Market Drivers
Increase in Aging Population Worldwide
Rising Pipeline Drugs for Age-Related Macular Degeneration
Opportunities:
Increasing Incidence of Lifestyle Associated Diseases
Challenges:
Growing Off Label Use
For more data or any query mail at [email protected] for customization in Report @ https://www.advancemarketanalytics.com/enquiry-before-buy/37693-global-age-related-macular-degeneration-market
Furthermore, the years considered for the study are as follows:
Historical year – 2017-2021
Base year – 2021
Forecast period** – 2022 to 2027 [** unless otherwise stated]
Highlighted of Global Age Related Macular Degeneration Market Segments and Sub-Segment: Market by Key Players: Novartis Ag (Switzerland), F. Hoffmann-La Roche Ltd (Switzerland), Bayer AG (Germany), Pfizer Inc. (United States), Regeneron Pharmaceutical, Inc. (United States), Acucela (United States), Neurotech Pharmaceuticals, Inc. (United States), GlaxoSmithKline Plc (United Kingdom), Santen Pharmaceutical Co. Ltd. (Japan), Synthetic Biologics Inc. (United States)Market by: by Type (Wet AMD, Dry AMD), Drugs (Lucentis, Eylea, Avastin, Others), Route of Administration (Intravenous, Intravitreal)
Regional Analysis for Worldwide Age Related Macular Degeneration Market:
• APAC (Japan, China, South Korea, Australia, India, and Rest of APAC; Rest of APAC is further segmented into Malaysia, Singapore, Indonesia, Thailand, New Zealand, Vietnam, and Sri Lanka)
• Europe (Germany, UK, France, Spain, Italy, Russia, Rest of Europe; Rest of Europe is further segmented into Belgium, Denmark, Austria, Norway, Sweden, The Netherlands, Poland, Czech Republic, Slovakia, Hungary, and Romania)
• North America (U.S., Canada, and Mexico)
• South America (Brazil, Chile, Argentina, Rest of South America)
• MEA (Saudi Arabia, UAE, South Africa)The study is a source of reliable data on: Market segments and sub-segments, Market trends and dynamics Supply and demand Market size Current trends/opportunities/challenges Competitive landscape Technological innovations Value chain and investor analysis.
Buy the Full Research report of Global Age Related Macular Degeneration Market@: https://www.advancemarketanalytics.com/buy-now?format=1&report=37693
Key Growths in the Market: This section of the report incorporates the essential enhancements of the marker that contains assertions, coordinated efforts, R&D, new item dispatch, joint ventures, and associations of leading participants working in the market.Key Points in the Market: The key features of this Age Related Macular Degeneration market report includes production, production rate, revenue, price, cost, market share, capacity, capacity utilization rate, import/export, supply/demand, and gross margin. Key market dynamics plus market segments and sub-segments are covered.
FIVE FORCES & PESTLE ANALYSIS:
In order to better understand market conditions five forces analysis is conducted that includes the Bargaining power of buyers, Bargaining power of suppliers, Threat of new entrants, Threat of substitutes, and Threat of rivalry.
• Political (Political policy and stability as well as trade, fiscal, and taxation policies)
• Economical (Interest rates, employment or unemployment rates, raw material costs, and foreign exchange rates)
• Social (Changing family demographics, education levels, cultural trends, attitude changes, and changes in lifestyles)
• Technological (Changes in digital or mobile technology, automation, research, and development)
• Legal (Employment legislation, consumer law, health, and safety, international as well as trade regulation and restrictions)
• Environmental (Climate, recycling procedures, carbon footprint, waste disposal, and sustainability)
Examine Detailed Index of full Research Study at@: https://www.advancemarketanalytics.com/reports/37693-global-age-related-macular-degeneration-market
Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Europe or Asia.
Contact US :
Craig Francis (PR & Marketing Manager)
AMA Research & Media LLP
Unit No. 429, Parsonage Road Edison, NJ
New Jersey USA – 08837
Phone: +1 201 565 3262, +44 161 818 8166
[email protected]
#Global Age Related Macular Degeneration Market#Age Related Macular Degeneration Market Demand#Age Related Macular Degeneration Market Trends#Age Related Macular Degeneration Market Analysis#Age Related Macular Degeneration Market Growth#Age Related Macular Degeneration Market Share#Age Related Macular Degeneration Market Forecast#Age Related Macular Degeneration Market Challenges
0 notes
Text
Sight Care Reviews: Know The Truth Before You Buying
In today's digital age, where screens and constant exposure to blue light are ubiquitous, the importance of maintaining optimal eye health has become more crucial than ever. SightCare, a leading player in the eye health supplement market, positions itself as a natural solution dedicated to supporting and enhancing vision. With a blend of natural ingredients, including eyebright, lutein, bilberry fruit, quercetin, and more, SightCare not only promises improved vision but also adopts a holistic approach to overall well-being. This 1500-word review will meticulously explore the ingredients, benefits, potential side effects, pricing, and the unique features that make SightCare a sought-after eye health supplement.
Understanding the Vision Struggle
Before delving into SightCare, it's crucial to understand the challenges faced by individuals in maintaining healthy eyesight. Factors such as age-related macular degeneration, prolonged exposure to blue light, and oxidative stress contribute to a decline in vision health.

Dietary supplements like SightCare emerge as a response to these challenges, claiming to harness the power of nature to combat these issues.
Analyzing the Ingredient Profile
SightCare distinguishes itself through a meticulously crafted blend of natural ingredients, each chosen for its potential contribution to vision enhancement. Let's dissect the core components:
Lutein: A naturally occurring carotenoid, lutein acts as a protective filter against blue light, reducing the risk of age-related macular degeneration (AMD) and providing antioxidant benefits.
Eyebright: Rich in rosmarinic acid, eyebright offers antioxidant and anti-inflammatory properties, safeguarding the retina from oxidative stress and potential damage.
Bilberry Fruit: Known for its anthocyanins, bilberry fruit serves as a powerful antioxidant, protecting the eyes from free radical damage.
Quercetin: With rutin and Quercetin-3-O-Glucoside, this compound provides antioxidant protection, reduces inflammation, and improves retinal function.
Zeaxanthin: This component absorbs blue light, shielding the eyes from harmful UV rays, enhancing visual acuity, and reducing the risk of AMD.
Astaxanthin: A potent antioxidant, astaxanthin protects the eyes from oxidative stress, offering higher antioxidant activity compared to other carotenoids.
The synergy of these ingredients forms the backbone of SightCare's commitment to optimizing vision health naturally.
The Visionary Creators
SightCare emerges from the collaborative efforts of professionals driven by a profound understanding of eye health. Their commitment is evident in the formulation of this supplement, prepared in an FDA-approved facility after extensive research and studies. The supplement undergoes rigorous testing in both its home lab and third-party labs, ensuring efficacy and safety before reaching consumers.
Unraveling the Mechanism for Healthy Vision
How does SightCare work its magic on vision health? The natural ingredients in SightCare act as a shield, protecting cells from damage caused by external factors such as cigarette smoke and blue light. These factors generate free radicals, leading to poor eyesight and blurred vision.
SightCare's anti-inflammatory properties come into play, keeping free radicals at bay and optimizing the health of eye cells. Consistent consumption aims to reduce dependence on corrective measures like contact lenses or glasses over time. Noteworthy ingredients like lutein, bilberry fruit, and more have demonstrated effectiveness in not just improving vision but also enhancing brain and memory function.
Clinical Validation of Ingredients
To ascertain the effectiveness of SightCare's key ingredients, it's essential to examine the scientific research supporting them:
Lutein: Acts as a natural filter for blue light, protecting against AMD. Its antioxidant properties neutralize free radicals, safeguarding the eyes.
Eyebright: Rich in rosmarinic acid, it protects the retina from oxidative stress and prevents AMD.
Bilberry Fruit: Anthocyanins in bilberry provide powerful antioxidant benefits, protecting against free radical damage.
Quercetin: Exhibits anti-inflammatory properties, reducing inflammation and improving retinal function.
Zeaxanthin: Absorbs blue light, protecting against UV rays, increasing visual acuity, and enhancing contrast sensitivity.
Astaxanthin: A potent antioxidant that outperforms other carotenoids in protecting the eyes from oxidative stress.
Unveiling the Health Benefits

SightCare doesn't limit its benefits to vision enhancement; it extends its positive influence to various aspects of health:
SightCare Supports Eye Health:
With its natural ingredients, SightCare aims to keep eye cells nourished and protected. The formula addresses issues like blurred vision caused by blue light exposure, promoting overall eye health.
Preventing Diabetic Retinopathy:
SightCare steps in to combat diabetic retinopathy and macular degeneration, offering protection against common eye problems that can hinder clear vision.
Promoting Healthy Vision:
By reducing the harmful effects of blue light, SightCare promotes healthy vision. The natural formula, enriched with vitamin C, lutein, quercetin, eyebright, and more, aims to maintain optimal eyesight and decrease dependency on corrective lenses.
Enhancing Visual Acuity and Preventing Macular Degeneration:
SightCare works to reduce swelling and toxicity in macula cells, supporting visual acuity and preventing the onset of macular degeneration.
Supporting Cognitive Function:
The supplement extends its benefits beyond vision health, promoting brain health by enhancing neurotransmitter production and optimizing the nervous system.
Supporting Healthy Digestion:
SightCare contributes to overall well-being by supporting healthy digestion, reducing inflammation in the digestive tract, and optimizing liver health.
The Right Dos
age for Optimal Results
To harness the maximum benefits of SightCare, it's recommended to take two capsules daily—one in the morning and one at night. Consistent and regular intake, coupled with a healthy diet and eye exercises, is emphasized for optimal results.
Minimal Reported Side Effects
The natural composition of SightCare translates into minimal reported side effects. Reviews from satisfied users echo the sentiment that the supplement has been effective in improving visual acuity without triggering any adverse reactions. SightCare's commitment to natural ingredients and the absence of artificial preservatives provide users with confidence in its safety.
The Procurement Process
SightCare's availability is limited to its official website, ensuring a secure and authentic purchase process. The company offers three distinct packages to cater to varied needs:
1-Bottle SightCare: Priced at $69 plus $9.99 shipping charges, this serves as an ideal trial for those new to SightCare.
3-Bottle SightCare: Positioned as a value pack, priced at $177 (or $59 per bottle), this package includes one free bottle of SightCare.
6-Bottle SightCare: Considered the best value and most popular pack, priced at $294 (or $49 per bottle), it comes with an additional free bottle of SightCare.
Money-Back Guarantee
SightCare instills confidence in its users through a robust 180-day money-back guarantee. The supplement's efficacy is complemented by a satisfaction guarantee, and the straightforward refund process offers a safety net for those not completely satisfied with the results.
Final Verdict on SightCare
In conclusion, SightCare emerges as a top contender in the eye health supplement market. With a blend of natural ingredients backed by scientific research, it positions itself as an effective and natural solution to vision-related concerns. The supplement's ability to not only enhance vision but also support cognitive function and overall well-being adds a layer of appeal.
SightCare's commitment to quality, safety, and transparency shines through its formulation and testing processes. The absence of reported side effects, coupled with positive user reviews, reinforces its reputation as a trustworthy eye health supplement.
For those navigating the challenges of maintaining optimal vision in the digital age, SightCare stands out as a holistic and nature-inspired solution. As with any dietary supplement, individual experiences may vary, but SightCare's comprehensive approach to eye health makes it a compelling choice for those seeking a natural boost to their vision and well-being.
0 notes