#positive acute phase proteins
Text
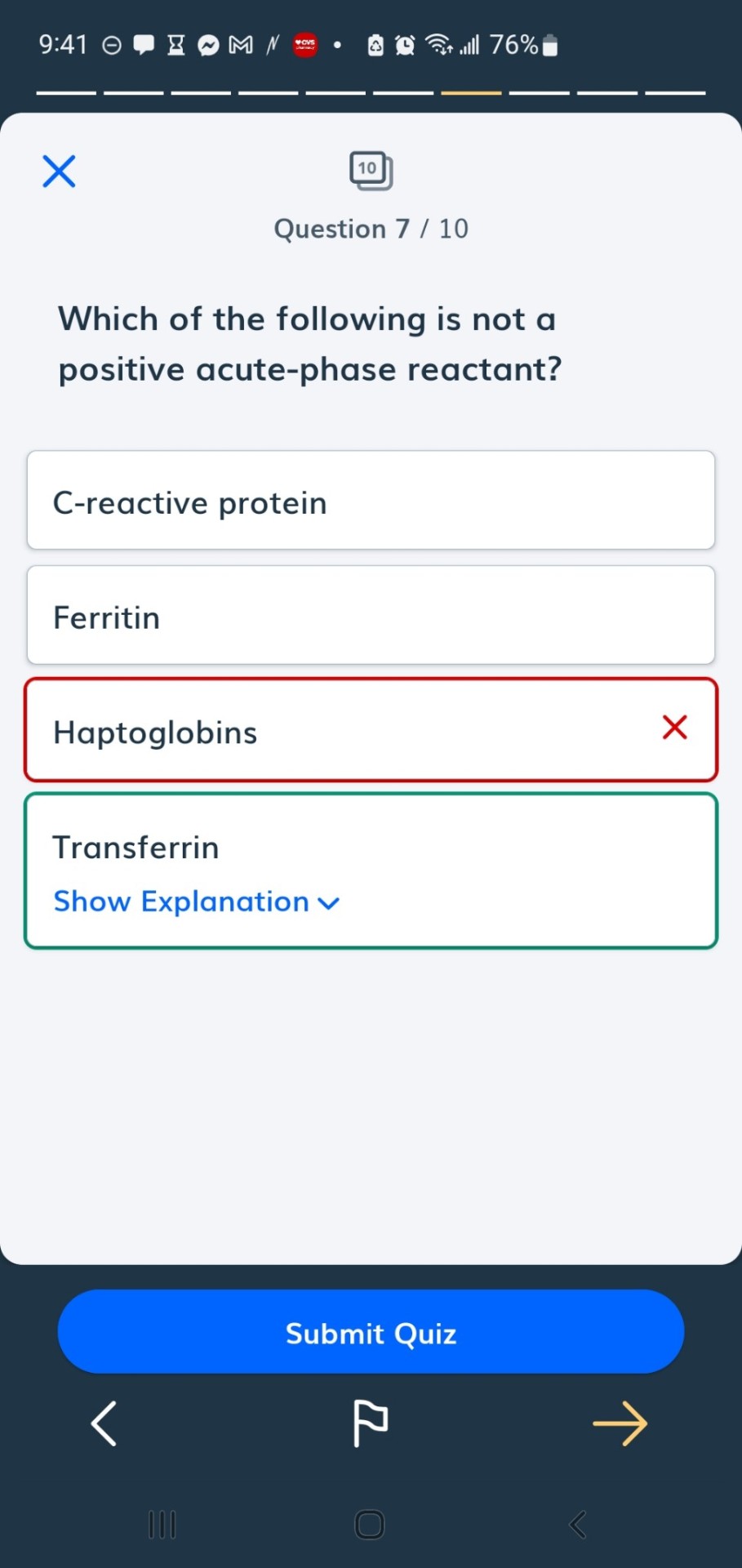
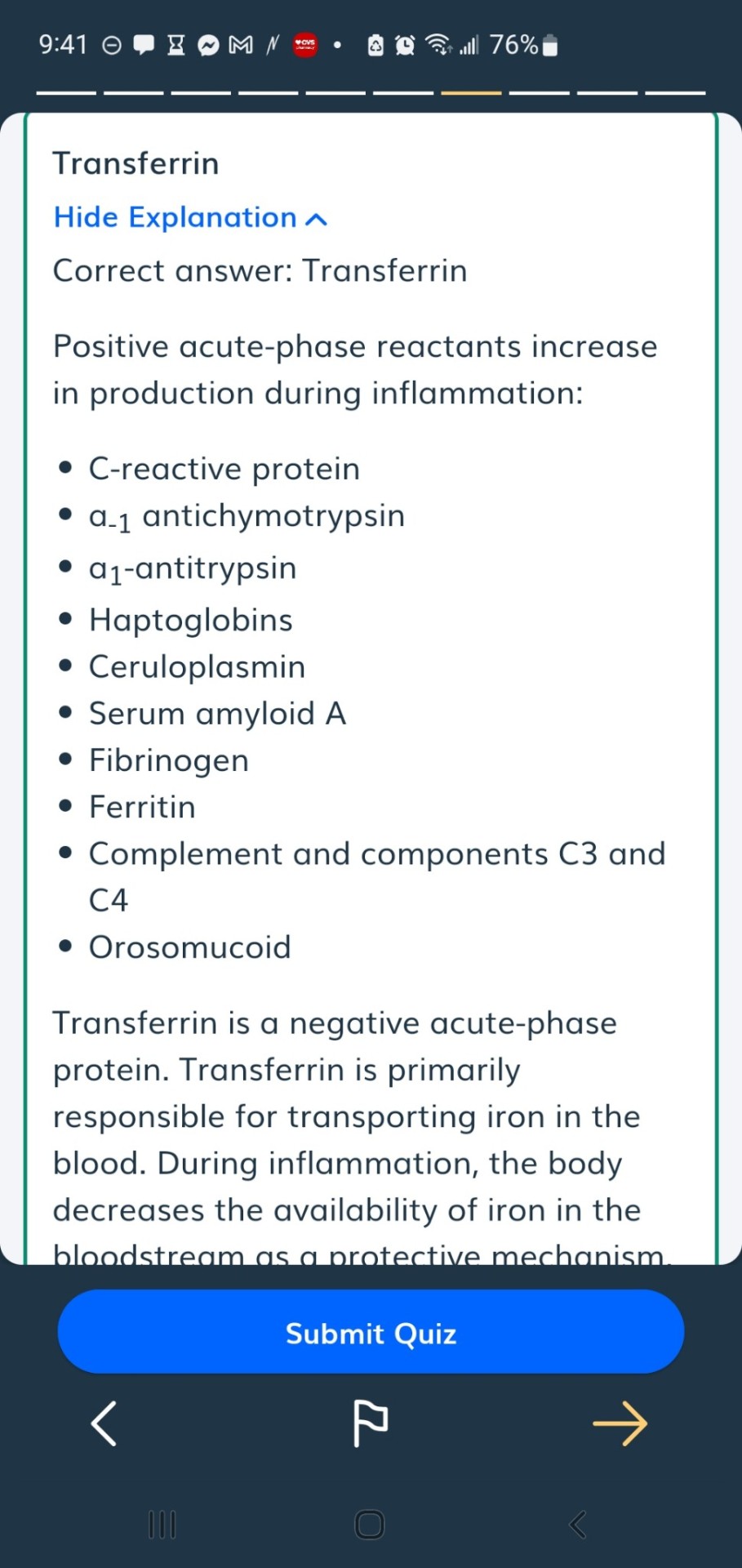

#dietetics#positive acute phase proteins#negative acute phase proteins#acute phase proteins#ferritin#transferrin#haptoglobin#CRP#C reactive protein#albumin#prealbumin
1 note
·
View note
Text
GP Vaccination Market to Get an Explosive Growth

Latest Global GP Vaccination Market study with 100+ market data Tables, Pie charts & Figures is now released by HTF MI. The research assessment of the Market is designed to analyze futuristic trends, growth factors, industry opinions, and industry-validated market facts to forecast till 2030. A significant region that is speeding up marketization is used to split the market study. Some of the leading players covered such as AstraZeneca Plc (United Kingdom), Johnson & Johnson Inc (Canada), Novavax (United States), BioNTech (Germany), Moderna (United States), Glaxo Smith Kline Plc (United Kingdom), CSL Ltd. (Australia), Abbott Laboratories (United States), Daiichi Sankyo Company Ltd (Japan), Astellas Pharma Inc. (Japan).
Download Sample Report PDF (Including Full TOC, Table & Figures) 👉 https://www.htfmarketintelligence.com/sample-report/global-gp-vaccination-market
According to HTF Market Intelligence, the Global GP Vaccination market to witness a CAGR of % during forecast period of 2024-2030. The market is segmented by Application (Hospitals, Clinics, Vaccination Centres, Academic and Research Institutes, Others) by Type (Whole-Pathogen Vaccines, Subunit Vaccines, Nucleic Acid Vaccines) and by Geography (North America, South America, Europe, Asia Pacific, MEA).
Definition:
A GP vaccination is a vaccine meant to grant received immunity in opposition to extreme acute respiratory syndrome coronavirus two (SARS-CoV-2), the virus that reasons coronavirus disorder 2019 (COVID19). A vaccine is an organic coaching that affords lively received immunity to a precise infectious disease. A vaccine usually consists of an agent that resembles a disease-causing microorganism and is regularly made from weakened or killed types of the microbe, its toxins, or one of its floor proteins. The agent stimulates the body's immune machine to understand the agent as a threat, smash it, and to similarly apprehend and smash any of the microorganisms related with that agent that it might also come upon in the future. The COVID19 vaccines are broadly credited for their function in decreasing the severity and loss of life precipitated via COVID19. Many international locations have carried out phased distribution plans that prioritize these at very best chance of complications, such as the elderly, and these at high danger of publicity and transmission, such as healthcare workers.
Market Trends:
Growing demand for Low-priced Vaccines
Rising Focus on the Development of New and Improved Vaccines
Market Drivers:
High Prevalence of Diseases Requiring Administration of Vaccines and Emerging Use of Vaccines for Different Infectious Diseases
Market Opportunities:
Increasing Healthcare Expenditure
GP Vaccination Market Competitive Analysis:Know your current market situation! Not just new products but ongoing products are also essential to analyze due to ever-changing market dynamics. The study allows marketers to understand consumer trends and segment analysis where they can face a rapid market share drop. Figure out who really the competition is in the marketplace, get to know market share analysis, market position, % Market Share, and segmented revenue.
Have a question? Market an enquiry before purchase @ https://www.htfmarketintelligence.com/enquiry-before-buy/global-gp-vaccination-market
Players Included in Research Coverage: AstraZeneca Plc (United Kingdom), Johnson & Johnson Inc (Canada), Novavax (United States), BioNTech (Germany), Moderna (United States), Glaxo Smith Kline Plc (United Kingdom), CSL Ltd. (Australia), Abbott Laboratories (United States), Daiichi Sankyo Company Ltd (Japan), Astellas Pharma Inc. (Japan)
Additionally, Past GP Vaccination Market data breakdown, Market Entropy to understand development activity and Patent Analysis*, Competitors Swot Analysis, Product Specifications, and Peer Group Analysis including financial metrics are covered.
Segmentation and Targeting:
Essential demographic, geographic, psychographic, and behavioral information about business segments in the GP Vaccination market is targeted to aid in determining the features the company should encompass in order to fit into the business's requirements. For the Consumer-based market - the study is also classified with Market Maker information in order to understand better who the clients are, their buying behavior, and patterns.
GP VaccinationProduct Types In-Depth: Whole-Pathogen Vaccines, Subunit Vaccines, Nucleic Acid Vaccines
GP Vaccination Major Applications/End users: Hospitals, Clinics, Vaccination Centres, Academic and Research Institutes, Others
GP Vaccination Major Geographical First Level Segmentation:
• APAC (Japan, China, South Korea, Australia, India, and the Rest of APAC; the Rest of APAC is further segmented into Malaysia, Singapore, Indonesia, Thailand, New Zealand, Vietnam, and Sri Lanka)
• Europe (Germany, UK, France, Spain, Italy, Russia, Rest of Europe; Rest of Europe is further segmented into Belgium, Denmark, Austria, Norway, Sweden, The Netherlands, Poland, Czech Republic, Slovakia, Hungary, and Romania)
• North America (U.S., Canada, and Mexico)
• South America (Brazil, Chile, Argentina, Rest of South America)
• MEA (Saudi Arabia, UAE, South Africa)
Buy Now Latest Edition of GP Vaccination Market Report 👉 https://www.htfmarketintelligence.com/buy-now?format=3&report=1943
0 notes
Text
C-Reactive Protein in Veterinary Practice
Abstract
Animal body reacts to all kinds of injuries and stress to keep up the homeostasis mechanism of the body. This homeostasis achieved either by or nonspecific mechanism. The nonspecific innate resistance of the body like cytological and cytokine reactions including fever, leukocytosis etc. is known as acute phase response. In this response, there will be increase or decrease of serum concentration of proteins. These proteins are known as acute phase proteins. Measurements of serum concentration of these acute phase proteins are found to be useful in assessment of health status and prediction of diseases of the man and animals. The serum concentration of these acute phase proteins returns to base levels when the triggering factor is no longer present. The acute phase response is now considered to be a dynamic process involving systemic and metabolic changes providing an early nonspecific defence mechanism against insult before specific immunity is achieved. Use of one of the acute phase proteins, C-reactive protein as biomarkers for animal disease diagnosis and health status assessment has got high potential in modern veterinary practice is discussed in this review
Keywords: Acute injury; C - reactive protein; Acute phase proteins; Veterinary practice
Abbrevations: CRP: C Reactive Protein; Hp: Haptoglobin; AGP: Acid Glycoprotein; SAA: Serum Amyloid A
Introduction
The acute phase reaction encompasses all the phenomena which take place in animals following tissue damage and is particularly associated with inflammation from whatever cause. During the acute phase reaction, the body mounts a multifactorial response to remove and replace damaged tissue and one of the mechanisms involved is the production and secretion by the liver of a number of ‘acute phase proteins’ [1]. The concentrations of these proteins increase during the reaction are called as Positive acute phase proteins (APPs) such as C reactive protein (CRP), Serum Amyloid A (SAA), Haptoglobin (Hp), Ceruloplasmin, α2- Macroglobulin, α1 Acid Glycoprotein(AGP), Fibrinogen and Complement (C3,C4) while those of others, including albumin, Transferrin, Transthyretin and Retinol-binding protein decrease as the liver switches production of protein towards the synthesis of the proteins required to deal with the damage; is called as negative APPs [2] (Figure 1).
Biological functions of C-reactive Protein
The protein was named the C-reactive protein because of its ability to bind pneumococcal C-polysaccharide. The presence of CRP has also been described in human patients during acute infections caused by acute lobar pneumonia, active rheumatic fever and bacteraemia caused by “colon bacillus”. Among the biological functions described in the literature are Complement activation and opsonisation [3,4] Modulation of monocytes and macrophages, cytokine production [5] Binding of chromatin [6] Prevention of tissue migration of neutrophils
CRP in Bovines
During the early stages of infection, the serum concentration of CRP increases [3]. This increase has been described to be evident before an elevated rectal temperature is observed. Even though increased concentrations of bovine CRP during naturally occurring infections and a correlation with herd health status have been reported, CRP is generally not considered an acute phase protein in cattle. As stress increases to a critical point, the liver rapidly synthesizes large amounts of CRP and releases it into the blood to provide immediate protection against stress [7]. Diseases in a dairy herd elevated the serum CRP level. The serum CRP level was also correlated with milk production. The greater the milk production, the higher the level of serum CRP. Diseases, especially acute infections, induced much higher levels of CRP production than stress or lactation. Also showing that plasma C-reactive protein concentration is related to different kind of stress (poor health, high lactation, blood collection). Strong correlation was observed in cows after delivery (0-1 month) between fibrinogen and CRP values [8]. Obtained results suggest that not only inflammations but also physiological factors such as pregnancy, delivery and/or state of lactation may have a significant impact on APPs values in the blood plasma of dairy cows. It would be worth in the future to check whether there is a relationship assessing the animal health status obtained using acute phase proteins method relatively to other indicators, such as milk yield, length of lactation or others. Morimatsu [9] also discovered Elevation of bovine serum C-reactive protein by lactation when compared to other Acute Phase protein such as serum amyloid P component levels (Figure 2).
CRP in Swine
In the pigs, as in the dogs and humans, C-reactive protein (CRP) is the prototypical acute phase protein with major diagnostic value. CRP concentrations are useful for evaluating the health status of a swine herd, but not for the health status of an individual animal or the differentiation of diseases. Serum haptoglobin (HP) concentration is better than serum CRP concentration as an indicator of inflammatory reactions in pigs, and HP is an important marker for swine health status [10]. On the other side, pigs undergone experimental study had CRP serum concentration below 22μg/ml (mean 18.64 ± 2.59). Twenty-four hour after coinfection with swine influenza virus (H1N1) and Pasteurella multocida, the mean concentration of CRP reached 62.85 ± 35.55μg/ml. Significant difference were noticed as compared to control animals [11]. The presence of elevated serum Hp and CRP concentrations in apparently healthy pigs at slaughter could provide important information to a veterinary inspector about the presence of sub-clinical lesions that could lead to condemnations or a decrease in the quality of carcasses [12].
CRP in Canines
In canines CRP is the major APP used as marker for systemic inflammation / infection. Normally the level of CRP is less than 1.5 mg/ dL or even lower than 0.5 mg/dl. The normal range may be 0.08 to 2.26 mg/dl [13]. The level rises within 4 to 6 hrs after onset of inflammation / infection. Serum CRP level above 3.5 mg/dl, indicates presence of systemic inflammation. Level above 5 mg/dl is a strong evidence of systemic inflammation. Strong correlation was observed between CRP and animal’s temperature and Total Leukocyte counts of canine patients naturally infected with Leptosporosis [14]. CRP levels could be used to monitor early responses to antibiotic treatment and might alert veterinarians to the need for further evaluation or additional treatment. Serum CRP concentrations provide useful information about the severity of inflammation inside the Urinary Bladder. These correlations suggest that CRP concentrations can represent a safe, convenient, and alternative method for evaluating the status of bacterial cystitis [15]. Significantly high CRP values were observed in cases like Lympoma, pyometra, panniculitis, acute pancreatitis, polyarthritis, leptospirosis, babesiosis, parvo viral enteritis, glomerulonephritis, immune mediated disease and malignant neoplasia [16]. Rise in CRP may not be observed in local tumours like leiomyosarcoma, upper respiratory tract infection, diabetes, neurological problems involving intracranial disorders. Since the CRP concentration did not increase in patients with intervertebral disk protrusion, it might be useful in distinguishing arthritis from spinal / brain diseases in patients with lameness. Thus, CRP is a nonspecific inflammatory marker, it could facilitate the diagnosis by indicating the presence and the extent of inflammation
CRP in Elephants
The reference interval for CRP reported herein for Asian elephants (1.3–12.8 mg/l) is like CRP intervals reported in harbor seals (Phoca vitulina) and bottlenose dolphins (Tursiops truncatus). When compare to the CRP, SAA is demonstrated to be the most responsive major APP in elephants [17]. This agrees with previous reports where SAA elevations were noted consistently in elephants with Elephant endotheliotropic herpesvirus (EEHV) and in 2 captive elephants with inflammatory lesions. Further studies are needed to address the reactivity of the CRP reagents with elephant proteins and to consider the use of either elephantspecific reagents or non–antibody-based assays
CRP in Chicken
The birds positive for E.Coli, Pasturella multocida and Staphylococcus aureus infections as well as histomoniasis and adjuvant injection found to be positive for CRP. CRP also positive in clinically normal population. These birds on post-mortem, had lesions consistent with chronic respiratory disease, which is highlighting the use of CRP as a potential biomarker for nonclinical disease [18]. CRP did not rise in chickens as quickly as it does in humans, whereby CRP was detectable 36-48 hours post infection in chickens, compared to 16-18 hours in humans. A more recent study investigated CRP serum concentrations using a human CRP kit and found CRP concentrations to increase
CRP in Equines
A high serum concentration was also found in horses with pneumonia, enteritis, arthritis and after castration. Increased plasma concentration has been observed in carbohydrate induced laminitis. Increased serum concentration of CRP has been found in horses suffering from aseptic inflammation induced by intramuscular turpentine injections [3].
To Know More About Journal of Dairy & Veterinary sciences
Please click on: https://juniperpublishers.com/jdvs/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#wild life rehabilitation#Veterinary sonography#Veterinary Therapeutics#Veterinary Virology#Juniper Publishers#open access journals
0 notes
Text
Abstract
Background Autoimmunity has been reported in patients with severe coronavirus disease 2019 (COVID-19). We investigated whether anti-nuclear/extractable-nuclear antibodies (ANAs/ENAs) were present up to a year after infection, and if they were associated with the development of clinically relevant post-acute sequalae of COVID-19 (PASC) symptoms.
Methods A rapid-assessment line immunoassay was used to measure circulating levels of ANAs/ENAs in 106 convalescent COVID-19 patients with varying acute phase severities at 3, 6 and 12 months post-recovery. Patient-reported fatigue, cough and dyspnoea were recorded at each time point. Multivariable logistic regression model and receiver operating curves were used to test the association of autoantibodies with patient-reported outcomes and pro-inflammatory cytokines.
Results Compared to age- and sex-matched healthy controls (n=22) and those who had other respiratory infections (n=34), patients with COVID-19 had higher detectable ANAs at 3 months post-recovery (p<0.001). The mean number of ANA autoreactivities per individual decreased between 3 and 12 months (from 3.99 to 1.55) with persistent positive titres associated with fatigue, dyspnoea and cough severity. Antibodies to U1-snRNP and anti-SS-B/La were both positively associated with persistent symptoms of fatigue (p<0.028, area under the curve (AUC) 0.86) and dyspnoea (p<0.003, AUC=0.81). Pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α and C-reactive protein predicted the elevated ANAs at 12 months. TNF-α, D-dimer and interleukin-1β had the strongest association with symptoms at 12 months. Regression analysis showed that TNF-α predicted fatigue (β=4.65, p=0.004) and general symptomaticity (β=2.40, p=0.03) at 12 months.
Interpretation Persistently positive ANAs at 12 months post-COVID are associated with persisting symptoms and inflammation (TNF-α) in a subset of COVID-19 survivors. This finding indicates the need for further investigation into the role of autoimmunity in PASC.
0 notes
Text
The particular Long-term Results of Epidermal growth factor receptor in People Along with Kind Only two Person suffering from diabetes Renal system Illness
(H) Last year Elsevier Limited. Just about all privileges set aside.The particular lymphatics began receiving consideration from the technological neighborhood as soon as 1622, while Gasparo Aselli mentioned each side milky-white vessels inside the mesentery of a well-fed dog. Because period this website , the particular lymphatic system continues to be in the past thought to be your sewage of the vasculature, passively draining smooth and healthy proteins from your interstitial spaces (as well as lipid from your belly) in to the blood vessels. Recent surveys, however, declare that the actual lymphatic system function inside lipid transfer can be an lively and intricate method, understanding that when lymphatic purpose can be affected, you can find systemic implications to be able to fat metabolic process and carry. This kind of evaluate illustrates these types of latest results, and implies long term directions with regard to understanding the interaction among lymphatic system and also fat the field of biology within wellness condition.Qualifications: Pneumonia due to adenovirus contamination is often extreme particularly with adenovirus serotype Seven frequently related to lower respiratory system illness acne outbreaks. Many of us documented an outbreak regarding 80 cases of significant pneumonia together with one demise of children in Shaanxi Land, The far east. Sample showed adenovirus 7 (Ad7) because the main pathogen with some co-infections. Results: A couple of stresses involving adenovirus and 2 ranges of enterovirus had been separated, the 21 pharynx swabs showed Fourteen positive amplifications regarding adenovirus; three co-infections with respiratory syncytial trojan, two positive for rhinovirus, a single beneficial regarding parainfluenza 3, and 4 damaging. Adenovirus keying in showed seven in the eight adenovirus positive examples were HAdV-7, a few ended up HAdV-3 and two have been also fragile to do sequencing. Your entire hexon gene of adenovirus has been sequenced along with analyzed for the check details a pair of adenovirus serotype Several isolates, showing the actual nucleic acidity homology has been Ninety nine.8% forwards and backwards stresses as well as 97.5% when compared to research strain HAdV-7 (GenBank accession quantity AY769946). To the 21 years old serious period serum examples Epidermal growth factor receptor in the 21 years of age sufferers, half a dozen biological materials acquired pluses results for ELISA detection involving HAdV IgA, along with the neutralization titers with the convalescent-phase examples ended up 4x greater than that relating to your acute-phase trials in eight sets. Conclusions: Many of us came to the conclusion adenovirus has been the viral pathogen, mainly HAdV-7, with many co-infections accountable for the particular outbreak. This can be the first record associated with an toddler pneumonia herpes outbreak a result of adenovirus serotype Seven within Shaanxi Domain, Tiongkok.Medium-chain-length poly(3-hydroxyalkanoate)utes (mcl-P(3HA)utes) with various side-chain duration starting from C3-C9 had been produced via 2-alkenoic fatty acids of C6-C12 simply by using a metabolically engineered stress involving Escherichia coli. The consequence involving side-chain length of mcl-P(3HA)azines upon cold weather components along with crystallization behaviours ended up being looked at by simply DSC and also X-ray studies. Almost all mcl-P(3HA)utes created the chain-packed crystalline composition within the solvent-cast films. Burning conditions of solvent-cast video involving mcl-P(3HA)azines initial lowered from 59 degrees H to be able to Forty-five levels D with all the change involving side-chain from C3 for you to C4 and afterwards elevated to be able to Sixty nine diplomas C with the expansion associated with side-chain to be able to C9. The particular X-ray diffraction patterns indicate the organization of an split construction arranged your main-chains inside airplanes involving side-by-side packing regarding side-chains which has a regular long distance of merely one.
0 notes
Text
The training Portfolio
This assessment outlines concepts DSP-5990 involving sophisticated CT image order as well as energy in acute cerebrovascular event operations.The strategies of based substantially about located practical information on imitation continues to be termed capital breeding and it is in contrast to revenue propagation, exactly where requires associated with duplication are usually pleased through exogenous (nutritional) sources. Many species likely drop anywhere between these two extreme conditions, as well as the position of your living thing alongside this particular incline may influence several essential life-history characteristics. Frequent eiders (Somateria mollissima) would be the simply hurtling parrots which are nonetheless usually regarded real money collie breeders, recommending that they can depend entirely in endogenous stocks to form their eggs and incubate. All of us investigated the particular yearly and seasons variance inside advantages of endogenous and also exogenous means in order to egg creation inside eiders breeding in the Eastern side Bay community within the Canada Arctic. Many of us collected prey things in addition to ladies in addition to their ova in the course of various phases regarding reproduction and also used a pair of supporting systematic techniques: body hold characteristics and also dependable isotope [delta(13)H, delta(16)N] mixing designs. Search engine spiders regarding protein reserves remained stable from pre-laying for you to post-laying periods, even though fat stocks declined considerably through installing. In the same way, secure isotope looks at established that (One) exogenous nutrition derived from sea invertebrates strongly contributed to the formation involving lipid-free ovum elements, along with (Two) yolk fats have been constituted generally from endogenous lipids. We also discovered proof of seasonal alternative from the utilization of entire body stores, along with first collie breeders using proportionally more exogenous meats to form every eggs than overdue collie breeders. Based on these kind of results, we all decline the actual speculation that will eiders are usually pure money tiers. In these traveling chickens, the fitness fees of the rigid funds reproduction strategy, like non permanent loss of trip ability along with constraint associated with clutch as well as ovum measurement, may be greater than positive aspects like a decrease in eggs predation price.History: The purpose of this research ended up being to appraise the effectiveness as well as tolerability involving photodynamic therapy (PDT) when compared with intravitreal vascular endothelial development element (VEGF) inhibitors from the treatment of polypoidal choroidal vasculopathy (PCV). Approaches: Pertinent studies were selected via an intensive search from the PubMed, EMBASE, World wide web regarding Scientific disciplines, as well as Cochrane Catalogue sources. Eating habits study attention integrated visual benefits, anatomic variables, as well as unfavorable occasions. Outcomes: 6 reports enrolling a total of 346 people have been incorporated.
#Kinase Inhibitor Library#HDAC inhibitor#Tomivosertib#E-64#Geldanamycin#Molidustat#SGC-CBP30#Dynasore#UNC0642#Halofuginone#Avasimibe#Fasudil#Thymidine#BAPTA-AM#Fimepinostat#AP-III-a4#CFI-400945#PF-8380#BMS-1#kira6#Aspirin#Vancomycin#Amiloride#lurasidone#Dexmedetomidine#Raloxifene#Menadione#Tolvaptan#Thiotepa#Grazoprevir
1 note
·
View note
Text
Viruses, Vol. 14, Pages 2825: Development of Fluorescence-Tagged SARS-CoV-2 Virus-like Particles by a Tri-Cistronic Vector Expression System for Investigating the Cellular Entry of SARS-CoV-2
Severe acute respiratory syndrome-related coronavirus-2 (SARS-CoV-2) has caused the pandemic that began late December 2019. The co-expression of SARS-CoV-2 structural proteins in cells could assemble into several types of virus-like particles (VLPs) without a viral #RNA genome. VLPs containing S proteins with the structural and functional properties of authentic virions are safe materials to exploit for virus-cell entry and vaccine development. In this study, to generate SARS-CoV-2 VLPs (SCoV2-SEM VLPs) composed of three structural proteins including spike (S), envelop (E) protein and membrane (M) protein, a tri-cistronic vector expression system was established in a cell line co-expressing SARS-CoV-2 S, E and M proteins. The SCoV2-SEM VLPs were harvested from the cultured medium, and three structure proteins were confirmed by Western blot assay. A negative-stain TEM assay demonstrated the size of the SCoV2-SEM VLPs with a diameter of about 90 nm. To further characterize the infectious properties of SCoV2-SEM VLPs, the VLPs (atto647N-SCoV2-SEM VLPs) were fluorescence-labeled by conjugation with atto-647N and visualized under confocal microscopy at a single-particle resolution. The results of the infection assay revealed that atto647N-SCoV2-SEM VLPs attached to the surface of the HEK293T cells at the pre-binding phase in a ACE2-dependent manner. At the post-infection phase, atto647N-SCoV2-SEM VLPs either fused with the cellular membrane or internalized into the cytoplasm with mCherry-rab5-positive early endosomes. Moreover, fusion with the cellular membrane and the internalization with early endosomes could be inhibited by the treatment of camostat (a pharmacological inhibitor of TMPRSS2) and chlorpromazine (an endocytosis inhibitor), respectively. These results elucidated that SCoV2-SEM VLPs behave similarly to the authentic live SARS-CoV-2 virus, suggesting that the development of SCoV2-SEM VLPs provide a realistic and safe experimental model for studying the infectious mechanism of SARS-CoV-2. https://www.mdpi.com/1999-4915/14/12/2825?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Alnylam Pharmaceuticals, Inc. and Glaxosmithkline Plc.– Major Players in RNAi Therapeutics Market
RNAi refers to the interference RNA which is responsible to control and manipulate protein translation in the cell. These RNAi are used to silence particular genes which are responsible for diseases. Currently, the awareness about RNAi is increasing extensively due to its ability to offer target specific mechanism, leading to superior treatment outcomes. The approach is widely utilized for drug development.
Market leaders operating in the market have undertaken various organic growth strategies in the RNAi therapeutics market. The RNAi therapeutics market majorly consists of the players such Alnylam Pharmaceuticals, Inc., Sanofi, Olix Pharmaceuticals, Inc., Glaxosmithkline Plc., Benitec Biopharma, Arbutus Biopharma Corporation, Silence Therapeutics, Rexahn Pharmaceuticals, Inc., Arrowhead Pharmaceuticals, Inc, and Quark amongst others. Several organic approaches, such as product launches, and expansion/relocation in the RNAi therapeutics market, have resulted in the positive growth of the market. Product launches help the company to strengthen its product offering and the customer base, which allows the company to hold a strong position in the market. Similarly, utilizing expansion activities, it is easy to venture into untapped economies and use the opportunities being offered.
Below is the list of the growth strategies done by the players operating in the RNAi therapeutics market:
Year
News
Nov-2019
Alnylam Pharmaceuticals, Inc got FDA approval for its Givlaari, a very first subcutaneously administered RNAi therapy for the treatment of acute hepatic porphyria (AHP).
Dec-2018
Benitec Biopharma completed phase 2 clinical trial of its RNAi therapy candidate BB-401 for the treatment of head and neck squamous cell carcinoma (HNSCC). The study was conducted in Russia and Australia.
Mar-2018
OliX Pharmaceuticals, Inc. got clinical trial approval from Medicines and Healthcare products Regulatory Agency (MHRA), UK to conduct phase 1 trial for the drug candidate OLX10010. Olix Pharma is developing this drug for the treatment of hypertrophic scar.
Aug-2017
Rexahn Pharmaceuticals, Inc. received New U.S. Patent for Supinoxin which is a small molecule modulator of the β-catenin pathway- a key biological pathway that is activated in tumor cells.
0 notes
Text
Any computational strategy to realize structure-activity relationship of a single,3-disubstituted imidazole 1,5-α pyrazine types identified as Dupracetam aggressive inhibitors with the IGF-1 receptor in connection with Ewing sarcoma
The objective populace of the review can be a hypothetical cohort associated with Sixty years previous ladies in the past helped by breast-conserving surgical procedure with regard to node-negative, estrogen receptor-positive cancer of the breast with growths < 1 cm. Enough time horizon is actually#keep##links# 15 years, as well as the viewpoint is interpersonal. Your interventions tend to be total breast RT, 3-D CRT, along with brachytherapy breasts irradiation. The end result steps are usually expenses (08 US$), quality-adjusted living years (QALYs), and slow cost-effectiveness rates. The base-case outcome was: 3-D CRT was the preferred strategy, pricing typically $10,800 along with producing 11.21 years old QALYs. On-time complete breasts RT charges $368,000/QALY compared to 3-D CRT, higher than the $100,000/QALY WTP patience. 3-D Cathode ray tube was also preferred around delayed whole breast RT. Brachytherapy was not ever chosen. Awareness analysis established that the final results were responsive to the rate involving repeat outside of the initial tumor quadrant ("elsewhere failure") in one-way evaluation. Probabilistic awareness evaluation revealed that results were responsive to parameter uncertainness, knowning that the particular elsewhere-failure charge along with remedy choices may possibly travel results. The limitation with this examine is effectiveness estimations derive from studies that may not totally represent the population patterned. Like a bottom line, 3-D CRT was chosen over whole breasts RT and then for girls more likely to postpone RT, implying that will 3-D Cathode ray tube could possibly be specific better ahead of randomized test proof.Many of us yet others recently revealed that psychological and also physical activation in form involving environmental enrichment minimizes cerebral beta-amyloid (A new experiment with) deposition in transgenic mouse styles of Alzheimer's disease. This specific influence ended up being unbiased via amyloid forerunner health proteins (APP#keep##links#) term or running as well as fairly because of increased wholesale of your try out. However, the comprehensive mechanisms remain cloudy. In today's review, many of us show ecological enrichment inside TgCRND8 these animals (carrying man Software(Swedish+Indiana)) impact the neurovascular product by greater angiogenesis as well as differential unsafe effects of The experiment with receptor/transporter compounds, specifically up-regulation involving LRP1, ApoE and A2M in addition to down-regulation associated with RAGE to ensure brain to body A new 'beta' clearance is actually facilitated. These kind of outcomes advise a previously unidentified effect of environment enrichment counteracting the general disorder within Alzheimer infected mental faculties.Infection shortly after propagation inhibits establishment of being pregnant. Shot regarding peptidoglycan-polysaccharide (PG-PS), a part of gram-positive bacterias, into lambs about evening 5 after multiplying reduces being pregnant rate. Findings specified to gauge the particular acute-phase result (Interest rates) throughout ewes for you to shot involving PG-PS about morning A few#keep##links# right after propagation (day time 3). Catheters had been placed in to the jugular and posterior vena cava upon morning Four. Upon morning Your five, ewes were challenged along with saline or perhaps 30 mu g/kg weight (BW) PG-PS (Exp A single) or 60 mu g/kg BW PG-PS (Exp A couple of). Blood samples had been gathered each and every 20 min for 6 h (Exp One particular) and every 16 min for just two l, by the hour for 14 h, at 24, Thirty five, as well as Forty-eight l (Exp A couple of). Body temperature and medical signs of contamination had been watched in Exp 2.
0 notes
Text
Atorvastatin enhances motor purpose, depression and anxiety by NOX2-mediated autophagy and also oxidative stress inside Ibrutinib-lesioned mice
We implement this method in a magic size assembler, called Genome Assemblage through Bayesian Inference (GABI), and also illustrate its software on the bacteriophage Phi X174. The trying method achieves the two very good blending along with convergence about Illumina test files with regard to Phi X174, displaying the actual feasibility of our tactic. All of us sum it up the particular posterior submission regarding assemblage hypotheses created by simply GABI as a majority-rule consensus construction. We evaluate the particular rear submitting in order to exterior devices of the same examination files, along with annotate people devices simply by setting posterior probabilities to be able to characteristics that are that is similar to GABI's assemblage chart. GABI will be openly accessible within a GPL licenses coming from https://bitbucket.org/mhowison/gabi.Cell decline soon after transplantation is often a significant issue with regard to mobile substitute strategies in regenerative remedies. To gauge the particular success kinetics of caused pluripotent stem cell (iPSC)-derived cardiomyocytes (Centimetres #Link# ) we all produced transgenic murine iPSC traces which in turn, in addition to CM-specific term associated with puromycin N-acetyl-transferase and enhanced green phosphorescent proteins (EGFP), also constitutively show firefly luciferase (FLuc) for bioluminescence (BL) inside vivo imaging. Although undifferentiated iPSC traces made by simply random incorporation from the transgene in to the genome retained steady FLuc exercise above several paragraphs, the actual BL sign intensity ended up being firmly lowered in pure iPS-CM compared to undifferentiated iPSC. Targeted incorporation of FLuc-expression cassette to the ROSA26 genomic locus making use of zinc oxide #Link# hand nuclease (ZFN) engineering highly reduced transgene silencing within iPS-CM, resulting in any several-fold larger BL when compared with iPS-CM expressing FLuc through hit-or-miss genomic loci. To analyze the actual emergency kinetics regarding iPS-CM in vivo, purified Centimetres extracted from iPSC lines articulating FLuc from your haphazard or perhaps the ROSA26 locus have been transplanted straight into cryoinfarcted hearts of syngeneic mice. Engraftment regarding workable cells has been checked through BL image resolution more than 30 days. Adopted iPS-CM ended up poorly stored in the myocardium independently with the mobile or portable series employed. Nonetheless, as much as 8% associated with tissue made it through for Four weeks to begin regarding shot, which has been confirmed simply by immunohistological diagnosis associated with EGFP-positive iPS-CM in the sponsor muscle. Transplantation of iPS-CM failed to #Link# impact the surgical mark creation as well as capillary density in the periinfarct place associated with number myocardium. This statement is the initial to discover the survival kinetics associated with drug-selected iPS-CM inside the infarcted cardiovascular utilizing BL image and signifies that transgene silencing for the duration of iPSC difference might be cut down tremendously by making use of genome modifying engineering. FLuc-expressing iPS-CM made within this research can allow further scientific studies to reduce their loss, boost long-term tactical and useful plug-in on hair transplant.Goal. Pentraxin Three or more (PTX3), fresh discovered irritation sign, is part of acute-phase meats. The actual hypothesis, functionality of gingival tissue along with serum PTX-3 increases within the new periodontitis model (using 10-day along with 40-day intervals), has been analyzed simply by sensing gingival cells along with serum PTX-3 quantities in subjects together with new periodontitis. Approaches.
0 notes
Text
Matching-Adjusted Indirect Comparison involving Ribociclib Additionally Dihydrotestosterone versus Palbociclib Plus Letrozole while First-Line Treating HR+/HER2- Innovative Cancer of the breast
Among breast malignancies, the particular NF-kappa B-dependent induction associated with JAG1 as well as the NOTCH-dependent growth of the cancer come mobile or portable population arise only inside the basal-like subtype. Jointly, our benefits suggest that will NF-kappa B features a non-cell-autonomous position inside managing cancer malignancy come mobile or portable numbers by developing intratumoural microenvironments composed of JAG1-expressing non-cancer originate cellular material with a basal-like subtype.The particular liver disease Elizabeth computer virus #Link# (HEV) causes intense well-liked liver disease, but its characterization is actually distracted with the lack of a competent inside vitro contamination system that can be used to review the effects involving HEV protein about cellular procedures. Earlier research declare that the viral ORF3 health proteins (pORF3) is essential pertaining to an infection throughout vivo and is planning to regulate the actual host response. Below, we report that pORF3 localizes for you to early and these recycling endosomes to cause a new postpone from the postinternalization trafficking associated with epidermis development factor receptor (EGFR) in order to past due endosomes/lysosomes. The actual cytoplasmic phosphorylated indication transducer as well as activator involving transcription Three (pSTAT3) protein call for expansion factor receptor endocytosis for translocation through the cytoplasm in order to nucleus. Therefore, 'abnormal' amounts of pSTAT3 were found within the nuclei involving ORF3-expressing Huh7 man hepatoma cellular material triggered along with EGF. This leads to downregulation in the acute-phase response, an important determining factor of inflammation inside the web host. We advise which through their outcomes upon EGFR trafficking, pORF3 extends endomembrane expansion element #Link# signaling as well as helps bring about cellular success. The results in STAT3 translocation would cause a lowered inflammatory response. Both these occasions will likely contribute absolutely for you to popular reproduction.Purpose: All of us screened regardless of whether rosiglitazone (RGZ), a new peroxisome proliferator-activated receptor-gamma agonist, can easily bring back alveolar development and general rise in a rat model of bronchopulmonary dysplasia (BPD). Resources and techniques: A new rat style of BPD had been caused through intra-amniotic shipping involving lipopolysaccharide (LPS) and also postnatal hyperoxia (80% regarding 7 days). RGZ (Three or more mg/kg/d, we.r.) or even car was presented with every day for you to rat pups regarding 14 days. This kind of style provided 4 trial and error organizations: Simply no BPD+vehicle (V), Zero BPD+RGZ, BPD+V, and also BPD+RGZ. Upon D14, alveolarization, lungs vascular denseness, and right ventricular hypertrophy (RVH) were looked at. Outcomes: Morphometric investigation says the BPD+RGZ team got significantly smaller plus more complex airspaces and larger alveolar floor as opposed to BPD+V team. The actual BPD+RGZ party had drastically increased lung general density compared to the BPD+V party. Western bare examination revealed that significantly diminished levels of vascular endothelial expansion aspect (VEGF) and it is receptor VEGFR-2 through the mixed experience intra-amniotic LPS as well as postnatal hyperoxia have been restored from the RGZ treatment RVH ended up being considerably reduced within the BPD+RGZ party than in the particular #Link# BPD+V party. Summary: These types of outcomes suggest that RGZ can easily restore alveolar and lung general advancement and lower pulmonary blood pressure inside a rat model of BPD.
0 notes
Text
09 | scientific inspiration

pairing — spider-man!vernon x ofc
featuring — joshua, yeji (itzy), felix (skz), yangyang (nct)
word count — 3.6k
genres — spider-man au, marvel au, fluff, action, angst, humor
warnings — none.
note — so here it is, the big Science Dump that will form the basis for one of the major arcs of the story. now, i don’t pretend to know too much of what i’m talking about, but hopefully all the hours of scrolling through obscure genetics articles will hold up. hell, they probably won’t, but this is superhero fiction about a sixteen-year-old man-spider vigilante, so please excuse it !!! a lot of this is borrowed from the ultimate spider-man comics lore by brian michael bendis.

Vernon was still thinking about Luce’s offer when he got to work later that day.
Normally, he would have tried to keep his head clear during his work, but since all he had to do that day was log data entries, it didn’t really matter. Doc hadn’t come back to the lab yet, so it was just him and the janitor, but from the open holograph display on his table, Vernon figured he’d be back pretty soon. Despite the state of his office, the doctor didn’t like messes, especially not in his workplace.
He hadn’t expected Luce to even consider inviting the others, even though she had been friendly with them. Movie night was something that belonged to just the four—three—of them, something sacred and untouched by outsiders. The thing that had surprised him even more was his own willingness. For someone who had been so acutely ticked off by their unannounced arrival, he sure had warmed up to his new teammates quickly.
Vernon was only a few entries in when Dr. Connors entered the lab, holding a cup of steaming coffee from the cafeteria. He smiled at Vernon when he came in, not bothering to glance at the screen to check what he was doing before making his way over to the work table. One of the things Vernon liked the most about this place was that despite being nothing more than a research assistant, he was still allowed to help out in more impactful ways than simply entering and saving data.
“You’re here early,” Dr. Connors said, setting down the Styrofoam cup on his table. He looked tired, Vernon noticed, probably why he had bought that cafeteria coffee despite it being a thick, dark color and tasting like tar. There were dark circles under his blue eyes, and his usually neatly combed brown hair was slightly disheveled.
“I came here directly after school was over,” Vernon said. “Figured I’d save a lot more time that way, and I don’t really have much left to do.”
“Hm?” The scientist faced the holographic model, hitting a few keys on the pad below it. His movements were listless, but his shoulders were still tense. Reminds me of seniors before finals, Vernon thought. It wasn’t exhaustion like he had assumed, but stress. “Then perhaps you’d like to help me out here.”
“Really?” Vernon tried to keep the excitement out of his voice, but failed. Probably for the better, because it sparked a small smile on Dr. Connors’s face. “What are you working on right now?”
He didn’t get an answer for a long moment. Vernon spun in his chair and pulled himself to his feet, ignoring the remains of exhaustion weighing his body down as he made his way over to the doctor’s table.
“It’s something your father and I were working on before…before this project was abandoned, almost a decade ago,” Dr. Connors said. He was looking at the display with a different kind of intensity in his eyes, like it was something to be defeated rather than discovered. “When I lost your father, I gave up all hope of ever getting back to it, but after all my recent failures, I think I need to revisit my roots.”
Vernon pursed his lips at failures, but said nothing. The hologram looked like a DNA strand—a double helix blown to the size of a poster tube. It shone with a dull blue light, lighting up Dr. Connor’s features, illuminating the creases around his mouth and eyes that Vernon wasn’t sure had been there before. Standing next to him made his own tiredness feel like a minor inconvenience.
“This was your father’s brainchild, after all,” the man said, still staring at the display. “A completely independent protoplasmic model based on the body’s own genetic edifice built to fortify the weaker structure of a sick body.”
“A protoplasmic model?” Vernon’s eyes widened. “I thought it was supposed to be controlled AI, like nanobots or something.”
“Imagine that, except a sentient being with the ability to detect and eradicate weaknesses in the body on its own, without any direction,” Dr. Connors said. “Something to cure everything—the right combinations of proteins able to use the body’s own natural resources to fight any infection, overcoming the problem of grafting and able to treat everything from neural atrophy to genetic diseases to cancer, contained in a small tubule.” His eyes shone. “The perfect cure.”
The perfect cure. Vernon glanced back at the holographic model, now seeing the inconsistencies in its structure when compared to normal human DNA. The idea was intoxicating and exhilarating, made even more amazing by the fact that it had been proposed by his father. It made his chest ache with longing, thinking of the possibilities of fulfillment if his father had been alive still—not just for the experiment, but for Vernon himself.
“He was way ahead of his time, Richard Parker—in that sense, you are a lot like him,” Dr. Connors murmured in a low, wistful voice, as if speaking to himself. “It had become almost impossible for us to receive any support or funding for our project, because of how wildly imaginative it was. We were ridiculed, discredited, called mad for our ideas before we finally got the deal with Oscorp. We had worked on the cure for so long, and just a couple of days before the deal’s signing, your father called me one night, sounding excited about a fresh prospect.” He shook his head. “But then…”
He didn’t need to complete his sentence. Vernon caught the drift of it, and turned away to hide the pained expression brought onto his face by the flood of emotions. He didn’t know if he felt good about being so close to his father’s work, or bad about being so far away from his father himself. Even the mere presence of his old colleague, still alive while he wasn’t, seemed to taunt Vernon.
Snap out of it, he told himself firmly. His father’s death hadn’t been Dr. Connors’s fault—he knew that, but still had to avoid even thinking of that idea, because once the seeds had been planted in his brain, Vernon knew he wouldn’t be able to work with Dr. Connors in harmony. Plus, watching him talk about the work he and his dad had done together, no one could say that the scientist didn’t care about his former partner.
“What did he discover?” Vernon prompted.
Dr. Connors’s eyes turned sad. “I never did get to find out,” he said. “Just two days after the call, he was finally going to come back to the state to share his discoveries with me, so we could compare notes and build on what was lacking. The first step to phase two, he called it.” His jaw tightened. “And just when we thought something was going to go right for once…”
Vernon hung his head. Maybe knowing his father had been on the verge of a breakthrough should have made him feel better about his achievements, but he only thing that Vernon could think about was what all the world had lost when he had lost his dad. A revolution in medicine. A father. He was almost a little uneasy thinking about which kind of loss affected him more. The world could have been a much better place, but all Vernon wanted was his dad back.
“I’ve been unfair to you, Vernon,” Dr. Connors said, breaking him out of his reverie. He straightened while keeping his eyes fixed on the DNA hologram, then faced Vernon with a sad look. “You should have had someone to help you come to terms with your father’s death, someone who could have told you about his great ideas and even greater work. I shouldn’t have left you alone to deal with everything, but I simply couldn’t bring myself to…”
His voice had lowered with every syllable until he trailed off, making Vernon think that his voice had finally become too small for anyone to hear. Vernon swallowed, unable to think of anything to say. He was usually good at talking to people, even heart-to-hearts, but when the subject touched his obscure past, words failed him.
“I understand,” he said, the first words that came to his blank mind. He tried for a reassuring smile, unsure of what the result actually looked like. “You shouldn’t blame yourself for it. And anyway, I am here now.”
Dr. Connors smiled a little. “That, you are,” he said. “I feel like I’ve been doing your genius intellect a great injustice by assigning you all these menial tasks.”
“Hey, someone’s gotta do the menial tasks, right?” He smiled back. “My experience with research is next to nothing compared to that of the other people in this lab, so I’m fine with where I am. And not all the tasks are exactly menial.”
“Still.” The man sighed. “Since it was your father’s genius that came up with this idea, it feels only right to have you develop it further—or at least play a role in its creation.”
“I’m here whenever you need me,” Vernon said, glad about the lightening of the atmosphere. He wasn’t sure how much more of that weight he could have taken. He cocked his head, studying the listed proteins. “What made you want to work on this ‘cure’ again after so long?”
“A lot of different reasons,” the scientist said. “I think I had been avoiding this project for so long because I couldn’t bear to continue it without Richard by my side, but meeting you, his son, and having you take up a position in my lab felt like a sign.” He gave the boy a sideways smile. “And from a scientific viewpoint—before this, I’d been working on a different kind of cure, a serum with a principle based in cross-species genetics. It was supposed to be give a person the ability to regenerate lost limbs like a lizard, but the premature human trials went off the rails.”
Vernon nodded, keeping his mouth clamped shut. “I see,” he said, not wanting to bring up the Lizard incident unless he was sure Dr. Connors was ready to address it.
“However, after someone helped…fix the problem by making an anti-serum, the new formula for it gave me an idea,” the man continued. “Scientific inspiration, I guess you could call it. There’s a lot to be done, but I still have the anti-serum here in the lab, and have been studying it for over a month now.”
The gears had already begun turning in Vernon’s head. He had been the one to create the anti-serum as Spider-Man, and no one knew the methodology better than the original creator. Most of it had stemmed from the original Lizard formula, and with a bit of recalibration and measured reversal, the formula had worked. That makes me wonder…
“Hey, doc,” he murmured, brow pinched into a thoughtful frown, “if you had a sample of perfectly bonded human and non-human cell structure, do you think you would be able to mimic it and engineer a matching structure for the cure?”
The man frowned. “How do you mean?”
“I mean…” Vernon hesitated. Because of the OZ formula transferred into his blood by the spider bite, his DNA was perfectly bonded to spider DNA, which gave him what they called in post-human-speak a ‘healing factor’. It wasn’t as effective as Wolverine’s, but it was still something—and it was based on the same principle as the cure. Like the OZ formula helped his body develop a natural cure for anything he could be hit by—be it a paper cut or a head wound—by using its own resources.
The only difference was that it heightened his facilities by combining human abilities with spider abilities, which gave him things like his spider sense. However, if Vernon could use his own blood to develop a kind of skeletal structure for the cure. If it did work, it would only work on enhanced spider/human DNA, but at least then he’d have a start. The possibilities after that were endless.
“If there already existed a perfect sample of blood which had an in-built system like the cure,” Vernon said, trying not to give away too many details.
“Like mutant DNA?” Dr. Connors asked. “They have a completely different genetic structure in place, though, Vernon. They have the X-Gene. Their nucleotide sequence itself is mutated.”
“No, not like that,” Vernon said. “Like human DNA, just…enhanced. Bonded with something like the cure, just not—not living.”
Dr. Connors raised his eyebrows. “Well, having a perfect sample would reduce the needed brainwork to a tenth,” he said. “But you couldn’t acquire a sample like that, because, well, it exists only in theory.”
“Right,” Vernon muttered, but already the beginnings of a smile had started to curve his lips. “Only in theory.”

Vernon’s mind was buzzing with so much excitement from his idea for the cure that even web-slinging hadn’t been able to distract him from it.
He and the rest of the S.H.I.E.L.D. team had spent the evening scouring the city for any signs of something that could substantiate Vernon’s theory, but had come up with nothing except a few petty criminals, who had been easily stopped. The other three had left early, telling him to use their new communication devices (which looked an awful lot like kitschy wrist bands, except for the fact that they could turn invisible) if anything came up.
Nothing did.
It was nine p.m. and Vernon had still not changed out of his Spidey suit, spending the free hour to swing around the city and try and clear his head. Too much had happened in one day, and his mood was seesawing between elation at his new project and trepidation because of the dreaded return of movie night. Funny that a high school hangout was a source of more nervousness for him than trying to imitate his own radioactive blood sample to finish his dad’s decades old design.
When I put it like that, it sounds even more absurd, he thought, scrolling through the usual evening homework-help texts on his phone as he waited in line to buy eggs and a carton of milk at the not-so-local grocery store. Even Spider-Man had to obey queues when he was out doing chores for Aunt May.
He paid for the eggs and milk without the tattooed cashier giving him a second glance, and stepped out into the street with the bags. Aunt May wouldn’t be back until ten; he had about an hour to kill until curfew, but he wanted to get home early to talk to her about movie night (yet another reaction to dread) and hopefully study his spidery OZ-bonded radioactive blood under the lens of his old microscope that Uncle Ben had gotten him over a year ago.
“Yo, Spidey!”
Vernon looked up to see a chubby, tanned guy in his late twenties beaming at him like an old friend as he jogged up to meet him. “Hey, I remember you,” he said, pointing at the guy. “You’re uhhh…” He squinted at him, trying to remember when he’d last seen him. “That pizza delivery guy who almost got abducted by aliens!”
“That’s me! Paulo!” the guy exclaimed, his wide smile widening even more upon being recognized. “You saved me from those killer robot aliens last month, remember? And I promised you free pizza in case you ever needed it,” he added. “How’s it going?”
“As usual.” He raised the bag containing the groceries he’d just bought.
“Running errands when you get a break from crime-fighting, eh?” Paulo asked, giving his thick dark curls a shake. His smile refused to dim even a bit, like someone had switched on a light bulb with a permanent power source. “Keeps the superheroes humble.”
“Tell that to Captain America.” Vernon checked the comm device on his wrist, almost groaning out loud when he saw it was almost half past nine already. “Great. Uh, Paulo, I’ll have to catch you later. It’s late, and I gotta get back well before curfew in case there are delays on the way.”
“Of course! Go do your Spider-Man thing.” Paulo lifted his hands, mimicking the thwip-thwip gesture of shooting webs, and grinned. “See you later, Spidey!” he called out from behind him as Vernon swung himself up to a lamppost before launching himself into the air. “Remember the offer with the free pizzas still stands!”
“I will!” Vernon yelled back as he swung away. And he wasn’t just saying that, either—free pizzas were free pizzas.
He had to change in an alleyway again, but thankfully this time it didn’t have an open dumpster or smell like someone had thrown out a decayed cheese slab in the trash. By the time he got back home, Aunt May was already back, as indicated by the lights in the kitchen. Just perfect, he thought miserably, as he unlocked the front door with his spare key and trudged into the hallway.
“Vernon! You’re back early,” a voice yelled from the kitchen when she heard the door shut behind him. A woman with short silver hair, clad in a comfortable t-shirt and yoga pants came out into the living room as he entered it, wiping her hands with a hand towel. “Did you get the milk and eggs like I asked you to?” Aunt May asked.
For an older lady, she sure has great hearing. “Yep,” he said, swinging his bag off his shoulders and unzipping it, internally praying he hadn’t squashed the milk carton from all his swinging like last time. Thankfully, they were undamaged. “Did you come back from yoga classes early?”
“Oh, Denise pulled a muscle in her back, poor thing,” May said. “I offered to bring her back home, but she refused to let me ice it for her, saying she’d get Mac to do it instead.” She disappeared into the kitchen once again, coming out without the hand towel this time. “Put the groceries in the fridge, won’t you?”
For an older lady, Aunt May also had a lot of things going for her. Yoga classes on Monday-alternating weekdays, squash sessions over the weekend, classes for baking and music and whatnot—she might even have been busier than Vernon himself.
“Will do,” he said, obeying. His mind was still swimming with all the older thoughts, but now that he was standing right in front of Aunt May, the worry about movie night had pushed itself to the forefront, demanding all of his nervous attention.
He stood at the fridge even after closing the door, chewing his lip and wondering how to bring it up. Words really had failed him today. “Aunt May?” he ventured, unable to keep the hint of nerves from his voice. “I wanted to talk to you about something.”
“What is it, honey?” she asked, poking her head out of the kitchen. Around her waist was an apron that said Don’t Kiss the Cook. “Vernon?”
He kissed his teeth, teetering back and forth on the balls of his feet. “It’s about movie night.”
She stilled. “What about movie night?”
Maybe I shouldn’t have said anything, Vernon thought, pursing his lips. Aunt May hadn’t exactly been close with the Osborns, but he knew she had cared about Harry in her own way, the same way she cared about any neglected kid that Vernon brought home like an abandoned cat. She gave them as much comfort as she could, tried to give them the family they never really had, even if she knew she couldn’t completely replace them. It had happened before: Harry, and Luce—and now, Vernon thought with a little sigh, maybe even the team.
“Luce asked me to ask you if you were okay with us doing movie night this weekend,” he said slowly. “And there are these new kids, and she told me to ask them too, but if you’re busy we can always—”
“Vernon!” Aunt May smiled widely, coming out of the kitchen to rest her hands on his shoulders and give them a big squeeze. “Of course I’m okay with it! Oh, you don’t know how I wished you kids would do one of those again, I’m sure that’s what Harry would have wanted too.” She gave him a motherly smile, one that was soft and sad at the same time. “I’ll leave the house to you kids that day.”
“Oh, no, Aunt May, that’s not necessary—” he started, but she cut him off with a wave of her hand.
“Don’t be so formal with me, kiddo,” she said. “I know movie night means a lot to you, and if you have new friends coming over, I’m sure you don’t want a chaperone around.” She raised her eyebrows. “Although I would like to meet them before I go out.”
Vernon sighed, but there was a tiny smile on his face. “God, you’re the best.”
“And don’t you forget it.” She winked. “Besides, even an old woman like me needs to go out with her friends every once in a while, too. This might just turn out to be a good break for both of us.”
He nodded, feeling a welling of emotion in his chest that wouldn’t go down no matter how much he tried to push it away. One less thing to worry about, he thought half-heartedly, trying not to think about how Aunt May’s agreement meant movie night was on, which had the potential to be an even more worrying prospect. “I hope so.”
#kwritersworldnet#caratwritersclub#svtcreations#seventeen#svt#vernon#seventeen imagines#seventeen fluff#seventeen scenarios#seventeen fanfic#vernon imagines#vernon fluff#vernon scenarios#vernon fanfic#svt imagines#svt fluff#svt scenarios#svt fanfic#hansol fluff#hansol scenarios#hansol fanfic
58 notes
·
View notes
Text
We have a pt who has rhabdomyolysis from taking a statin and being on the floor for 3 days. She's old, so she had muscle breakdown. The attending is Dr. Giraldo, who is really nice and awesome. He asked me what urine test you use to diagnose rhabdo and I totally forgot about myoglobinuria so I said I wasn't aware. He told me to look it up. Then after we were rounding for a bit, my brain remembered myoglobinuria! So I told him. But now I want to look up the details of diagnosing rhabdo so I can present to him tomorrow. But we also have a pt with CKD who may have kidney stones and the attending and the resident didn't realize that you can diagnose a kidney stone with a non-contrast CT. I remembered learning that last year during my emergency medicine rotation. They had ordered a KUB because they thought you would need to use contrast for the CT and so they didn't want to get the CT because the contrast would hurt the pt's kidney. But you don't use contrast to diagnose nephrolithiasis with CT. So at least I remembered something! This is from UpToDate:
●The clinical manifestations of rhabdomyolysis include myalgias, weakness, red to brown urine due to myoglobinuria, and elevated serum muscle enzymes (including creatine kinase [CK]). The degree of myalgias and other symptoms varies widely, and some patients are asymptomatic. Fever, malaise, tachycardia, and gastrointestinal symptoms may be present. Muscle swelling may occur with rehydration.
This pt was actually tachycardic in the ED. So that tracks.
●The laboratory findings that characterize rhabdomyolysis include an acute elevation in the CK and other muscle enzymes and a decline in these values within three to five days of cessation of muscle injury. The other characteristic finding is the reddish-brown urine of myoglobinuria, but this finding is often absent because of the relative rapidity with which myoglobin is cleared. The serum CK is generally entirely or almost entirely of the MM or skeletal muscle fraction, although small amounts of the MB fraction may be present.
●Other manifestations include fluid and electrolyte abnormalities, many of which precede or occur in the absence of acute kidney injury, and hepatic injury. Hypovolemia, hyperkalemia, hyperphosphatemia, hypocalcemia, hyperuricemia, and metabolic acidoses may be seen. [I think the pt also had hypocalcemia, but it wasn't true hypocalcemia because the albumin was low, so her corrected Ca2+ was in the normal range based on the lab values at the hospital; she did have acidosis too I think]. Hyperkalemia may result in cardiac dysrhythmias. Later complications include acute kidney injury (AKI), hypercalcemia, compartment syndrome, and, rarely, disseminated intravascular coagulation.
●We diagnose rhabdomyolysis in a patient with an acute muscular illness or injury based upon a marked acute elevation in serum CK; the CK is typically at least five times the upper limit of normal and is frequently greater than 5000 international units/L. Key diagnostic laboratory studies include the creatine kinase and urinalysis, including dipstick and microscopic evaluation. Myoglobinuria (present in 50 to 75 percent of patients at the time of initial evaluation) results in a positive test for blood on the urine dipstick but without red blood cells on the microscopic examination of the urine. And for this pt, the UA showed a small amount of blood, so that could have been myoglobin in the urine, but we didn't order a microscopic analysis. She also has a UTI, so that could be from the UTI as well. Also, the other day Dr. Agarwal asked how long you treat UTIs. When in the hospital, you can treat with ceftriaxone until the pt has clinically improved.
●The differential diagnosis depends upon the combination of findings present. It includes myocardial infarction, other causes of red or brown urine, inflammatory myopathy, and local causes of pain, such as deep vein thrombosis or renal colic.
The characteristic triad of complaints in rhabdomyolysis is muscle pain, weakness, and dark urine. Additional symptoms that are more common in severely affected patients include malaise, fever, tachycardia, nausea and vomiting, and abdominal pain. Altered mental status may occur from the underlying etiology (eg, toxins, drugs, trauma, or electrolyte abnormalities).
The hallmark of rhabdomyolysis is an elevation in CK and other serum muscle enzymes. The other characteristic finding is the reddish-brown urine of myoglobinuria, but because this may be observed in only half of cases, its absence does not exclude the diagnosis. Routine lab tests, including complete blood count (CBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), vary greatly depending on the underlying cause of rhabdomyolysis. Infections and crush injuries are associated with marked elevation of the acute phase reactants and peripheral white blood cell (WBC) count, while these markers of inflammation would likely be normal or only minimally raised in patients with other etiologies, such as drug-induced or electrolyte derangements.
Serum CK levels at presentation are usually at least five times the upper limit of normal, but range from approximately 1500 to over 100,000 international units/L. The mean peak CK reported for each of a variety of different causes and for patients with both single and multiple causes ranged from approximately 10,000 to 25,000 in the largest series; exceptions were the three patients with malignant hyperthermia, whose values averaged almost 60,000.
I googled the normal serum CK level:
In a healthy adult, the serum CK level varies with a number of factors (gender, race and activity), but normal range is 22 to 198 U/L (units per liter). Higher amounts of serum CK can indicate muscle damage due to chronic disease or acute muscle injury.
The CK is generally entirely or almost entirely of the MM or skeletal muscle fraction; a small proportion of the total CK may be from the MB or myocardial fraction. The presence of MB reflects the small amount found in skeletal muscle rather than the presence of myocardial disease. Elevations in serum aminotransferases are common and can cause confusion if attributed to liver disease. In one study, aspartate aminotransferase (AST) was elevated in 93.1 percent and alanine aminotransferase (ALT) in 75 percent of rhabdomyolysis cases in which the CK was greater than or equal to 1000 units/L. In only one instance was the ALT greater than the AST, although the AST declines faster than the ALT as the rhabdomyolysis resolves, such that the two may equalize after a few days.
The serum CK begins to rise within 2 to 12 hours following the onset of muscle injury and reaches its maximum within 24 to 72 hours. A decline is usually seen within three to five days of cessation of muscle injury. CK has a serum half-life of about 1.5 days and declines at a relatively constant rate of about 40 to 50 percent of the previous day's value. In patients whose CK does not decline as expected, continued muscle injury or the development of a compartment syndrome may be present.
Urine findings and myoglobinuria — Myoglobin, a heme-containing respiratory protein, is released from damaged muscle in parallel with CK. Myoglobin is a monomer that is not significantly protein-bound and is therefore rapidly excreted in the urine, often resulting in the production of red to brown urine. It appears in the urine when the plasma concentration exceeds 1.5 mg/dL. Visible changes in the urine only occur once urine levels exceed from about 100 to 300 mg/dL, although it can be detected by the urine (orthotolidine) dipstick at concentrations of only 0.5 to 1 mg/dL . Myoglobin has a half-life of only two to three hours, much shorter than that of CK. Because of its rapid excretion and metabolism to bilirubin, serum levels may return to normal within six to eight hours.
Thus, it is not unusual for CK levels to remain elevated in the absence of myoglobinuria. In rhabdomyolysis, myoglobin appears in the plasma before CK elevation occurs and disappears while CK is still elevated or rising. Therefore, there is no CK threshold for when myoglobin appears. As above, rhabdomyolysis does not occur unless CK is elevated five times or more above the upper limit of normal. Routine urine testing for myoglobin by urine dipstick evaluation may be negative in up to half of patients with rhabdomyolysis. Pigmenturia will be missed in rhabdomyolysis if the filtered load of myoglobin is insufficient or has largely resolved before the patient seeks medical attention due to its rapid clearance.
Both hemoglobin and myoglobin can be detected on the urine dipstick as "blood;" microscopic evaluation of the urine generally shows few red blood cells (RBC) (less than five per high-powered field) in patients with rhabdomyolysis whose positive test results from myoglobinuria. Such testing is not a reliable method for rapid detection of myoglobin if RBC are present or in patients with hemolysis due to its lack of specificity for myoglobin. Hemoglobin, the other heme pigment capable of producing pigmented urine, is much larger (a tetramer) than myoglobin and is protein-bound. As a result, much higher plasma concentrations are required before red to brown urine is seen, resulting in a change in plasma color.
Hypocalcemia, which can be extreme, occurs in the first few days because of entry into damaged myocytes and both deposition of calcium salts in damaged muscle and decreased bone responsiveness to parathyroid hormone. During the recovery phase, serum calcium levels return to normal and may rebound to significantly elevated levels due to the release of calcium from injured muscle, mild secondary hyperparathyroidism from the acute renal failure, and an increase in calcitriol (1,25-dihydroxyvitamin D).
Severe hyperuricemia may develop because of the release of purines from damaged muscle cells and from reduced urinary excretion if acute kidney injury occurs.
●Metabolic acidosis is common, and an increased anion gap may be present. Our pt did have an anion gap and I wondered why. I guess it's because there's more uric acid in the blood.
Acute kidney injury — Acute kidney injury (AKI, acute renal failure) is a common complication of rhabdomyolysis. The reported frequency of AKI ranges from 15 to over 50 percent. The risk of AKI is lower in patients with CK levels at admission less than 15 to 20,000 units/L; risk factors for AKI in patients with lower values include dehydration, sepsis, and acidosis. [Our pt had peed a lot and was on the floor for 2 to 3 days, so she was probably dehydrated, increasing her risk for AKI]. Volume depletion resulting in renal ischemia, tubular obstruction due to heme pigment casts, and tubular injury from free chelatable iron all contribute to the development of renal dysfunction. Reddish-gold pigmented casts are often observed in the urine sediment.
Compartment syndrome — A compartment syndrome exists when increased pressure in a closed anatomic space threatens the viability of the muscles and nerves within the compartment. Compartment syndrome is a potential complication of severe rhabdomyolysis that may develop after fluid resuscitation, with worsening edema of the limb and muscle. Lower extremity compartment syndrome can also be a cause of rhabdomyolysis, as may occur after tibial fractures.
Disseminated intravascular coagulation — Infrequently, severe rhabdomyolysis may be associated with the development of disseminated intravascular coagulation due to the release of thromboplastin and other prothrombotic substances from the damaged muscle.
EVALUATION AND DIAGNOSIS
Indications for diagnostic testing — Diagnostic testing should be performed in individuals with:
●Both myalgias and pigmenturia.
●Either myalgias or pigmenturia, with a history suggesting the presence or recent exposure to a potential cause or event.
●The absence of myalgias and pigmenturia in a clinical setting associated with increased risk for rhabdomyolysis, as symptoms may be vague or absent in up to 50 percent of patients. The diagnosis should be suspected following prolonged immobilization [like our pt who was on the floor for 2 to 3 days], in any stuporous or comatose patient, or in a patient who is otherwise unable to provide a medical history and has one or more of the following:
•Muscle tenderness
•Evidence of pressure necrosis of the skin
•Signs of multiple trauma or a crush injury
•Blood chemistry abnormalities suggesting the possibility of increased cell breakdown, such as hyperkalemia, hyperphosphatemia, and/or hypocalcemia
•Evidence of acute kidney injury
●Acute muscle weakness and marked elevation of creatine kinase (CK).
Diagnostic evaluation — We obtain the following key diagnostic laboratory studies:
●Creatine kinase – In addition to elevation of the CK, other muscle enzymes are typically elevated (eg, aldolase, aminotransferases, lactate dehydrogenase), but such testing is not usually necessary to make the diagnosis. However, elevations in aminotransferases or lactate dehydrogenase may suggest the need for CK testing if it has not been performed in a patient in whom such abnormalities may potentially be due to muscle injury rather than hepatic injury or another cause.
●Urinalysis, including dipstick and microscopic evaluation – Evidence of myoglobinuria should be sought by routine urine dipstick evaluation combined with microscopic examination. Testing of the unspun urine or the supernatant of the centrifuged urine will be positive for "heme" on dipstick if myoglobinuria is present, even if red to reddish brown urine is not evident macroscopically. The visual and microscopic examination of the sediment from a fresh urine specimen is required to exclude the presence of red blood cells (RBC) as the cause of positive testing; RBC in an older specimen may hemolyze over time, confounding the results.
In patients with persistent red to reddish-brown urine, myoglobinuria is suggested when the urine tests positive for heme by dipstick after centrifugation, while the plasma has a normal color and tests negative for heme.
Myoglobinuria lacks sensitivity as a test for rhabdomyolysis; it may be absent in 25 to 50 percent of patients with rhabdomyolysis due to the more rapid clearance of myoglobin, compared with CK, following muscle injury. Myoglobin also decreases rapidly in a similar fashion in patients with renal failure, suggesting a role for extrarenal metabolism and clearance in such patients.
We also obtain the following tests, which may help in prompt recognition of other potentially dangerous manifestations, in differential diagnosis, and in identifying the cause:
●Complete blood count, including differential and platelet count
●Blood urea nitrogen, creatinine, and routine electrolytes including potassium
●Calcium, phosphate, albumin, and uric acid
●Electrocardiography
Additional testing, such as evaluation of suspected metabolic myopathy or toxicology screening for drugs of abuse, depends upon the clinical context.
Diagnosis — We make the diagnosis of rhabdomyolysis in a patient with either an acute neuromuscular illness or dark urine without other symptoms, plus a marked acute elevation in serum creatine kinase (CK). The CK is typically at least five times the upper limit of normal, and is usually greater than 5000 international units/L. No absolute cut-off value for CK elevation can be defined, and the CK should be considered in the clinical context of the history and examination findings.
MANAGEMENT
The major issues in the treatment of patients with rhabdomyolysis include:
●Recognition and management of fluid and electrolyte abnormalities, which should be initiated regardless of renal function and which may prevent severe metabolic disturbances and acute kidney injury
●Identification of the specific causes and the use of appropriate countermeasures directed at the triggering events, including discontinuation of drugs or other toxins that may be etiologic factors
●Prompt recognition, evaluation, and treatment of compartment syndrome in patients in whom it is present
7 notes
·
View notes
Text
Vaccine hesitancy, molecular mimicry, and blood clots (oh my!)
There were many mixed messages in the world of coronavirus last week. Just as it appears that Michigan is the lead state in the fourth wave of the virus, the US is about to hit a “vaccine wall” as demand drops for vaccinations even though the supply is greatly improved. In the first three months of the rollout for the Pfizer, Moderna, and the Johnson & Johnson/Jantzen (J&J) vaccines, getting shots into arms of the most vulnerable has required a full court press from public health departments and the healthcare establishment, as well as persistence on the part of those trying to wrangle an appointment. The results from state to state have been uneven.
Figure 1

So far 14 states have administered fewer than 75% of the doses distributed to them with Alabama having the lowest vaccination rate per capita. Twenty states have administered more than 80% of the doses distributed to them with the most vaccinations per capita in New Hampshire.[1]
Vaccine Hesitancy:
The good news for those who want to get vaccinated is that it is a whole lot easier to get an appointment now. That said, the goal of herd immunity is a long way off and with demand dropping for jabs, we may not get there. Vaccine hesitancy is an important reason for declining demand and that is a shifting picture.
In a study that was put out by the Kaiser Family Foundation (KFF) in December, 52% of Black Americans said they would “wait and see” before signing up for the vaccine while only 20% said they wanted the shot as soon as possible. The share of Black people who were skeptical of the vaccines was higher than for White respondents (36%) and Latinx (43%).[2]
By March of this year, 55% of Black respondents to another KFF survey said they had been vaccinated or wanted the vaccine as soon as possible. Twenty-four percent were still holding back. Blacks have been one of the hardest hit demographics of COVID-19 and that has, no doubt, played a part in changing minds. Another possible reason for the turnaround in willingness to get vaccinated is because there has been a concerted outreach effort tapping trusted sources such as Barack Obama, sports stars, and other influencers such as Black ministers to address vaccine hesitancy among Black people.
The Urban Institute’s September 2020 Coronavirus Tracking Survey, a nationally representative survey of adults ages 18-64, asked people whether in the last 12 months they had ever felt a doctor, other health care provider, or their staff judged them unfairly or discriminated against them based on their race/ethnicity, gender, gender identity, sexual orientation, a disability, or a health condition and about the consequences of these experiences. This survey indicated that perceptions of discrimination and unfair judgement while seeking health care were higher among Black adults than among Hispanic and White adults in the previous 12 months (September 2019-September 2020).[3]
Figure 2
A “food desert” is described as an urban area that does not have a grocery store within one mile or a rural area that does not have one within ten miles. There is also a “pharmacy desert” that generally occurs in primarily Black neighborhoods in urban areas as well as in rural areas. People of color are less likely to have a family primary care provider and so access to information about the individual’s risks and benefits of getting vaccinated from a trusted source, much less getting an appointment for the vaccine itself, is often more challenging than it is for White people. My guess is that these experiences and the barriers to appropriate care contributed to the initial skepticism among people of color generally and Black people specifically in the initial rollout phase.
FIGURE 3
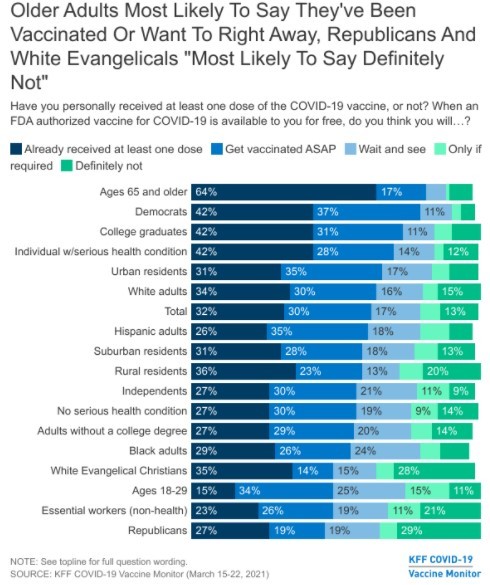
Meanwhile, Republicans and Evangelical Christians were the most likely groups to say they will not get vaccinated, according to the KFF survey.[4] I do not have an explanation for that. I also don’t know how wearing masks got politicized last year. If anyone has an explanation that doesn’t involve a gang of Democratic, cannibal pedophiles, I am really interested in hearing it.
Molecular mimicry and autoimmune disease:
There are science-based reasons that some are reluctant to get vaccinated. An issue that has been little discussed publicly is molecular mimicry. The theory is that some part of the spike protein of SARS-CoV-2, the virus that causes COVID-19 and is replicated in our cells, is similar enough to our own tissues that the immune system starts attacking our own cells thinking that those cells are the virus. Thus, the vaccine could trigger an autoimmune disease like rheumatoid arthritis, lupus, multiple sclerosis, or other autoimmune condition.
In one study looking for similar protein sequences between the SARS-CoV-2 virus with protein sequences in humans and other mammals, as well as other human coronaviruses, the number of shared protein sequences at two particular sites was quite high for humans, rats, and mice but miniscule or not at all with other human coronaviruses, cats, dogs, rabbits, chimpanzees, gorillas, or macaques.[5] Sadly, the investigators did not include bats, which I think of as flying rats, but that’s just me. It has been hypothesized that the original source of COVID-19 was from bats. Could the virus have molecular mimicry with bats? If so, what does that mean for the species?
These authors believe that much of the damage seen in the “cytokine storm” that causes the worst damage in COVID-19 may, in fact, be due to this molecular mimicry between the virus and, for example, lung tissue. It should also be noted that molecular mimicry from the whole SARS-CoV-2 virus is much more likely than it is from a small part of the virus (the spike protein). If the vaccine can trigger an autoimmune disease, so can the whole virus.
The presence and level of autoantibodies (AAbs) that attack our own cells, frequently detected in patients with COVID-19, are significantly associated with hospitalization and more severe prognosis. Clinically, these patients are more likely to have respiratory distress, acute cardiac injury, acute kidney injury, multi-organ dysfunction with such common complications as coagulopathy and thrombocytopathy (put a pin in this one as it is also at play with blood clots). [6]
Blood Clots and the J&J and AstraZeneca (AZ) vaccines:
Last week the J&J vaccine rollout was put on pause by the Food and Drug Administration (FDA) because six women developed unusual blood clots after receiving this vaccination. This was six out of seven million shots given. Some saw this as an over-reaction by the FDA that would likely lead to more vaccine hesitancy. However, these blood clots are different from clots that occur from “the usual suspects” like oral contraceptives and smoking.
Figure 4
A normal number of platelets is between 150,000-450,000 per microliter of blood (there are 1,000 microliters in one milliliter). If you have less than 150,000 platelets per microliter, you have a deficiency called thrombocytopenia. In the clots associated with the viral vector vaccines (J&J in the US and AZ in Europe), the platelets tend to stick together in the veins of the brain, which causes a blockage known as a cerebral venous system thromboembolism (CVST). This creates back pressure of blood in the brain itself, causing damage in the same way a hemorrhagic stroke would. [7]
“Normal” clots are usually treated with a blood thinner called heparin. With vaccine-induced prothrombotic immune thrombocytopenia (VIPIT), there is a deficiency of platelets and so that treatment would only make things worse. While the Centers for Disease Control and Prevention (CDC) and the FDA are getting the word out to doctors not to use heparin, they are also looking for ways of figuring out which people are more at risk for this extremely rare complication. Putting the vaccine on pause was clearly the ethical thing to do and this kind of transparency gives me greater confidence in the vaccine rollout.
As is the case with molecular mimicry, the danger of VIPIT happening if a person gets COVID-19 is much higher than it is from either the J&J or the AZ vaccine.
“…If the mechanism is the same, one can speculate that the high occurrence in COVID-19 vs. vaccination is because the whole virus is more thrombogenic [likely to cause clots] than the spike protein alone.” Paolo Madeddu, professor of experimental medicine at the University of Bristol[8]
Symptoms associated with VIPIT include headache, tiny red spots under the skin, blurred vision, fainting or loss of consciousness, impaired movement in parts of the body, or coma. With either of these vaccines these blood clots, so far, only occurs 4-20 days after vaccination. Scientists believe that symptoms before or after that window are likely due to another cause.
It is important to note that COVID-19 itself has been reported to lead to thrombocytopenia (low blood platelets) in up to 41% of positive patients, with the figure going up to 95% of those with severe disease.[9]
Cause for cautious optimism:
Two separate studies published in the New England Journal of Medicine on April 9 indicated that in the case of the AZ vaccine, used in Europe, VIPIT was due to rogue antibodies against platelet factor 4 (PF4). This complication is similar to heparin-induced thrombocytopenia (HIT) and is diagnosed and treated the same way. It can be diagnosed with a lab test called ELISA that is pre-treated with PF4. If there is a big immune response, that means the patient has VIPIT. To be clear, there are lots of things that can cause blood clots and health professionals want to know what the cause is because the appropriate treatment is dependent on what is causing the problem. VIPIT from the AZ vaccine is treated with the administration of intravenous immunoglobins (IVG) and anti-coagulants. The J&J vaccine was not used in either of these studies and so we do not yet know if the same is true for that vaccine, but both are the same type of (viral vector) vaccine and both use an adenovirus as the viral vector.[10]
If we can get the one-and-done J&J vaccine back in use safely, that would be especially helpful for vaccinating unsheltered people. It would also be much easier to use in rural areas because J&J can be stored in a regular refrigerator unlike the Pfizer and Moderna vaccines that must be kept frozen.
My take:
For those who choose not to get vaccinated, for whatever reason, hoping to ride the coronavirus out, you should know that even without a vaccine, the SARS epidemic that hit Asia in 2002 did eventually go away, or, more likely, mutated to a less lethal virus. It took four years, but it can happen. However, that is not what always happens. Case in point, smallpox, which was around since at least the fourth century until it was declared eradicated by the World Health Organization in 1980. I don’t think I know anyone who has had smallpox and I may not know anyone who knows anyone who has had smallpox. In that case, the vaccine worked as intended.
Maybe you may feel like you are strong and healthy and even if you got COVID-19, you are unlikely to get significantly sick. Consider the possibility that you could be asymptomatic but still spread the disease. There are just no options that are completely risk free. Choose wisely.
[1]Romeo, A. (4/15/2021). America is about to hit a “vaccine wall” as demand drops—with or without Johnson & Johnson, Yahoo News.
[2]Bunn, C. (4/12/2021). Vaccine hesitancy among Black Americans has turned a corner. Here’s why.”, NBC News.
[3]Gonzalez, D., Skopor, L., McDaniel, M., Kenney, G.M. (4/2021). Perceptions of discrimination and unfair judgement while seeking health care, findings from the September 11-28 Coronavirus Tracking Survey, Urban Institute Health Policy Center. Retrieved from: https://www.urban.org/sites/default/files/publication/103953/perceptions-of-discrimination-and-unfair-judgment-while-seeking-health-care_0.pdf
[4] Hamel, L., Lopez, L., Kearney, A., Brodie, M.(3/30/2021) KFF COVID-19 monitor: March 2021. Retrieved from: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-march-2021/
[5]Kanduc, D., Shoenfeld, Y. (9/18/2020). Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine, Immunol. Res. doi: 10.1007/s12026-020-09152-6
[6]Macela, A, Kubelkovak, K. (3/22/2021). Why does SARS-Co-V-2 infection induce autoantibody production? Pathogens, 10(3). doi: 10.3390/pathogens10030380
[7]Taylor, A. (4/16/2021). Blood clot risks: comparing AstraZenica vaccine and the contraceptive pill, The Conversation. Retrieved from: https://theconversation.com/blood-clot-risks-comparing-the-astrazeneca-vaccine-and-the-contraceptive-pill-158652
[8]Russell, P. (4/15/2021). Vaccines carry far lower risk for rare blood clots than COVID, study shows, Medscape News UK
[9] Op cit Taylor, A. (4/9/2021).
[10] Grenacher, A., et. al. (4/9/2021). Thrombocytic thrombocytopenia after ChAdOx1 nCoV-19 vaccination, NEJM. doi:10.1056/NEJMoa2104840Schulz,NH, et. al. (4/9/2021). Thrombocytic thrombocytopenia after ChAdOx1 nCoV-19 vaccination, NEJM. doi: 10/1056/NEJMoa2104882
#vaccination#covidー19#astrazeneca vaccine#johnson and johnson#blood clots#molecular mimicry#autoimmune disease
4 notes
·
View notes
Text
Are You Experiencing Some of the Common Symptoms of Ascension?
(Long Post)
Some of you may find this helpful, please only take what helps and do not worry or fret if something in this list does not resonate with you. You can take what resonates and leave what doesn’t. That is okay.
Those who need to hear this will be drawn to it, for that is the way of our great Universe.
~ Blessings to All ~
I was just looking at quite a few pages of COVID long haulers support groups...where a COVID-positive was indicated and they have not fully recovered their well being. 85% of the descriptions of their symptoms (which are found as negative health concerns) are labelled as COVID...some are being told it’s Lyme, fibromyalgia and chronic fatigue among other unknown causes. As I reviewed the endless lists and common symptoms I found that they are very similar to ascension symptoms.
However most do not realize that they are going through a physical change in their DNA structure. Those who are aware and have gone through the many waves of change understand and roll with the fatigue and other symptoms in gratitude and not fear, trusting and seeing the positive change in who they are as this occurs. It is a leaving or shedding what has been known... to reveal the true self within.
I felt it would be supportive for you, and for your friends and loved ones to review to find a “positive outlook” on a changing body and world. May this fill you with hope, for all are emerging through this great transformation.
PLEASE NOTE: This article is based on work presented by Samuel Greenberg's original list and is not authored by Dr. Nickerson. Before you read this, realize that you are okay and that what you are experiencing is "The SHIFT". This is a normal process when the universal vibrational energy forces you to rise above your normal 3D level of existence here on Earth. It’s all okay. When in doubt, please see your doctor to confirm to alleviate fear.
Ascension Symptoms:
1. Feeling as though you are in a pressure cooker or in intense energy; feeling stress. Remember, you are adjusting to a higher vibration and you will eventually adjust. Old patterns, behaviors and beliefs are also being pushed to the surface. There is a lot going on inside of you.
2. A feeling of disorientation; not knowing where you are; a loss of a sense of place. You are not in 3D anymore, as you have moved or in the process of moving into the higher realms.
3. Unusual aches and pains throughout different parts of your body. You are purifying and releasing blocked energy vibrating at 3D, while you are vibrating in a higher dimension.
4. Waking at night between 2 and 4 a.m. Much is going on in your dream state. You can’t be there for long lengths of time and need a break. This is also the ‘cleansing and releasing’ hour.
5. Memory loss. A great abundance of short term memory loss and only vague remembrances of your past. You are in more than one dimension at a time, and going back and forth as part of the transition, you are experiencing a ‘disconnect’. Also, your past is part of the Old, and the Old is forever gone. Being in the Now is the way of the New World.
6. ‘Seeing’ and ‘hearing’ things. You are experiencing different dimensions as you transition, all according to how sensitive you are and how you are wired.
7. Loss of identity. You try to access the Old you, but it is no longer there. You may not know who you are looking at in the mirror. You have cleared much of your old patterns and are now embodying much more light and a simpler, more purified divine you. All is in order, You are okay.
8. Feeling ‘out of body’. You may feel as though someone is talking, but it is not you. This is our natural defense mechanism of survival when we are under acute stress or feeling traumatized or out of control. Your body is going through a lot and you may not want to be in it. My ascension guide told me that this was a way of easing the transition process, and that I did not need to experience what my body was going through. This only lasted a short time. It passes.
9. Periods of deep sleeping. You are resting from all the acclimating and are integrating, as well as building up for the next phase.
10. Heightened sensitivities to your surroundings. Crowds, noise, foods, TV, other human voices and various other stimulations are barely tolerable. You also overwhelm very easily and become easily overstimulated. You are tuning up. Know that this will eventually pass.
11. You don’t feel like doing anything. You are in a rest period, ‘rebooting’. Your body knows what it needs. In addition, when you begin reaching the higher realms, ‘doing’ and ‘making things happen’ becomes obsolete as the New energies support the feminine of basking, receiving, creating, self-care and nurturing. Ask the Universe to ‘bring’ you what you want while you are enjoying yourself and having fun.
12. An intolerance for lower vibrational things of the 3D, reflected in conversations, attitudes, societal structures, healing modalities, etc. They literally make you feel ‘sick’ inside. You are in a higher vibration and your energies are no longer in alignment. You are being ‘pushed, to move forward; to ‘be’ and create the New.
13. A loss of desire for food. Your body is adjusting to a new, higher state of being. Also, part of you does not want to be here anymore in the Old.
14. A sudden disappearance of friends, activities, habits, jobs and residences. You are evolving beyond what you used to be, and these people and surroundings no longer match your vibration. The New will soon arrive and feel so-o-o-o much better.
15. You absolutely cannot do certain things anymore. When you try to do your usual routine and activities, it feels downright awful. You are evolving beyond what you used to be, and these people and surroundings no longer match your vibration. The New will soon arrive and feel so-o-o-o much better.
16. Days of extreme fatigue. Your body is losing density and going through intense restructuring.
17. A need to eat often along with what feels like attacks of low blood sugar. Weight gain, especially in the abdominal area. A craving for protein. You are requiring an enormous amount of fuel for this ascension process. Weight gain with an inability to loose it no matter what you do is one of the most typical experiences. Trust that your body knows what it is doing.
18. Experiencing emotional ups and downs; weeping. Our emotions are our outlet for release, and we are releasing a lot.
19. A wanting to go Home, as if everything is over and you don’t belong here anymore. We are returning to Source. Everything is over, but many of us are staying to experience and create the New World. Also, our old plans for coming have been completed.
20. Feeling you are going insane, or must be developing a mental illness of some sort. You are rapidly experiencing several dimensions and greatly opening. Much is available to you now. You are just not used to it. Your awareness has been heightened and your barriers are gone. This will pass and you will eventually feel very at Home like you have never felt before, as Home is now here.
21. Anxiety and panic. Your ego is losing much of itself and is afraid. Your system is also on overload. Things are happening to you that you may not understand. You are also losing behavior patterns of a lower vibration that you developed for survival in 3D. This may make you feel vulnerable and powerless. These patterns and behaviors you are losing are not needed in the higher realms. This will pass and you will eventually feel so much love, safety and unity. Just wait.
22. Depression. The outer world may not be in alignment with the New, higher vibrational you. It doesn’t feel so good out there. You are also releasing lower, darker energies and you are ‘seeing’ through them. Hang in there.
23. Vivid, wild and sometimes violent dreams. You are releasing many, many lifetimes of lower vibrational energy. Many are now reporting that they are experiencing beautiful dreams. Your dream state will eventually improve and you will enjoy it again. Some experience this releasing while awake. My mother commented one day that she believed I was having nightmares in the daytime.
24. Night sweats and hot flashes. Your body is ‘heating’ up as it burns off residue.
25. Your plans suddenly change in mid-stream and go in a completely different direction. Your soul is balancing out your energy. It usually feels great in this new direction, as your soul knows more than you do. It is breaking your ‘rut’ choices and vibration.
26. You have created a situation that seems like your worst nightmare, with many ‘worst nightmare’ aspects to it. Your soul is guiding you into ‘stretching’ into aspects of yourself where you were lacking, or into ‘toning down’ aspects where you had an overabundance. Your energy is just balancing itself.
Remember.....Finding your way to peace through this situation is the test you have set up for yourself. This is your journey, and your soul would not have set it up if you weren’t ready. You are the one who finds your way out and you will.
Looking back, you will have gratitude for the experience and realize that you are a different person.
I hope this helps.
#ascension#awakening#enlightenment#5d#3d#spiritualguidance#spiritual gangster#spiritual development#spirituality#dna#dna activation#covid#covid-19#coronavirus#new age#it's a new world
5 notes
·
View notes
Text
The Covid 19 Scam
This document was copied from the moderna web site which is proof that they were working on coved 19 before the first cases were ever detected which is absolute proof that it is all a devious and deadly scam perpetuated by all the worlds governments against the people of the world Nuremberg two should be demanded by the people for justice to be recognised or democracy is dead
Moderna Announces Progress in Prophylactic Vaccines ...
https://www.businesswire.com/news/home/20200210005823/en/Moderna-Announces-Progress-Prophylactic-Vaccines-Modality-CMV
The development candidates announced today are mRNA vaccine candidates against Epstein-Barr virus (mRNA-1189), respiratory syncytial virus (mRNA-1345) in young children, and the novel coronavirus ...
Moderna Announces Progress in Prophylactic Vaccines Modality with CMV Vaccine Phase 2 Study Data Now Expected in Third Quarter 2020 and Expands Investment in This Core Modality with Three New Development Candidates
Phase 2 CMV vaccine dose-confirmation study more than sixty percent enrolled
mRNA-1189 to prevent infectious mononucleosis and Epstein-Barr virus (EBV) infection
mRNA-1345 to prevent respiratory syncytial virus (RSV) disease in young children, with the intent to combine with mRNA-1653 to create a pediatric respiratory vaccine against RSV, hMPV and PIV3
mRNA-1273 to prevent novel coronavirus (2019-nCoV) disease, in collaboration with the National Institutes of Health; physical manufacturing of first batch complete, awaiting analytical testing
February 10, 2020 05:11 PM Eastern Standard Time
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Moderna, Inc. (Nasdaq: MRNA), a clinical stage biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines for patients, today announced that the Phase 2 dose-confirmation study of its mRNA vaccine against cytomegalovirus (CMV) is enrolling ahead of plan with data now expected in the third quarter of 2020. The Company also announced three new vaccine development candidates, which support the Company’s strategy, announced January 12, to accelerate new development candidates in its core modalities, prophylactic vaccines and systemic secreted & cell surface therapeutics. The three new infectious disease vaccine candidates complement the January announcement of two autoimmune development candidates, PD-L1 (mRNA-6231) and IL-2 (mRNA-6981), in the Company’s other core modality, systemic secreted & cell surface therapeutics.
Moderna’s CMV vaccine (mRNA-1647), the first mRNA vaccine for an infectious disease to enter a Phase 2 study, is enrolling ahead of plan with the second cohort nearly completed. This Phase 2 study will investigate the safety and immunogenicity of mRNA-1647 in approximately 252 healthy adults in the U.S. at three dose levels (50, 100 and 150 μg) in both CMV-seronegative and CMV-seropositive participants administered in a three-dose vaccination schedule (0, 2 and 6 months). The first interim analysis, expected in the third quarter of 2020, will evaluate safety and immunogenicity at three months (one month after the second vaccination) and is intended to inform Phase 3 dose selection. The Company is actively preparing for a Phase 3 pivotal study, which will evaluate prevention of primary CMV infection in a population that includes women of childbearing age.
The development candidates announced today are mRNA vaccine candidates against Epstein-Barr virus (mRNA-1189), respiratory syncytial virus (mRNA-1345) in young children, and the novel coronavirus (mRNA-1273). mRNA-1345 will be evaluated in early clinical trials with the intention of combining the vaccine with mRNA-1653, Moderna’s hMPV/PIV3 vaccine, to address RSV, hMPV and PIV3, viruses that cause significant respiratory diseases in young children, in one vaccine. Each of these new development candidates utilizes the Company’s proprietary lipid nanoparticle (LNP) technology.
Today’s announcement reflects the Company’s belief that positive Phase 1 safety and immunogenicity data across nine studies with more than 1,000 participants have validated the Company’s prophylactic vaccine modality. Clinical data demonstrate that Moderna’s proprietary vaccine technology has been generally well-tolerated1 and can elicit durable immune responses to viral antigens. The Company also believes that it has demonstrated the ability to leverage shared technology, digital systems and its flexible manufacturing infrastructure to advance a large portfolio quickly and efficiently.
“Our investments in science and manufacturing have resulted in six positive Phase 1 infectious disease vaccine readouts. These data validate the technology used in our prophylactic vaccines modality, which has allowed us to accelerate research and development timelines, and advance our mRNA vaccines into new areas of high unmet need. I am pleased with the continued progress of our late-stage CMV vaccine program as we prepare for a pivotal Phase 3 study and commercial readiness. The three new development candidates reflect the continued productivity of our platform and the potential of our mRNA technology. Moderna now owns global rights to three vaccines, which we believe have blockbuster potential – CMV, EBV and the potential combination RSV/hMPV/PIV3 vaccine for young children,” said Stéphane Bancel, Moderna’s Chief Executive Officer. “I am proud of the team’s ability to rapidly respond to the ongoing public health crisis posed by the novel coronavirus and to be working with the National Institutes of Health and Coalition for Epidemic Preparedness Innovations.”
Moderna currently has 24 mRNA development candidates in its portfolio with 12 in clinical studies. Across Moderna’s pipeline, more than 1,500 participants have been enrolled in clinical studies.
About Moderna’s New Development Candidates
mRNA-1189 is an mRNA vaccine against Epstein-Barr virus (EBV) containing five mRNAs that encode viral proteins in EBV, gp350, gB, gp42, gH and gL. Similar to Moderna’s CMV vaccine (mRNA-1647), the viral proteins in mRNA-1189 are expressed in their native membrane-bound form for recognition by the immune system. There is no approved vaccine for EBV.
mRNA-1345 is an mRNA vaccine against respiratory syncytial virus (RSV) in young children encoding for a prefusion F glycoprotein, which elicits a superior neutralizing antibody response compared to the postfusion state. The Company intends to combine mRNA-1345 with mRNA-1653, its vaccine against hMPV and PIV3, to create a combination vaccine against RSV, hMPV and PIV3. There is no approved vaccine for RSV.
mRNA-1273 is an mRNA vaccine against the novel coronavirus encoding for the viral Spike (S) protein, which was selected by Moderna in collaboration with the National Institutes of Health, the manufacture of which was funded by the Center for Epidemic Preparedness and Innovations (CEPI). The S protein complex is necessary for membrane fusion and host cell infection and has been the target of vaccines against the coronaviruses responsible for Middle Eastern Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). On January 13, the NIH and Moderna’s infectious disease research team finalized the sequence for the 2019-nCoV vaccine and Moderna mobilized toward clinical manufacture. The first clinical batch, including fill and finishing of vials, was completed on February 7. This mRNA vaccine was designed and manufactured in 25 days and is undergoing analytical testing prior to release to the NIH for use in their planned Phase 1 clinical trial in the U.S. Currently, there are no approved vaccines specific to 2019-nCoV.
About Epstein-Barr Virus (EBV)
EBV is a common herpesvirus that is spread through bodily fluids, most commonly saliva, and contracted primarily by young children and adolescents (approximately 50% and approximately 89% seropositivity, respectively). It is a major cause of infectious mononucleosis (IM) in the U.S., accounting for over 90% of the approximately 1-2 million cases annually. IM can debilitate patients for weeks to months and, in some cases, can lead to hospitalization and splenic rupture. EBV infection is associated with the development and progression of certain lymphoproliferative disorders, cancers, and an increased risk of autoimmune diseases including multiple sclerosis (MS), an autoimmune disease of the central nervous system. There is no approved vaccine for EBV.
About Respiratory Syncytial Virus (RSV)
RSV is the leading cause of unaddressed severe lower respiratory tract disease and hospitalization in infants and young children worldwide, with most children infected at least once by two years of age. The virus is transmitted primarily via contamination of environmental surfaces with infectious secretions, and symptoms typically begin within several days of exposure. The illness may manifest as wheezing, bronchiolitis, pneumonia, hospitalization or even death.
In the United States, it is estimated that over two million children younger than five years of age receive medical attention and more than 86,000 are hospitalized due to RSV infection annually. Globally, RSV is estimated to be responsible for over approximately 33 million episodes of acute lower-respiratory tract infection, 3.2 million hospitalizations and as many as 118,000 deaths per year in children younger than five years of age. Infections with RSV follow a seasonal pattern, occurring primarily in the Northern hemisphere between the months of November and April, and in the Southern hemisphere primarily between March and October. There is no approved vaccine for RSV.
About Coronavirus (2019-nCoV)
Coronaviruses are a family of viruses that can lead to respiratory illness, including Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). Coronaviruses are transmitted between animals and people and can evolve into strains not previously identified in humans. On January 7, 2020, a novel coronavirus was identified as the cause of pneumonia cases in Wuhan, Hubei Province of China. Estimates from the World Health Organization as of February 9, 2020 indicate that there are approximately 37,000 confirmed cases and over 800 deaths worldwide. The suspected number of infections is likely to be substantially higher. It is important to note that there is not yet a good understanding of the rate of asymptomatic infection. Currently, there are no approved vaccines specific to 2019-nCoV.
About Moderna’s Prophylactic Vaccines Modality
Moderna scientists designed the Company’s prophylactic vaccines modality to prevent infectious diseases. More than 1,000 participants have been enrolled in Moderna’s infectious disease vaccine clinical studies under health authorities in the U.S., Europe and Australia. Based on clinical experience across six Phase 1 studies, the Company deems prophylactic vaccines a core modality and intends to accelerate development of its infectious disease vaccine candidates.
The potential advantages of an mRNA approach to prophylactic vaccines include the ability to mimic natural infection to stimulate a more potent immune response, combining multiple mRNAs into a single vaccine, rapid discovery to respond to emerging pandemic threats and manufacturing agility derived from the platform nature of mRNA vaccine design and production.
Moderna currently has nine development candidates in its prophylactic vaccines modality, including:
Vaccines against serious respiratory infections
Respiratory syncytial virus (RSV) vaccine for older adults (mRNA-1777 and mRNA-1172 or V172 with Merck)
RSV vaccine for young children (mRNA-1345)
Human metapneumovirus and parainfluenza virus type 3 (hMPV/PIV3) vaccine (mRNA-1653)
Novel coronavirus (2019-nCoV) vaccine (mRNA-1273)
Influenza H7N9 (mRNA-1851)
Vaccines against serious infections transmitted from mother to baby
Cytomegalovirus (CMV) vaccine (mRNA-1647)
Zika vaccine (mRNA-1893) with the Biomedical Advanced Research and Development Authority (BARDA)
Vaccines against common viral infections with high unmet need
Epstein-Barr virus (EBV) vaccine (mRNA-1189)
To date, Moderna has demonstrated positive Phase 1 data readouts for six prophylactic vaccines (H10N8, H7N9, RSV, chikungunya virus, hMPV/PIV3 and CMV). Moderna’s CMV vaccine is currently in a Phase 2 dose-confirmation study. Moderna’s investigational Zika vaccine (mRNA-1893), currently in a Phase 1 study, was granted FDA Fast Track designation.
Moderna has built a fully integrated manufacturing plant in Norwood, MA which enables the promise of the technology platform.
About Moderna
Moderna is advancing messenger RNA (mRNA) science to create a new class of transformative medicines for patients. mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane or secreted proteins that can have a therapeutic or preventive benefit and have the potential to address a broad spectrum of diseases. Moderna’s platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, providing the Company the capability to pursue in parallel a robust pipeline of new development candidates. Moderna is developing therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases, and autoimmune and inflammatory diseases, independently and with strategic collaborators. Moderna has 24 mRNA development candidates in its portfolio across all modalities, with 12 in clinical studies. Four of these programs are in or preparing for Phase 2 studies and the Company is preparing for its first Phase 3 study.
Headquartered in Cambridge, Mass., Moderna currently has strategic alliances for development programs with AstraZeneca, Plc. (Nasdaq: AZN) and Merck, Inc. (Nasdaq: MRK), as well as the Defense Advanced Research Projects Agency (DARPA), an agency of the U.S. Department of Defense; the Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS). Moderna has been named a top biopharmaceutical employer by Science for the past five years.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding plans for Moderna’s development candidates, plans to create a pediatric respiratory vaccine against RSV, hMPV and PIV3, plans regarding regulatory submission and clinical testing for mRNA-1273, expected timing of data from the Phase 2 study of mRNA-1647, and Moderna’s strategy, business plans and focus. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, uncertainties related to market conditions and the completion of the public offering on the anticipated terms or at all. These and other risks and uncertainties are described in greater detail in the section entitled “Risk Factors” in Moderna’s most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) and other filings that Moderna has made or may make with the SEC in the future. Any forward-looking statements contained in this press release represent Moderna’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Moderna explicitly disclaims any obligation to update any forward-looking statements.
Moderna has filed a registration statement (including a prospectus) with the SEC for the offering to which this communication relates. Before you invest, you should read the prospectus in that registration statement and other documents the issuer has filed with the SEC for more complete information about the issuer and this offering. You may get these documents for free by visiting EDGAR on the SEC Web site at www.sec.gov. Alternatively, the issuer, any underwriter or any dealer participating in the offering will arrange to send you the prospectus if you request it by calling toll-free 1-866-471-2526.
1 The most common adverse reactions in Moderna’s Phase 1 clinical trials in prophylactic vaccines include injection site pain, headache, myalgia, and fatigue.
Contacts
Moderna Contacts:
Media:
Colleen Hussey
Senior Manager, Corporate Communications
203-470-5620
[email protected]
Investors:
Lavina Talukdar
Head of Investor Relations
617-209-5834
[email protected]
Release Summary
Moderna announces progress in prophylactic vaccines modality with CMV vaccine Phase 2 study data now expected in third quarter 2020.
Contacts
Moderna Contacts:
Media:
Colleen Hussey
Senior Manager, Corporate Communications
203-470-5620
[email protected]
Investors:
Lavina Talukdar
Head of Investor Relations
617-209-5834
[email protected]
TIME LINE
mRNA-1273 to prevent novel coronavirus (2019-nCoV) disease, in collaboration with the National Institutes of Health; physical manufacturing of first batch complete, awaiting analytical testing
February 10, 2020 05:11 PM Eastern Standard Time
January 2020
31 January – The first two cases of coronavirus (2019-nCoV) in the United Kingdom are confirmed.[1]
February 2020
6 February – A third case of coronavirus is confirmed in the UK.[2]
10 February – The total number of cases in the UK reaches eight as four further cases are confirmed in people linked to an affected man from Brighton.[3][4]
11 February – A ninth case is confirmed in London.[5]
23 February – The DHSC confirms a total of 13 cases in the UK as four new cases in passengers on the cruise ship Diamond Princess are detected. They are transferred to hospitals in the UK.[6]
28 February – The first British death from the disease is confirmed by the Japanese Health Ministry; a man quarantined on the Diamond Princess cruise ship.[7]
1 note
·
View note