#vpoc has cool rocks
Text
Let's Talk Pyrite Disease
Okay. Pyrite is amazing. Pyrite, or fool’s gold, has a simple formula (FeS2), forms beautiful cubes, and has all sorts of cool features. I'll talk later about how iron minerals can move and change and how they're related to theories about cells, but first, let's talk about Pyrite disease.

Image ID: Several pyritized ammonite shells in various stages of decay. They all have a light-grey color and a velvety appearance. There are a few in the center that are severely decayed and appear to be just a pile of dark grey and yellow dust, with a similar appearance to mold. One ammonite in the top-center is a more gold-brown color and has cube-shaped pyrite crystals on it. End ID.
[Image by https://twitter.com/MSidKelly/status/667548523475288064]
TLDR: Pyrite disease is a form of rust that impacts pyrite. It produces iron oxides, sulfuric acid, and sulfur dioxide, damaging mineral specimens, fossils, and storage materials. It causes expansion and acid damage that cracks and erodes specimens.
oh and @simple-potato-farmer, @team-clockers, @edthefatmagicturtle, y’all expressed interest in this so here you go! warning: it’s 10 pages long
How Can Fossil Come in Contact with Pyrite?
Pyrite is the most abundant sulfide mineral. Pyrite is found in quartz veins, coal mines, sedimentary rock, metamorphic rock, and in fossils as a replacement mineral. Pyrite forms in anoxic environments, which makes it a good environmental indicator. It also means that pyrite is found in association with many deep ocean fossils.
Fossils can be replaced by pyrite. This happens in a process called pyritization, a form of replacement diagenesis where the original material of the organism (in some cases, even soft parts) is replaced by pyrite. For instance, due to the anoxic and high-sulfide concentration in the deep ocean, calcite shells may be replaced by iron pyrite. This is aided by the presence of sulfur-reducing bacteria that facilitate the creation of iron sulfides, and therefore facilitate the precipitation of pyrite during decay(1) as sulfides are produced by the decaying organism(2). In a unique case, high microbial activity led to documentation of pyrite formation on the shells of living mollusks(3). This process leads to beautiful pyritized fossils that look like metal casts but actually are the fossil completely replaced by pyrite.

Image ID: An ammonite cut in half to show pyritization. The entire ammonite appears metallic gold, and the internal cavities contain fine drusy pyrite crystals. End ID.
[Image by: Replacement/Recrystallization (petrifiedwoodmuseum.org)]
Not all fossils end up beautifully pyritized, and not all pyritized fossils stay that way. Pyrite disease, also called pyrite oxidation, pyrite rot, or pyrite decay, is a form of rust. Millions of years of history, only to be destroyed by poor archiving, humidity, and pyrite decay.

Image ID: A brachiopod specimen with pyrite replacement and a patchy outer coating of pyrite. Unlike the ammonite, the pyrite has grown over the shell of this brachiopod in addition to replacing the original structure. End ID.
[Image by: jsjgeology.net/Replacement.htm]
What does Pyrite Disease Do?
Since pyrite is formed in anoxic conditions, most pyritized specimens remain stable for a long time. The reason we observe so much pyrite decay in museum settings and other fossil collections is because the specimens have been removed from the rock and sediment that were limiting their exposure to oxygen. Pyrite decay can occur with or without water, but when pyrite oxidizes in humid air, it reacts with both oxygen and water. This creates not only iron oxide ‘rust’, but also iron sulfate, sulfuric acid, and sulfur dioxide gas4 (corrosive and toxic materials)(1). This chemical reaction eventually destroys the specimen through a combination of factors. The sulfuric acid is corrosive and damages the specimen. Oxidized pyrite is unstable and may crumble over time. There is also another damaging factor: the risks of pyrite replacement and iron sulfate production.

Image ID: A tray of mollusk fossils, each with separate boxes, with evidence of pyrite decay. There is dark grey dust in the bottom of most boxes, and a line of what appears to be water damage across the boxes. The apparent water damage is the result of the sulfuric acid produced. It has also discolored fossils and their labels. End ID. [Image by me]
Pyrite disease is a progressive process that affects fossils over time. Many pyritized specimens may last for years in perfect condition, while many others progressively decay from undetected pyrite disease. The reason that pyrite replacement is notorious for decay has to do with the crystal structure of pyrite. The famous cuboid structure of pyrite crystals is the most stable form of pyrite(4) since the compact form does not easily absorb moisture. Pyrite may occur in a compact, crystalline, stable form or a porous microcrystalline form that is unstable. Certain formations produce more stable pyrite with fewer impurities, but certain fossils are also more susceptible to unstable forms of pyrite. Marcasite is a less common dimorph of pyrite that is more unstable(5). Ammonites, especially those from the Charmoth clays, are notorious for pyrite decay while South American specimens and Yorkshire coast fossils are much more stable(2). The iron sulfate that is produced by the oxidation process is considered an efflorescent mineral(5), a term that means “flowering.” This is because this material has a higher molar volume (the ratio of the volume occupied by a certain amount of a substance) than iron pyrite and wedges the specimen apart as it expands past the constraints of the original crystal structure(5).
Unfortunately, the creation of efflorescent minerals, including other hydrous sulfate minerals, creates a feedback loop. These materials produce acids and provide water that causes the pyrite to further decay, producing more Fe3+ and more efflorescent materials, which feed the cycle of oxidation(5).

Image ID: Two images of the same pyrite disk, taken two years apart. In the left image, the disk has a few cracks and white discoloration in the center, with a yellow outline around the discoloration from sulfur. In the right image, the disk is shown two years later, cracked into four large pieces. The discoloration now extends almost to the edges of the disk, with a much larger yellow margin. End ID.
[Image by Ed Clopton, General: Need 'Pyrite Disease' Photos (mindat.org)]
(For an interesting, related topic – pyrite decay is a risk in coal mining for a multitude of reasons, including the increase of pulmonary and respiratory disease after pyrite inhalation, the risk of silicosis, and the risk of spontaneous combustion caused by the exothermic reaction involved in the decay of high-sulfide deposits.)
What Does Pyrite Disease Look Like?
Pyrite disease may appear grey, greenish, black, red-orange, white, or sulfur yellow. A redder color indicates rust from other iron minerals other than pyrite. Since pyrite contains sulfur and pyrite oxidation produces sulfuric acid, it may have the rotten-egg smell often associated with sulfur. A metallic-iron smell may also be present. Inhaling the result of pyrite decay can be hazardous (in my experience, it caused migraines even when I double-masked before entering the room due to the severity of the condition of our collections. In most cases it is much less of a concern). If pyrite disease is found, wear gloves and masks and try to disturb the dust as little as possible. Remember that pyrite disease is worse in humid conditions, and these same conditions may encourage the growth of mold. The early stages of pyrite disease may appear as spots of discoloration on a fossil, a slightly duller appearance, additional crystallization, or cracking. Often, the first noticeable sign that is clearly identifiable is dust in the bottom of the box the specimen is in. In these early stages, the severity of the pyrite decay may not be detected until the specimen is moved or handled, and subsequently begins to crumble or leaves dust and a metallic smell on your hands. Later stages appear like mold and may have a fuzzy, cloud-like appearance. If you handle a specimen with pyrite disease, take care to wash your hands and dry them well before handling any other specimens, and check the container or the contaminated specimens and those that surround it so that they can be replaced if needed. In the worst stages of pyrite decay, what is left is not a fossil that may crumble if handled, but a pile of dust. Pyrite disease can affect vertebrate fossils, invertebrate fossils, botanical specimens, mineral specimens with pyrite, and the containers and shelving used.

Image ID: Two images of cubic pyrite crystals. Left: Four cubic pyrite crystals growing into each other, they are a solid metallic gold color with few visible imperfections. Right: a cubic pyrite crystal in a dark matric. This piece is affected by pyrite decay, and the cubic shape is no longer perfect due to cracks in the specimen. It has orange and yellow sections that appear like water stains due to the sulfuric acid produced by pyrite oxidation. End ID.
[Image by Pyrite Disease - Canadian Museum of Nature]
Why Is It A “Disease?”
If the damage to the fossils themselves was not bad enough, consider another factor: it’s often called pyrite disease, instead of pyrite oxidation, for a reason. That reason is that pyrite disease spreads and progresses over time. Historically it was thought that this was the result of a bacterial component, and it was even recommended to treat fossils with antibacterial ointments(5). While bioleaching of pyrite is studied, bacterial theories are no longer supported. The spread of pyrite disease is due to the spreading of acid and the flaking of iron sulfates that may trigger decay on other specimens that already contain pyrite. Although this process is not contagious due to bacteria, it is still an irreversible process that causes fossils to crumble to dust and is, to an extent, a contagious process. The sulfuric acid and sulfur dioxide created in pyrite oxidation can damage the containers and fossils near the contaminated specimen.
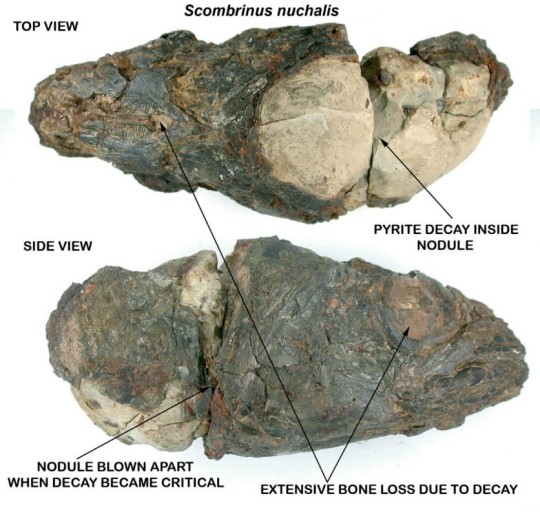
Image ID: An annotated example of a fish skull preserved in a nodule, which decayed and broke due to pyrite disease. There is a top view (top) and a side view (bottom, fish facing right). There are labels pointing to the top of the skull and to the eye socket showing cavities and red-orange rust labeled “extensive bone loss due to decay.” A white section of the nodule that is split and crumbled is labeled “pyrite decay inside nodule.” The side shows extensive decay to both the fossil and nodule, labeled “nodule blown apart when decay became critical.” End ID.
[Image by Trouble with pyrite – Deposits Mag]
One of the reasons that it is considered a disease is the progressive nature of pyrite decay, which visibly worsens over time. Another reason is the way that sulfuric acid seems to spread and contaminate even the container that the fossil is held in, leaving damage marks on the fossil and its labels. Another factor is the fact that the products of pyrite oxidation expand, appearing like mold or growths that further damage the fossil. The expansion of these oxidation products is one of the most damaging aspects of pyrite disease, as the instability in the crystal structure of the specimen causes cracking and flaking. This is because pyrite oxidation causes the conversion of iron pyrite (FeS2), an iron sulfide mineral that has a cubic crystal structure, to iron sulfate (FeSO4), which has an orthorhombic crystal structure. Not only does this cause the specimens to crack and crumble, but the cracking of the specimen causes flakes of pyrite to break off and spread. These flakes may land on other specimens, spreading the contamination of pyrite decay to other fossils that were previously stable. In many cases, museums must face difficult decisions to remove scientifically valuable specimens, or even portions of specimens, to prevent further spread of pyrite disease.

Image ID: Left: The crystal structure of iron pyrite. Right: The crystal structure of iron sulfate. End ID.
[Images by: mp-226: FeS2 (Cubic, Pa-3, 205) (materialsproject.org)]
How Can Pyrite Disease Be Managed?
Now, I’ve said that pyrite disease is unreversible. But can it be stopped from progressing once it contaminates a specimen? The simple answer is no. The more complicated answer follows:
Several factors that contribute to pyrite disease: temperature, humidity, oxygen levels, pH, exposure, crystal structure, and bacteria(3). Some factors, such as bacteria and pH, require specific conditions that are uncommon (Thiobacillus bacteria are present over 95% humidity(3) and pH is often affected by the decay process and the methods used to manage it). Other factors are closely linked (e.g., temperature and humidity).
Pyrite disease is most closely linked to humidity and high oxygen conditions. The best way to stop pyrite disease is prevention. Most museums use some form of climate control to preserve their specimens, but the issue here is that vertebrate fossils (bones) have a moderate recommended relative humidity level (~45-55%) in order to avoid cracking or warping6, while the recommended humidity level to prevent pyrite disease is 30%(7) (although below 50% is also stated by some sources(2,8)). In contrast, humidity above 60% is known to accelerate the decay, even to the point that decay begins within a few days of exposure(3, 2). This low humidity level is not always possible, especially for public display, although it is used in some invertebrate collections. Using humidity and oxygen scavengers (e.g., silica gel packets) when collecting can reduce the exposure of specimens to these factors. Using humidity and oxygen barriers when storing specimens can prevent pyrite disease(3). Contained storage of an already contaminated specimen may trap moisture with the specimen. Although, I would say it is better to have an isolated specimen that decayed than have an open specimen contaminate others.

Image ID: The unpolished appearance of a pyritized ammonite. The ammonite is held in front of a rocky area. It is a dull grey color with a matte appearance. It has lumps of rounded pyrite surrounding it. End ID.
[Image by Martin Curtis, Pyrite Decay in Fossil Collections – ZOIC PalaeoTech Limited (zoicpaleotech.com)]
Since the damage from pyrite disease is exacerbated by sulfuric acid, one of the other prevention methods used relies on acid neutralization(5), although this is less common. Currently, the use of humidity and oxygen buffers is the most common method, but some groups rely on extensive testing of specimens to determine which have unstable forms of pyrite incorporated into the fossil or matrix before acting. Another preventative method is removing salt (which may speed up the decomposition) and removing any matrix that may contain pyrite or trap moisture (e.g., clays)(5). Washing specimens to remove salts or matrix remains could damage specimens that are already unstable or could cause damaging moisture exposure (although this could be avoided by washing with alcohol).
Many studies have attempted to find effective ways to stop, reverse, or prevent pyrite decay. From acid treatments to preemptively coating specimens in resin, these tested methods were often damaging to the specimens, especially over the long term. However, were they damaging enough to be worse than pyrite disease? Not necessarily, but a combination of the damage done, the cost, the time investment, and the ineffectiveness of these treatments means that prevention is still the only reliable method for pyrite disease.
Coating specimens in resins and varnishes may provide a buffering effect, but the resin only slows oxidation, it does not stop it. This becomes an issue when the vanishes cannot be removed, and the specimen cannot be accessed for other treatment methods(2). Varnishes may delay the effects, but they have effects of their own, are usually not moisture-proof, and may yellow and crack over time. Coating with resin or embedding the fossil in resin creates the risk of an explosion as the oxidative reaction builds heat and pressure. Some museums coat their fossils in plastic glues (such as Paraloid B-67 or B-72), these glues are reversible, and B-67 is hydrophobic, unlike most resins.

Image ID: Examples of varnished ammonite specimens, showing progressive stages of pyrite disease as the varnish aged and cracked. Left: Complete pyritized specimens with no signs of decay and a glossy finish from the varnish. Center: Pryitized specimens with patches of yellow-orange decay in sections where the varnish cracked. Right: specimens reduced to dark grey and white dust. End ID.
[Image by Chris Andrew, Pyrite Decay in Fossil Collections – ZOIC PalaeoTech Limited (zoicpaleotech.com)]
There are two “cures” to pyrite decay. However, these do not cure pyrite decay in the sense that they reverse it. Instead, these are methods to neutralize the products of pyrite disease to prevent further harm to the specimens. These two methods involve the use of ammonium gas or ethanolamine thioglycolate to remove pyrite byproducts (ammonia converts the iron sulfate to iron oxide(8)) and neutralize any generated sulfuric acid(2). Both methods are intensive, potentially hazardous, and relatively expensive. As a ‘cure,’ they are effective at temporarily halting the progression of pyrite disease, but not preventing it or reversing it. These treatments have been proven to be ineffective at preventing pyrite decay without the use of controlled microclimate (oxygen and humidity exposure)(3).
References:
1 Replacement/Recrystallization (petrifiedwoodmuseum.org)
2 Pyrite Decay in Fossil Collections – ZOIC PalaeoTech Limited (zoicpaleotech.com)
3Minerals | Free Full-Text | Pyrite Decay of Large Fossils: The Case Study of the Hall of Palms in Padova, Italy (mdpi.com)
4Pyrite Disease - Canadian Museum of Nature
5Pyrite disease (palaeo-electronica.org)
6Shells Eggs Bone and Related Materials 160229 (welshmuseumsfederation.org)
7PowerPoint Presentation (vertpaleo.org)
8Trouble with pyrite – Deposits Mag
#vpoc writes#rockposting#vpoc yells#pyrite disease#pyrite oxidation#vpoc has cool rocks#other cool rocks#long post#paleontology#geology
61 notes
·
View notes
Text
Another rock post be upon ye!! How about.... OPTICAL CALCITE
Also called Icelandic Spar or even Viking sunstone!

Despite all of the fancy names, optical calcite is essentially just exceptionally clear calcite. However, calcite is so cool and deserves all the love <3
Fun properties, double refraction, and the reason behind the names below the cut!

Calcite is called Icelandic spar, due to the area it was most famously mined. It may be called optical calcite if it is exceptionally clear. Calcite (CaCo3) has several notable qualities! Some that are commonly used for its identification are: 3 on the Mohs hardness scale, twinning, birefringence (if y'all ever want a brain melting post I will totally deep dive into birefringence for y'all, I'll talk about a lot of the basics of it here!), effervescence with hydrochloric acid, and distinct cleavage planes. The cleavage is probably the most well know! Look at those perfect 6-sided polyhedrons below! It has three perfect cleavage planes at 60-120 degree angles.

However! What I will be talking about most are the optical properties that give this mineral it's nicknames!
Calcite has a very cool property called double refraction! This is a principle of birefringence (which means that light passing through the crystal refracts at different angles for different polarizations. think polarized lenses in sunglasses that block light moving at a specific angle!). Unpolarized light passes through the calcite and is polarized by the crystal! Splitting it into two rays, one moving in the original direction and one splitting off, causing images to appear doubled!
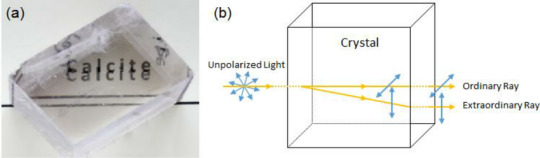
Since the double-image is caused by the distortion of light as it passes through the crystal, the size of the crystal impacts the spacing of the images (as shown above in the chart and below in an example with three pieces of calcite)

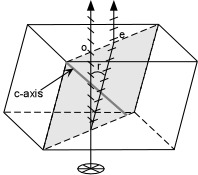
The 'ordinary ray" (original path of light) makes an image that stays in place, but rotating the crystal causes the double of the image (the extraordinary ray) to move. The double image occurs if the direction of the polarization is at an angle to the ordinary ray, rotating the crystal 90 degrees can cause the polarizing direction and the ordinary ray to align, eliminating the double image (shown below).

Interestingly, trilobite eyes are made out of calcite! The many lenses of their eyes are all oriented at the C-axis of the calcite crystal, the only axis that aligns the incoming light and polarized direction, eliminating the double image!
NOW TO THE MOST FUN: WHY IS IT CALLED A VIKING SUNSTONE?
When most people think of a sunstone, they think beautiful feldspars with shimmery orange flecks, or of the man-made, glitter-filled orange stone.
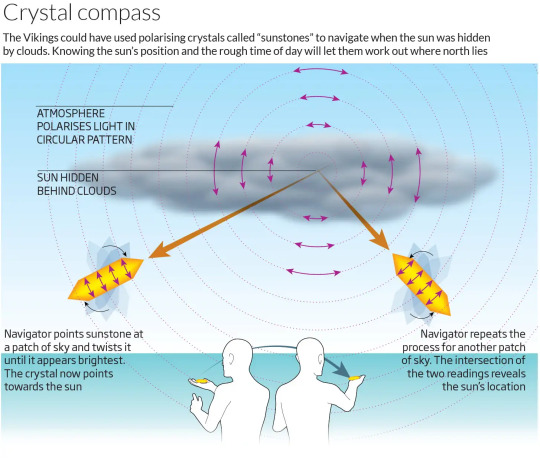
When historians came across discussions of sunstones in Norse texts, they at first thought it was a reference to some mythical stone, or perhaps a real stone tied to mythology in its origin. Then, more texts discussed the use of this "sun stone" in navigation. It wasn't until years later that it was proposed that the sunstone could be calcite, because there was no clear description of how to use a sunstone.
There are two proposed methods for using a sun stone in navigation, the simpler dot method and the more commonly accepted color method (not official names, just the easiest way for me to describe them).


The 'Dot method' involves drawing a mark on one side of the calcite piece. Using the properties we discussed before, and holding the stone at the correct angle, you can calculate the direction of the light source by looking through the stone until the double image of the dot on the opposite side turns into one image. Because rotation affects this process, and because some texts refer to the color yellow, this is the less supported theory.
The 'color method' involves moving the stone until it appears yellow, which is caused by a phenomenon called Haidinger's brush. This makes it so that polarizing minerals may show a yellow pattern when facing away from the sun. This pattern appears more distinct against blue backgrounds (e.g., the sky) and is brightest 90 degrees away from the sun. When tested, it was found that you could calculate the East-West direction within a few degrees by finding the two points where the yellow color was brightest and calculating intersection, even when the sun was behind clouds or set beyond the horizon line.
Thanks @earthgeco for requesting more rocks!!! You have activated my trap card. As in. You are now trapped with me as I tell you about rocks. (I have more posts under my rockposting tag as well!)
Image sources: 1, 3, 4, 7, 9, 10
Images from me: 2, 5, 6, 8
#vpoc yells#rockposting#optical calcite#icelandic spar#vpoc has cool rocks#long post#vpoc writes#me writing this: why did my teachers not use these diagrams. why didn't they explain it this way instead. what. huh.
14 notes
·
View notes
Text
Alright folks, time for another rock post!
Now, gather around while I break out my notes from the worst class of my life (the class of the trip-from-hell-we-do-not-mention) to tell you about my good friend DOLOMITE.
Here’s Mine:

Now. I hear you clamoring, asking WHAT IS DOLOMITE AND WHY IS YOURS PINK? Great questions! Mine is pink because it is cooler than all that other dolomite.
(Actual explanations, cool pictures, and more detail under the cut!)
No, it’s because mine has manganese in it. Manganese has multiple oxidation states, and one is manganese (II) chloride, which is a pale pink color. Dolomite is usually white, but can be grey, pink, or even tan.



Dolomite is considered a double carbonate! This is because it has an alternative structure where the carbonate (CO3) is either paired with a magnesium or calcium ion. Interestingly, dolomite is also sometimes considered a type of limestone!
Dolomite, despite the groans of the poor geology students that had to memorize the 3 main formation methods and six methods of dolomitization (QUICK, POP QUIZ GEOLOGY STUDENTS! GO GO GO!), is pretty cool! It is a calcium and magnesium-based mineral (CaMg(CO3)2) that is much more common in ancient rock formations than modern ones. It can form directly from precipitation, which creates dolomite crystals, or from dolomitization and dolomitizing fluids. Dolomitization is a process of replacement diagenesis in limestone or other calcium carbonate rocks. This means that the limestone (CaCO3) is replaced by dolomite. This can happen because the Calcium ions and magnesium ions have the same charge, and magnesium rich fluids may cause a magnesium ion to replace a calcium ion (Mg + 2CaCO3 ➤ CaMg(CO3)2 + Ca), forming dolomite.
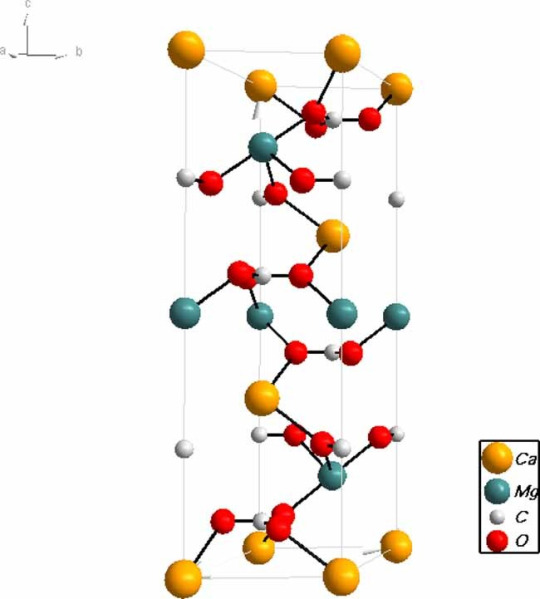
This means that dolomite is primarily found in areas with high evaporation since they require high concentrations of magnesium in solution. Evaporation causes calcite-based minerals to evaporate out, creating a higher Mg/Ca ratio. This means that dolomite can form at salt flats (sabkhas), tidal zones, seepage areas, and so on.
Image sources: 2, 3, 4, 5
Image 1 from me
#rockposting#vpoc yells#vpoc writes#vpoc has cool rocks#this is a reminder that at any moment you can say 'hey vpoc tell me about the rock closest to you' and i will#dolomite
8 notes
·
View notes
Text
Fulgurite post!!
Fulgurites (or sintered soil or petrified lightning) are super cool! They're branching tubes of glass formed when lightning hits sand, in layman's terms, fossilized lightning! If you think the ages of the rocks around you are cool, imagine holding the remnant of an ancient storm!
They can be found all over the world, but are much more common in areas with lots of sand, and are pretty delicate. Finding big ones or ones with the preserved lichtenberg branches are pretty rare.
This one was delicate enough that it was see-through

You can actually calculate how powerful the lightning strike was by the diameter of the interior of the fulgurite! They may look sandy on the outside, but all of them have a hollow smooth glass interior.
Here are some of the ones I found at a sand mine (estimated to be around 500,000 years old)!



#sorry for bad photos. lighting was weird#long post#vpoc has cool rocks#rockposting#geology#fulgurites#petrified lightning#fun facts
46 notes
·
View notes
Note
35!
Oooh 35 is calcite clam shells!
Clam shells are often replaced by calcite crystals, and these crystals can grow to fill the whole shell. They're considered mineral-fossil composites because of the role the shell plays in initiating the formation of the crystals.

3 notes
·
View notes
Text
@yokohamasonebraincell oh! #13 is One of my favorites!! Fibrous malachite! Also called velvet malachite! Malachite forms as a secondary mineral precipitating from water, and the fibrous samples are clusters of thin malachite crystals!

And your blog reminds me of honey calcite and amber and rose quartz :]
3 notes
·
View notes
Note
Rock number 8 >:3
Rock 8 is my pretty agatized coral!!

Fun fact: it's formed when silica in water replaces the skeleton of corals, turning it into a kind of agate called chalcedony! It comes in lots of different colors and can be crystalline or opaque. It's also the florida state rock :]
Also, your blog gives me tigers eye/ tiger iron vibes <3
2 notes
·
View notes
Text
New rock!!!! This cube is a septarian, which forms when mud dries and crystals form in the spaces between the cracks! It's one of my favorite rocks!

5 notes
·
View notes
Text
Eyyyy Rock post!! This one will be kinda long, so be prepared! We're gonna talk about Florida's State Rocks and why they're great but Florida is awful!
Florida has two State Rocks, the State Stone is agatized coral and the State Gemstone is moonstone! But VPOC, you say, those are very cool and pretty rocks. And you are correct!! Or you would be, if they were both rocks and both were from Florida.
Agatized coral is what happens when silica in ocean water replaces the original material with a form of quartz called chalcedony, creating a pseudomorph (the minerals are replaced by another, but the structure remains). You're probably more familiar with petrified wood, but it's the same concept. To me, this makes it a fossil! Not a rock. But Florida can maybe have a pass on this one because 1) stone and fossil were at one time used to refer to the same thing and 2) the other option was limestone. Agatized coral occurs in a variety of colors, from an opaque rainbow of colors to clear whites and browns. Some specimens even have pockets of druzy quartz!


Now here's where it gets dicey. Florida said "hey, our state rock is a little sketchy, let's add another" and they chose,, False Advertising!!! That's right everyone, the Moonstone is not native to Florida and cannot be found here. It is literally only the state gem because of the NASA base in Florida, and was adopted to commemorate the moon landing.
I can't be that mad about it though because it is very pretty. Moonstone is another name for gem quality feldspars. It comes in a variety of pastel colors and is called moonstone because when polished into a round surface it reflects crescents of light! Similarly to why star sapphires earned their name. A phenomena called adularescence reflects light over the surface of the stone, making the variety called rainbow moonstone appear to glow blue!

TLDR: Florida (Derogatory) chose to have two state rocks (affectionate). Both of them are very cool, but one is a fossil (classification as a rock is debatable) and the other isn’t from Florida.
#long post#florida is such a weird state I am so sorry for the people that live in Florida#vpoc yells#vpoc has cool rocks#rockposting#moonstone#agatized coral#geology#florida critical
2 notes
·
View notes
Text
Labradorite!
This is my first rock post, so I decided to go with my favorite rock! I love labradorite! It has a really cool trait called labradorescence where it reflects light at only one angle, giving it really fun color patterns and flash.
Here’s my prettiest piece in daylight:

She has blue! Green! Bands of orange!
Here’s what it looks like from other angles (on the back you can see the faintest shine of blue):

From any other angle, she looks mostly grey!
@reddpenn has a really cool post about it!
This piece is mostly blue, but very bright!!

I also have a little polished piece (shown in different lights) that has a bit of purple:

And a ring that shows the difference between the normal grey and the blue of the labradorescence really well:

8 notes
·
View notes
Text
Lapis Lazuli!
Lapis is considered to be one of the first rocks used for jewelry like beads and cabochons! It was mined as early as 7570 BCE!

By the middle ages it was being shipped to Europe, but not for its value as a gemstone. It was being ground into powder to make pigment! Specifically, lapis makes the pigment Ultramarine!
Here’s the piece that I have!

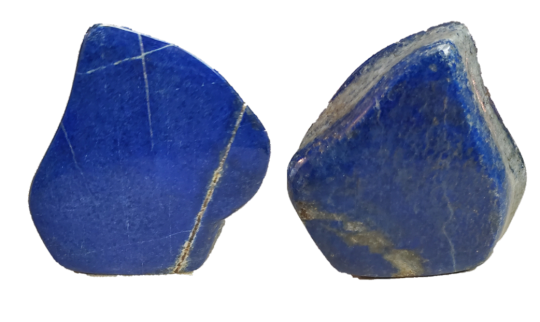
3 notes
·
View notes
Text
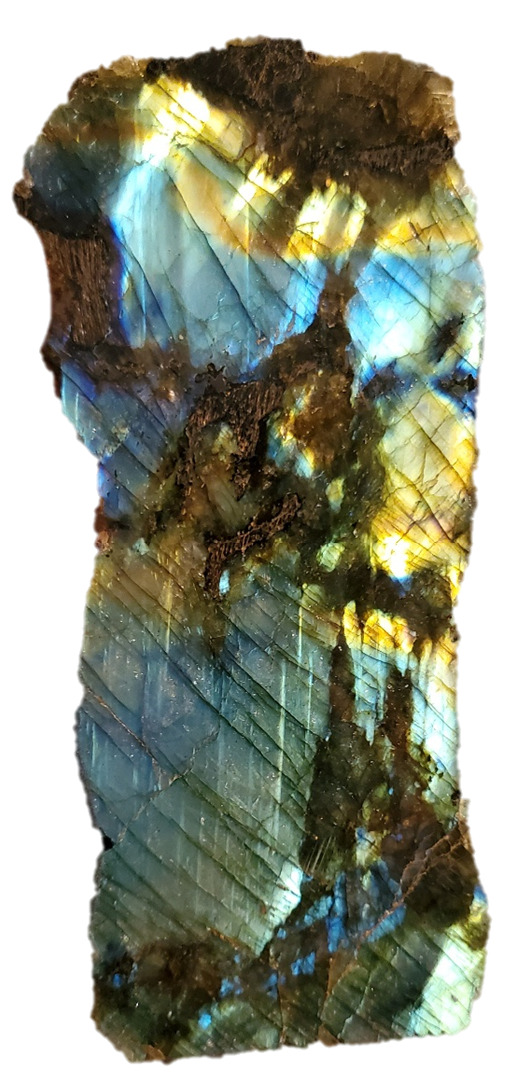
I just think, Labradorite, ya'know?

#I've been having. a week. so here's my favorite rock and another picture of my favorite rock. yep.#vpoc yells#rockposting#vpoc has cool rocks#vpoc pics
0 notes