Text
Recent Advance in Diagnosis, Pathogenesis and Risk Stratification of Essential Thrombocythemia

Abstract
In the 2016 version of WHO classification, bone marrow morphology is critical in the distinction between ET and pre-PMF. Reticulin-fiber grading becomes central: grade 1 or less is needed for ET. Furthermore, CALR assessment must be performed in all ET patients without JAK2 mutation. Some prognostic implications have been described for CALR mutations, i.e., a lower risk of thrombosis in ET.
Abbreviations: MPN: Myelo Proliferative Neoplasm; WHO: World Health Organization; JAK: Janus kinase; CALR: Calreticulin; bp: base pair; MPL: Myelo Proliferative Leukemia virus oncogene; TPO: Thrombo Protein; RARS-T: Refractory Anemia with Ring Sideroblasts associated with marked Thrombocytosis; PMF: Primary Myelo Fibrosis; IPSET: The International Prognostic Score for Essential Thrombocythemia; WBC: White Blood Cell; LMWH: Low-Molecular Weight Heparin; SVT: Splanchnic Vein Thrombosis
Introduction
ET is a Philadelphia-negative MPN characterized by sustained clonal proliferation of the megakaryocytic lineage in the bone marrow and an elevated platelet count in the peripheral blood [1]. According to the WHO classifications (especially the 2016 version), diagnostic criteria for ET include: major criteria (i) an elevated platelet count (≥ 450 X 109/L); (ii) bone marrow biopsy showing proliferation, mainly of the megakaryocyte lineage, with increased numbers of enlarged, mature megakaryocytes with hyperlobulated nuclei. No significant increase or left shift in neutrophil granulopoiesis or erythropoiesis and very rarely minor (grade 1) increase in reticulin fibers (iii) failure to meet diagnostic criteria for BCR-ABL1 CML, PV, PMF, myelodysplastic syndromes or other myeloid neoplasms; (iv) demonstration of clonal markers, such as JAK2V617F (JAK2), CALR or MPL mutations and a minor criterion: presence of a clonal marker or absence of evidence for reactive thrombocytosis. Diagnosis of ET requires meeting all four of the major criteria above or the first three major criteria and a minor criterion [2]. The incidence rate for ET is 0.2-2.5/100.000 people/year. The prevalence is higher at 38-57/100.000 people, because of the long life expectancy (in excess of 20 years) of ET patients [1].
Pathophysiology: Megakaryocyte progenitor cells in ET are hypersensitive to the action of several cytokines including IL3 and IL6 and possibly TPO. There is controversy regarding spontaneous megakaryocyte formation [3].
Genetic data: Driver mutation (JAK2V617F, CALR and MPL mutations): the frequency of JAK2V617F mutation is approximately 50-60%. Most patients with JAK2--unmutated ET express CALR or MPL mutations, with respective estimated incidences of 25-30% and 3-5% [4]. Most CALR mutations were deletions and insertions in exon 9, which cause frame shift mutations. Two CALR mutations are predominantly associated with ET. Type 1 result from a 52-bp deletion, and is found in approximately 50% of patients and type 2 mutation results from a 5-bp TTGTC insertion, and accounts for approximately 30% of patients [5].
Approximately 15% of patients are wild-type for all 3 mutations (i.e., triple-negative)[6]. However, a few triple negative patients carry activating mutation of MPL outside exon 10 [7]. Double-mutant JAK2V617F and CALR positive, patients make a specific subgroup that might be distinct from the JAK2-positive or CALR-positive subgroups and require a careful follow-up and management. The frequency of this cooccurrence was below 1%, in one study [8] and 6.8% in Lim et al. [9]study. In the last study, these patients had the oldest age, higher thrombotic events and higher major arterial thrombotic events after diagnosis and more patients were in the high-risk group for thrombo hemorrhagic complications [9]. Rashid et al. [10] (2015) reported 55 year old female ET patient who was in complete remission without cytoreductive therapy until their paper publication [10].
Non driver mutation: In ET, 46% of patients had somatic mutations. The most commonly mutated, non driver genes, included DNMT3A (6%), TET2 (13%), and ASXL1 (11%), SF3B1 (5%), CEBPA (4%), and TP53, SH2B3, EZH2, and CSF3R (2% each). Patients with ASXL1, EZH2, SRSF2, or IDH mutations are considered to have a "high molecular risk" profile and were at risk for premature death or leukemic transformation. However, only ASXL1 mutations remained significantly associated with survival [11]. The number of mutations also matters, the presence of 2 or more mutations predicted for worse outcomes; the reported median survival was 12.3 years for patients without a mutation compared with 2.6 years for those with 2 or more mutations. Those with post-ET-MF were more likely to have ASXL1 and EZH2 mutations, compared with those with post- PV-MF. The order in which mutations are acquired appears to influence clinical features. It was suggested that those patients who acquired JAK2V617F prior toTET2 are more likely to present with PV than ET [11].
Clinical picture: ET is an indolent myeloproliferative neoplasm [7]. The median age at diagnosis is 60 years. There may be higher prevalence in younger women (approximately 2:1). Many patients are asymptomatic at presentation [3]. The prevalence of constitutional symptoms is relatively high [7]. Patients might also have micro vascular symptoms such as headaches, acroparesthesia, erythromelalgia, peripheral ischemia, transient ischemic attacks, amaurosisfugax or scotom as [1]. Patients may present with splenomegaly (20%), thrombosis and bleeding. Hemorrhagic event, mostly from GIT is experienced in 13 to 37% of patients while thromboembolic events in the major vessels or microvasculature are experienced in 22-84% of patients [3].
Female patients in the child bearing period may experience: live birth rates of 50% to 70%, and spontaneous abortion rates of 25% to 50%, mostly during the first trimester. Age, parity, thrombophilia, platelet count, WBC count, and hemoglobin level have not been found to be predictive of pregnancy outcome in ET. Pregnancy complications in women with ET are associated with a higher risk of subsequent thrombosis [7].
Thrombotic risk factors: IPSET is a score which provides prognostic information on risk of thrombosis. It is based on widely accepted risk factors for thrombosis which are age ≥60 years (2 points), WBC count ≥11X109/L (1 point), and history of thrombosis (1 point) [7]. JAK2 (V617F) plays a major role in the pathogenesis of thrombosis, whereas CALR or MPL mutation and triple negativity identify patients with lower thrombo embolic risk [7]. Elevated platelet count (>1,000*109/L) was associated with lower arterial thrombotic risk [12].
Comparison of clinical and hematological features of patients with JAK2 mutation and CALR mutation: CALR- mutated ET patients had a lower risk of thrombosis than JAK2- mutated ET patients [6].Thrombotic events occurred in 26% of JAK2-mutated ET patients versus 7.7% of CALR-mutated ET patients. Genetic background such as race may influence the risk of thrombosis [12]. Previous studies have shown higher platelet counts and lower hemoglobin, leukocyte counts and granulocyte counts in CALR mutant compared with JAK2 mutant patients [6]. In addition, platelet counts are higher in type 2 CALR mutant compared with type 1 mutant patients [6]. CALR-mutated ET displaysa phenotype favoring megakaryopoiesis [12].
CALR-mutated ET patients had a longer survival compared with those with JAK2V617F mutation. A higher rate of post- ET-MF transformation was reported in CALR-mutated patients versus JAK2-mutated patients. Interestingly, no CALR-mutant ET patient evolved to PV [11].
Outcome: ET may transform into PV, MF, AML or MDS. However, ET is generally considered a benign illness because it is associated with prolonged survival and a low risk of leukemic transformation [13]. The 15-year cumulative risk of progression to myelofibrosis is about 10% on average, higher in type 1 CALR- mutant than in JAK2-mutant ET while the 15-year cumulative risk of leukemic transformation is about 3% on average [7].
Diagnostic Consideration
a) Screening for BCR-ABL1 is important in the diagnostic approach of ET patients to rule out chronic myeloid leukemia and atypical MPN associated with both BCR-ABL1 rearrangement and CALR mutation [7].
b) Assessment of JAK2 mutational status is mandatory in case of unexplained thrombocytosis [14].
c) CALR assessment must be performed in all patients without JAK2 mutations followed by MPL if CALR is absent. These mutations does not replace the need for BM morphologic examination in [1] confirming the diagnosis in triple-negative ET and [2] distinguishing ET from other MPN that share the same mutations, including masked PV and early/ prefibroticmyelo fibrosis [6].
d) JAK2 (V617F) assessment is recommended in patients with SVT because it may be the first marker of a latent MPN. Although, the incidence of CALR exon 9 is low in patients with SVT, CALR assessment can now also be included in the workup of these subjects. Genetic and acquired thrombophilia, in particular the presence of anti-phospholipid syndrome, should be studied in all cases of ET with SVT because ET patients with genetic or acquired thrombophilia are at high risk of recurrent thrombosis [7].
e) At present, there is little evidence to suggest the incorporation of testing for non driver mutation in routine clinical care of ET patients [11].
f) Von Willebr and factor antigen level and ristocetin cofactor activity have to be performed when platelet count is >1000 x 109/L or when an acquired von Willebrand syndrome is anyhow suspected [7].
Differential diagnosis: The most common cause of isolated thrombocytosis is a reactive (or secondary)causes [7] due to infections, acute or chronic inflammatory diseases, smoking, iron deficiency, chronic bleeding, postsurgical states, malignancies, hemolysis, rebound after immunosuppressive chemotherapy, and use of drugs (corticosteroids, adrenaline, and TPO mimetics [14].
a. Other clonal myeloid neoplasms [7].
b. Hereditary thrombocytosis and familial ET. Recent reports have described cases of hereditary thrombocytosis associated with non-canonical germ line mutations of JAK2. In familial ET, JAK2 (V617F) is always a somatically acquired event [7].
c. RARS-T, in which JAK2V617F mutational frequency has been reported to be as high as 50% [6]. Most patients have a combination of SF3B1 mutation (as a driver genetic lesion) and JAK2, MPL, or CALR mutation (as a subclonal genetic lesion); however, up to one-third of patients may have wildtype SF3B1 [7].
d. PV when the hemoglobin/hematocrit level is diagnostically equivocal (as in "masked" PV) [6].
e. Prefibrotic/early prePMF with a high platelet count. Leukocyte count, hemoglobin level, platelet count, serum LDH level, incidence of palpable splenomegaly and circulating CD34 cell count, all are greater in prePMF than ET. In ET, there is no or minor (grade 1) increase of reticulin fibers. The 2016 WHO classification allows the presence of reticulin fibrosis grade 1 in ET, but this is a very rare presentation [14]. On the other hand, age, gender distribution and JAK2V617F mutational frequencies were similar between both groups. The survival rates, leukemic transformation rates, and rates of progression to overt myelofibrosis were significantly worse in prePMF than in ET [15].
Treatment
Treatment of ET is based on risk stratification: Age >60 years, history of vascular complications (thrombosis or major bleeding), and platelet count ≥1500x109/L are the 3 risk factors used to classify patients with ET into low (no risk factors) and high risk (1 or more risk factors). Low-risk ET patients have an incidence of thrombosis similar to that of a healthy control population [7].
The indication for and goals of therapy: are to reduce the risk of life-threatening complications, such as venous or arterial thrombosis or severe bleeding, rather than induce remission or disappearance of the neoplastic clone. Treatment may also help to control vasomotor symptoms [1]. Treatment of ET is based on aspirin in the vast majority of ET cases and cytoreductive therapy [7].
Low-dose aspirin: All ET patients should be managed with low-dose aspirin if micro vascular disturbances are present.In low-risk ET, anti-platelet therapy reduces the incidence of venous thrombosis in JAK2-mutated patients [7] and the rate of arterial thrombosis in those with cardiovascular risk factors, with no effect on the risk of bleeding. By contrast, in CALR- mutated patients, anti-platelet therapy did not affect the risk of thrombosis whereas it was associated with a higher incidence of bleeding. In high-risk ET patients, anti-platelet therapy should be administered to all patients except those with extreme thrombocytosis (>1500 x 109/L) [7].
Cytoreductive therapy: Extreme thrombocytosis is frequently associated with acquired von Willebrand syndrome [7]. So, A platelet count >1500x109/L represents an indication to cytoreductive treatment [7] with hydroxyurea, or interferon, or an agrelide when indicated or ruxolitinib when inadequate response to hydroxyurea occurs [14].
Treatment of pregnant females: Aspirin is safe for both mother and fetus. It is recommended for all pregnant women with ET, unless contraindicated. Low-dose aspirin is effective in preventing preeclampsia [7]. LMWH is added to low- dose aspirin in case of previous major thrombosis or severe pregnancy complication. Consider interferon a if the platelet count is >1500 x109/L or in case of previous major bleeding. The optimal time to discontinue anti-platelet treatment is generally about 2 weeks before delivery for any possible instrumental delivery and epidural or spinal anesthesia. After delivery, treat all women with ET with LMWH for 6 weeks to prevent deep vein thrombosis [7].
Cytoreductive therapy and natural history of disease:Hydroxyurea does not specifically target the mutant clone and is therefore unlikely to modify the natural history of disease. Pegylated interferon α-2a has been shown to induce sustained complete molecular response in a subset of patients with JAK2 (V617F)-mutant ET. In addition, recent reports describe the positive effect of interferon α in patients with CALR-mutant ET [7]. Those with additional mutations had poorer molecular responses compared with those with CALR alone. Analyses of mutation type also suggested a differential effect from IFN-a on mutated clones, because some patients experienced a decrease in the CALR burden but an increase in the clonal burden of other somatic mutations [11]. These observations do not necessarily mean that interferon α can modify the natural history of disease but they at least indicate that this drug can target the myelo proliferative clone [7].
Future therapy: Whether JAK inhibitors can provide beneficial effects in patients with ET remains to be established in ad hoc clinical trials, but ruxolitinib has already proved to be effective in patients with a relatively aggressive disease [7]. A novel ET treatment concept involves telomerase inhibition. Response was seen regardless of the mutational profile, although more pronounced in those with JAK2 mutation. Mutant allele burdens were also reduced by 15% to 66% in those with MPL or CALR mutations [11].
Conclusion
There is a tremendous scientific advance in the last decade in diagnostic capability, and disease pathogenesis of essential thrombocythemia. However still there are unanswered questionsespecially as regards progression of disease.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers
0 notes
Text
Is The Begetting of Triplets Associated With The Developing of 3 Breast Primaries?

Abstract
A Birmingham (UK) group considered in 1980 that the establishment of a Histopathology data pool encourages epidemiologic analysis. Consequently, the senior author (WIBO) had such an advantageous opportunity. Interestingly, the junior author (GEN), working in a near Surgical Outpatient Clinic, was consulted by a gravid 5, para 8, patient, i.e., one twin and a set of triplets. Moreover, she also developed 3 breast carcinomas. Therefore, this paper is documented in order to stimulate worldwide interest in this peculiar combination. Is it only happenstance or an explicable natural event?.
Keywords: Breast, triple cancer, pregnancy, triplets, Igbos, epidemiology
Introduction
The breast has for centuries been the medical man’s talking point. Thus, as the physician/historian, Henry Sigerist, summed it up, "it was chiefly cancer of the breast that attracted the physician’s attention" [1]. Today, one such point is multiple cancers developing especially in this organ [2]. Perhaps, a most engaging view is also the delivery of triplets [3]. Therefore, we are persuaded that, if the one (triplet) heralds the other (triple tumors), the documentation of both of them is likely to constitute a good goal for recondite research [4].
Investigation
It was stated by a Birmingham (UK) group (5) that the establishment of a histopathology data pool aids in epidemiological analysis. Accordingly, what has gone between the Pathology Laboratory and the Surgical Outpatient Department in the University Teaching Hospital, Enugu, is the case of a woman bearing triplets and later developing breast cancer of triple types.
Case Report
At the Surgical Outpatient Clinic, a 34-year-old woman attended with the complaint of feeling a lump in her right breast. The junior author (GEN) queried fibroadinosis. The lesion was a mobile, non-tender, ovoid, firm mass 7 cm x 4 cm across with several tortuous veins on the skin over it. Menarche was at 18 years. The recent menses was 2 months before her attendance. Altogether, she was Gravida 5, Para 8 (1 twin delivery, 1 triplet delivery). At biopsy, the mass cut with a gritty feel. The obtained specimen was delivered to the senior author (WIBO), who discovered that there were 3 primaries, namely, invasive ductal carcinoma, papillary carcinoma, and medullar carcinoma.
Discussion
Here, there was set against the delivery of triplets the odd developing of triple carcinomas. Was this mere happenstance? Or, was Nature pointing to an explicable sequence? These questions are open to research. Incidentally, there is a known problem that arises in triplet pregnancy. It is described as a challenge in perinatal medicine [2]. However, we are dealing here with adult medicine, namely, the development of breast cancer not just in one but in three sites after the begetting of triplets.
Conclusion
One group [6], on looking anew at a nationwide study in Sweden, concluded that "Breast feeding patterns in mothers of twins also may modify their risk of developing breast cancer." However, emphasis should be made on triplet bearing patients. Unfortunately, work in USA [7] did not direct attention to such triplet’s bearers. Triplets were examined by some groups with reference to mortality strictly [8,9]. However, what of its effect, if any, on the rarity of triplet births with regard to malignancy of the breast, especially when it is up to three different primaries? Future researches should provide the answer!
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers
0 notes
Text
Drivers of Precision Medicine: Liquid Biopsy and Next-Generation Sequencing

Editorial
Targeted therapy specifically aims at tumor genetic alterations s the hallmark of precision medicine. Companion diagnostic testing utilized to determine the presence or absence of certain oncogenic mutations prior to targeted treatment under the current medical guidelines will enable improved clinical outcome, and thus serves as a vital component for precision medicine. Standard clinical practice to assess genetic mutations in cancer patients has historically been through direct sampling of tumor tissue with biopsy or surgical resection. Unfortunately, tissue biopsy is one-time single-site sampling, painful, costly, and can miss the dynamics of tumor clonally evolution during disease progression. The emerging liquid biopsy by circulating cell-free DNA (cfDNA), on the other hand, can fulfill the gap, not only providing actionable information for decision-making prior to treatment, but also longitudinal surveillance during and after the treatment regimen to assess for patient response, drug resistance[1-3] and cancer recurrence [4].
Genomic content of cfDNA that is collected from a befouled specimen must be tested with an appropriate analytical method in order to identify existing mutant alleles. Traditional methods for mutation detection include assays such as quantitative PCR and Sanger sequencing [5,6]. PCR-based assays are sensitive but provide only limited information on a handful of genes and hotspot mutations, whereas Sanger sequencing does not possess the sensitivity adequate for detection in the context of liquid biopsy [7]. Modern next-generation sequencing (NGS)-based technology involves sequencing DNA in a highly multiplex and parallel fashion with higher sensitivity [8].
Whole genome sequencing (WGS) or exome sequencing (WES) of tumor profiling using NGS is an unbiased approach that provides extensive and comprehensive genomic information about cancer. Although they can provide genetic resolution both at the single nucleotide level, as well as detect structural alterations such as large rearrangements, gross deletions and duplications [9-11],the cost of these sequencing are still too high and our current knowledge bases are too small for routine clinical application. Targeted gene panels are currently the best option for CLIA clinical utility in the field of liquid biopsy, as they allow multiple action able genes to be analyzed and can provide enough depth of coverage to detect minor allele frequencies in a clinically-relevant and cost-effective manner [12,13]. NGS gene panels can also eliminate the need for reflex testing and preserves precious specimens by limiting the number of tests required for full characterization. In order for NGS liquid biopsy diagnostics to be accurate and affordable, a balance must be reached between panel size and the level of multiplexing.
There are currently a handful of CLIA liquid biopsy companies who offer cfDNA NGS-based genetic testing. As more NGS gene panels begin to enter the market, it is important for users to understand the benefits and limitations of these methods and technologies being utilized. There are many nuances in designing NGS gene panels for liquid biopsy tumor profiling, and multiple options exist for each component of the workflow. Numerous factors can impact the accuracy, sensitivity and specificity of cfDNA sequencing including the sample preparation, target enrichment, library construction, sequencing platform and bioinformatics tool. Some degree of standardization of cfDNA NGS assays used for the detection of somatic mutations is essential before their deployment in clinical practice. Towards this direction, in addition to the concordance studies between tissue and liquid biopsies, comparison of cfDNA-based testing between different methodologies on different platforms in different laboratories are needed.
In the first side-by-side comparison study, the NGS results for a12-patient cohort from 2 commercially available gene panels (70 genes and 50 genes) are analyzed. These samples were processed and tested in two independent CLIA liquid biopsy laboratories, Guardant Health (GH) and Circulogene Theranostics (CT), with distinct sample volume input, sample preparation, library construction, NGS platform and different mutation calling bioinformatics. GH and CT tests resulted in a very similar 40- 50% rate of clinically relevant actionable information, i.e., either FDA drugs or active clinical trials. There are 2 cases reported “no mutation” by both tests. TP53 is the most frequent mutated gene identified in 41.7% (5/12) of cases. In 5 cases, there is at least one gene mutation matched. In another 5 cases, mutations did not match each other. It is noteworthy that there are 43 genes commonly covered by both panels, however, Guardant’s test surveyed all axons of these 43 genes, while Circulogene’s assay only scanned hotspot mutations within these genes. Nevertheless, the overall concordance between both test results was fairly high at the gene level, because the denominator in this calculation was 516 (43 genes for 12 patients), and the majority of genes were called wild-type alleles (no mutation detected).
A key issue in clinical oncology practice is the ability to accurately interpret what is truly clinically meaningful and actionable on a report from a commercially available NGS test. The majority of cancer care is not delivered at tertiary comprehensive cancer centers, but rather in busy community oncology practices. Treating physicians will have time constraints to read and digest NGS reports, so there is a need for clear, concise reporting of clinically relevant targets. With the integration of additional levels of content enhancement and scientific/clinical evidence, liquid biopsy NGS reports can eventually deliver patient-centered context and substance that will lead to better healthcare outcomes and toward the goal of precision medicine (Figure 1).
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers
0 notes
Text
Metastatic Adenocarcinomas of the Umbilicus in a Developing Community

Abstract
Popularized as the “Sister Joseph’s nodule” is the metastatic lesion of the umbilicus. Hitherto, cases had been reported worldwide. Therefore, this article aims to document the patterns of it obtained among an ethnic group in a developing community. Incidentally, a few indigenous doctors suspected the lesions to be of the Sister Joseph nodule type. The epidemiological data included equality of sex and the preponderance of adenocarcinomas.
Keywords: Carcinoma; Umbilicus; Metastasis; Age; Type; Sister Joseph Nodule
Introduction
Metastatic carcinoma of the umbilicus gained prominence when, “during the early days of the Mayo Clinic, Sister Mary Joseph, the superintendent of St. Mary’s Hospital and Dr. William Mayo’s frequent first assistant, imparted this clinical observation to Dr. Mayo after noting a firm nodule of the umbilicus in many patients with intra-abdominal cancer” [1]. Indeed, Sharaki and Abdel-Kader [2] published on 12 cases and entitled them “the Sister Joseph’s nodule.” Likewise, “Sister Joseph’s nodule” topped the title of the 7 cases published by Brustman and Seltzer [3], as well as the single case of Bank and Liberman [4] and also that of Samitz [5].
Individual cases are noteworthy especially as they centered on the different primary growth from the skin [6] and the uterine cervix [7]. Accordingly, deserving of documentation is the author’s series from a developing community.
Investigation
The findings were made in Nigeria concerning 16 patients of the Ibo/Igbo ethnic group [8]. Moreover, the data came through a histopathology data pool just as was recommended for epidemiological analysis by a Birmingham (UK) group [9]. Doctors serving the populace were encouraged to carry out biopsies, to preserve them in normal-saline and to give adequate clinical details in the Request Forms. The data were analyzed personally.
Results
Discussion
Of the more recent findings, the tendency is still to dwell on the Sister Joseph nodule nomenclature. Thus, Palaniappan [10] and group saw a case series in which 4 Sister Joseph nodules came from 4 different viscera, namely, gall bladder, ovary, rectum and gastrointestinal stroma. The literature came from Morocco [11], Taiwan [12], Greece [13] and India [14].
Conclusion
Therefore, my cases from Nigeria need documenting especially in terms of
the equal contribution from both sexes,
the equal contribution coming from Enugu as opposed to the rest of the towns,
the tendency to predominate in the 41 to 50 age group,
the preponderance of adenocarcinoma, and
Most of the doctors sending but a single specimen.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers
1 note
·
View note
Text
Pixel/Voxel-Based Machine Learning (PML) and Big Data in Medical Imaging: Detection and Regulation
Abstract
Medical imaging has always been an essential component of patient management. The pixel/voxel- based machine learning (PML) in medical imaging is gaining momentum as a computer aided diagnostic (CAD) tool if it can achieve better results than radiologists, in terms of detection and bringing down costs. CAD detection of breast cancer in mammograms is one area that has proved to be smarter, but again most radiologists are suspicious of eventual outcome. Machine learning using pixel/voxel values in medical images has shown emergence as a better diagnostic tools than the segmentation or feature based input. Pixel/Voxel based calculations avoid error that is inherent to segmentation based information. Once regulatory information is used as a metadata, new opportunities of machine learning in regulatory compliance would emerge and the regulatory framework would be based on the regulatory requirements and minimizing costs in regulatory compliance. Eventually, the potential for risk analysis may become automated.
Keywords: Computer aided diagnosis; Pixel; Voxel; Machine learning; Medical imaging; Regulatory
Go to
Introduction
Machine learning uses algorithms derived from empirical data and make informed decisions based on learning from prior. Analysis of medical images, segmentation, CAD, and other medical image involving methods such as image fusion or image guided therapy are all becoming possible by machine learning. Pixel and voxel metrics define the prior learning experience of the machine learning from previous examples and then un turn guides these machines to diagnose MRI, CT, PET-CT (positron emission tomography hybrid with CT) would require machine learning based on new algorithms and data. Pixel/voxel metrics would generate more diagnostic data than information obtained from segmentation of an organ, as errors can be avoided by pixel/voxel based machine learning.
a)What is pixel, voxel and an image?
A pixel, also known as picture element (pics for pictures, el for element), is the most basic and smallest unit of a digital image that could be displayed and controlled. It is a point sample and only exists at a point [1,2]. Pixel is thus a sample of its image. When there is a color image, there may be a combination of three colors, red, green and blue, and three such samples, making up a pixel. An image is, on the other hand, is a collection of many pixels. In a CT scan image, a pixel is a two dimensional sample matrix and is displayed by the attenuating tissue in the form of a grey scale based on Hounsfield unit [2]. Voxel, on the other hand, represents a volume unit and is a three dimensional unit. This means that the CT image is analyzed through many pixels and their brightness depends on the X-Ray absorption or attenuation capacity of voxels [2].
Go to
Pml Based Medical Imaging
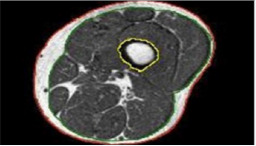
PML based machine learning incorporates the use of intelligent systems that use prior learning experiences derived from big data. Anatomical structures and pathological states are identified from normal state through pattern recognition and adjustment of its features in its algorithm. Data helps to identify abnormal from normal anatomy based on pixel and voxel characterization. In medical imaging, and in segmentation of pathology, use of lean methods to expedite diagnosis can save time [3,4]. This is why it is classified as Pixel/voxel based [5] and when compared to conventional forms of segmentation (Figures 1 & 2), PML is highly diagnostic in identification and diagnosis with a predictive value of labeling as benign or malignant (Figure 3). Proves to be a highly accurate method of segmentation of pathologic anatomy and diseased states, when compared to Segmentation can be performed in a semi-automated or manual representation detailing most external as subcutaneous adipose tissue, muscle bundle and the innermost bone [6]. PML is reproducible and thus of desired use with supervised, unsupervised or reinforcement learning. These learning methods could be data and label driven, only data driven and reacting to environment using an algorithm respectively (Figure 4) [7].
a)Disadvantages of PML
The most obvious disadvantage, at present, is a lack of informative data that may help to compute further as PML is data driven. It is expensive and can take only small identifier set as features and if there is a wide spread lesion, as in whole of left lung, it is difficult for PML to retrieve all the required information to diagnose. Discontinuous loss functions that are not differentiated are difficult to work with PML. Large amount of data is required to learn from prior experiences for PML and it is not always a must that PML would work in all scenarios [5]. The functional physiological mechanisms or physiologic modeling should correlate to the information received from the machine learning.
b)Regulation of PML
i FDA has classified two types of CAD devices: CADe and CADx [8]. CADe devices are “computerized systems that incorporate pattern recognition and data analysis capabilities (i.e., combine values, measurements or features extracted from the patient radiological data) and are intended to identify, mark, highlight or in any other manner direct attention to portions of an image, or aspects of radiology device data, that may reveal abnormalities during interpretation of patient radiology images or patient radiology device data by the intended user.” CADx devices are “computerized systems intended to provide information beyond identifying, marking, highlighting or in any other manner directing attention to portions of an image, or aspects of radiology device data, that may reveal abnormalities during interpretation of patient radiology images or patient radiology device data by the clinician”[8]. In 2012, FDA documented that CADe devices could follow the 510(k) process for their clearance and CADx would follow premarket approval.
Mammography CAD has still not been classified as CADe device through FDA and this implies that 510(k) route does not apply here. PML medical devices would also have to follow premarket approval route as no predicate devices would exist for FAD to allow a 510(k) route for these. No substantially equivalence would exist for these algorithms. This would mean a tougher route of FDA clearance and that PML medical devices may take a while to come to the market, even if the PML device companies start early on with FDA. However, CAD devices could offer another opinion for screening cancers, like lung cancers [9].
Go to
Conclusion
Machine learning requires an algorithm and a data. Data of patients and within a patient caries and algorithms may not be a feasible option in diverse use of machine learning in medical sciences, but certainly it has a ground of support for medical imaging. The brightness characterization of a pixel and its volume in a picture can give more information than a human eye or diagnose more is justified in some instances but needs more to explore. Cancer diagnosis, prognosis and predictions would become possible through PML [5,10]. Regulations for PML medical devices would not be in the very near future, if they follow premarket approval of FDA. PML will revolutionize the medical imaging, once it gets going, although this may happen outside US earlier than one would expect.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers
0 notes
Text
Sinonasal Adenoid Cystic Carcinoma: A Case Report and Review of the Literature

Abstract
Adenoid cystic carcinomas (ACC) represent 10% of all salivary tumors. It primarily affects the salivary glands particularly those located in the buccal cavity. Sinonasal location is rarely described [1]. We report a rare case of sinonasal adenoid cystic carcinoma (SACC), we discuss through a brief review of the literature its clinical, radiological, histopathological and therapeutic modalities and the prognostic factors of this tumor.
Keywords: Adenoid Cystic Carcinoma; Paranasal Sinus; Facial Pain
Introduction
ACC is a rare malignant neoplasm that accounts for 1-2% of all head and neck malignancies and approximately 10% of all salivary gland neoplasms [2]. It occurs predominantly among women, between the fifth and sixth decades of life [3]. Sinonasal Adenocarcinomas mainly arise from the respiratory epithelium or the underlying mucoserous glands (60%). It’s known by its slow persistent growth with a high risk of local recurrences and distant metastases. The treatment of choice is based on surgical excision of the tumor with adjuvant radiation therapy.
Case Report
A 44 year-old female presented to the ENT emergencies with a swelling of the latero-nasal area and the inner corner of the left eye evolving for the last year. It measured 2.5 cm at its largest diameter (Figure 1). The patient reported a progressive left-sided nasal obstruction of 8 months duration, with purulent,bloody and fetid nasal discharge, with a conservation of the general state. The anterior rhinoscopy highlighted nasal congestion with no visible tumor. Ophthalmologic examination revealed lateralization of the left eyeball with telecanthus. Facial CT scan revealed a tumor process of the left nasolabial furrow with lysis of the maxillary bone, its internal wall and also the inner wall of the orbit (Figure 2). A biopsy through the vestibular way has confirmed the diagnosis of a cribriformtype of ACC. The staging did not find distant metastases. The patient was managed surgically with adjuvant radiation therapy.
Discussion
Sinonasal Adenoid cystic carcinomas are considered as poor prognostic tumors, characterized by the possibility of late and frequent local recurrences, and poor survival. These tumors grow usually slowly, thus they can reach considerable size before the diagnosis is made. The initial prognosis may be good but the frequency of local recurrences and distant metastases (lung essentially) influence the long-term survival (10-year survival <10%) [4]. Local recurrences are more frequent for sinonasal locations (60% of clinically evident recurrences within 2 years following treatment) [5]. This is related to the difficulty of ensuring healthy resection margins at the base of the skull often because of the very advanced stage of the tumor by the time of the diagnosis, the anatomic complexity of the region, the frequent intracranial extension along cranial nerves and because of restriction on the resection margins caused by the proximity of critical neurovascular structures.
Among the most important prognostic factors involved are tumor stage, histological grade (The tubular- and cribriformtype ACCs are lower-grade tumors, whereas solid-type ACC is a higher-grade tumor), the existence of perineural invasion and cancerous resection margins [4,6,7]. Complete surgical resection is critical but often difficult to realize close to the skull base [4,8]. Postoperative radiotherapy improves the long-term prognosis of patients with large lesions especially if there are microscopic residues after surgery.
Conclusion
Adenoid cystic carcinoma of sinonasal cavities is a rare aggressive tumor with a high incidence of local recurrence and distance metastases regardless of therapeutic modalities used. Complete surgical excision and adjuvant radiation therapy for the extensive local infiltration offer to these patients the best chance to achieve high tumor control.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers
0 notes
Text
Update in Waldenström’s Macroglobulinemia

Abstract
Everolimus, an mTOR inhibitor, perifosine, an AKT inhibitor, enzastaurin, a phosphatidylinositide3 kinase/AKT inhibitor, panobinostat, a histone deacetylase inhibitor, ofatumumab, a third-generation anti-CD20 monoclonal antibody, and ibrutinib, a Bruton tyrosinekinase inhibitor, and newer drugs from known active subclasses, such as pomalidomide (immunomodulatory) and carfilzomib (proteasome inhibitor) arepromising drugs in various stages of study in WM. They may expand future treatment options.
Abbreviations: WM: Waldenström’s Macroglobulinemia, LPC: Lymphoplasmacytic, MYD: Myeloid Differentiation Primary Response Gene, IgM: Immunoglobulin M, CNS: Central Nervous System, DLBCL: Diffuse Large B-Cell Lymphoma, ATM: Ataxia Telangiectasia-Mutated, BTK: Bruton’s Tyrosine Kinase, IRAK1: Interleukin-1 Receptor-Associated Kinases, WHIM: Warts Hypogamma globulinemia Infections Myelokathexis, WT: Wild-Type, ERK: Extracellular Signal-Regulated Kinase, NF-κB: Nuclear Factor κB, LON: Late-Onset Neutropenia
Introduction
WM is defined by WHO as a lymphoplasmacytic lymphoma associated with a monoclonal IgM protein (regardless of its size) and bone marrow infiltration by clonal LPC cells [1]. Median age at diagnosis is70 years with male predominance the incidence is lower in non-Caucasians [2]. It accounts for 1%-2% of hematological neoplasms [3]. There is personal or family history of autoimmune, inflammatory and infective disorders particularly Sjogren syndrome and autoimmune hemolytic anemia. There is increased risk of WM and other B-cell disorders amongst relatives of patients with WM [2].
IgM-MGUS is characterized by the presence of an IgM monoclonal protein, less than 10% clonal lymphoplasmacytic bone marrow cells, and no symptoms attributable to tumor mass or infiltrations [4]. It is a precursor state for WM. Approximately 2% of IgM MGUS patients evolve to a B-cell malignancy per year, with most of these individuals progressing to WM [5]. Smoldering WM is characterized by an IgM monoclonal protein, clonal lymphoplasmacytic bone marrow infiltration greater than 10%, no symptoms attributable to tumor mass or infiltration, and no IgM-mediated symptoms [4]. Clinical features are related to disease burden, such as cytopenias, organomegaly and constitutional symptoms, or to IgM paraprotein such as hyper viscosity syndrome, hemolytic anemia, immune complex vasculitis and amyloidosis or to autoantibody specificity suchas peripheral neuropathy, cold hemagglutinin disease and acquired von Willebrand disease [2].
Bing Neel syndrome (rare) presents usually at WM relapse or at first diagnosis. Symptoms are diverse, non-specific and gradually progressive over weeks to months. They reflect LPC involvement of the CNS and rarely the peripheral nervous system. LPC may be detected in the cerebrospinal fluid, the meninges and/or the cerebral parenchyma [6]. WM patients are at increased risk for second malignancies, including transformation to DLBCL (5-10%), myelodysplastic syndrome, acute myeloid leukemia and solid cancers [4]. Development of bulky rapidly enlarging lymph node masses, extranodal disease and marked elevation in serum lactate dehydrogenase are suggestive of transformation to DLBCL [2]. The genomic landscape of WM is characterized by highly recurring MYD88 (>90% of cases) resulting in a protein change from leucine to proline at amino acid position 265 [4].
In tumor cells, MYD88L265P triggers activation of NF-κB through BTK or IRAK (IRAK1 and IRAK4) pathways. MYD88L265P was present in 50% to 80% of IgM MGUS, suggesting an early oncogenic event for this mutation [5]. MYD88 mutation is not unique to WM. It distinguished WM from overlapping entities such as marginal zone lymphoma, chronic lymphocytic leukemia, and multiple myeloma, wherein MYD88L265P was either absent or infrequently observed (10%) [4]. CXCR4 is mutated in 30% of WM patients. CXCR4 stimulation by its ligand CXCL12 activates AKT1 and mitogen-activated protein kinase family signaling, as well as facilitates cell migration and homing in WM cells [4]. The prolonged activation of CXCR4 signaling due to WHIM mutations may exaggerate these effects. Polymorphisms of CXCR4 ligand and CXCL12 have been associated with poor post treatment clinical outcomes [7].
MYD88 and CXCR4 mutation divide WM into three genomic groups (MYD88L265P CXCR4WT, MYD88L265P CXCR4WHIM, and MYD88WTCXCR4WT) on the basis of clinical manifestations and survival [7]. Other major pathway was the loss of chromatin remodeling proteins, ARID1A and ARID1B. ARID1Awas the third most common single nucleotide variant target in WM, they are thought to exert their effects via p53 and CDKN1A regulation [4]. BCR-signaling-associated mutations occur less frequently (15% of WM cases), and are restricted to the CD79A and CD79B genes [8]. Epigenetic dysregulation, aberrations in the phosphatidylinositol 3-kinase/mTOR, NFκB, JAK/STAT signaling pathways, as well as bone marrow micro environmental interactions, may be other key factors involved in WM pathogenesis [4].
Diagnosis
Bone marrow aspirate and trephine biopsies should be obtained and supplemented by flow cytometric and immunohistochemistry studies [2]. The bone marrow pattern is predominantly intertrabecular [9]. The immuno-phenotype of WM consists of expression of pan-B-cell markers (CD19, CD20, CD22), cytoplasmic immunoglobulin (cIg), FMC7, CD38, and CD79a[10]and typically negative for CD3 and CD103 [9]. The plasma cells number is generally in the normal range, but they differ from normal and myeloma cells by being positive for CD38, and commonly express CD19, CD45, and CD20, but lack CD56 [10].
Workup
IgM levels by densitometry or total serum IgM quantitation by nephelometry must be determined. IgM values assessed by nephelometry are higher than M protein values determined by densitometry that is why sequential response assessments for individual patients must be carried with the same methodology [11]. Quantification of serum viscosity might be helpful [3]. Hyperviscosity syndrome is evident when IgM M-protein >40 g/l and/or [2] the serum viscosity exceeds 4 centipoise. Serum viscosity does not always correspond to the clinical severity of hyperviscosity. Venous engorgement ‘sausaging’ in the retinal veins by fundoscopy is an excellent indicator of clinically relevant hyperviscosity [3].
Evaluation of anti-myelin associated glycoprotein, antigangliosides M1 and anti-sulfatide IgM antibodies may support the diagnosis of IgM-related neuropathy. Also, the possibility of amyloid light-chain amyloidosis in association with peripheral neuropathy needs to be considered [3]. Screening for hepatitis B and C viruses is required prior to the introduction of rituximab-containing treatments [2]. An ultrasound or CT scan should be carried out to documentorganomegaly/ adenopathies. PET scanning is indicated when a large cell lymphoma transformation is suspected [3]. Testing for MYD88 is essential for patient’s candidates for ibrutinib therapy [12]. Cytogenetic analysis is not required for the routine diagnostic assessment of WM [2]. Partial or whole 6q deletion is the most common recurrent chromosomal abnormality (approximately 50% of patients) and was associated with a complex karyotype, hypoalbuminemia, high 2-microglobulin levels [4] and an adverse prognosis [9].
Other cytogenetic aberrations, include trisomy 18 (15%) and 13q14 deletion (13%). Less than 10% of patients had trisomy 4, 17p13 (TP53) deletion, 11q22 (ATM) deletion, trisomy 12, or 14q32 (IGH) translocations. Deletion of 6q, 11q and trisomy 4 had adverse effects on survival. Recurrent deletions on 13q14 and 17p13 have been mostly seen in more advanced stages of the disease [4]. Although not unique to WM, inactivating mutations of TRAF3 (located on cytoband 14q32.32) lead to constitutive activation of NF-κB pathways and are recurrent findings in a small percentage (~5%) of WM patients [10].
Risk stratification
In International Prognostic Scoring System for WM I (IPSSWM), 5 covariates (age > 65 years, hemoglobin ≤11.5 g/ dL, platelet counts ≤100x109/L, 2-microglobulin>3mg/L, serum monoclonal protein >70 g/L) defined 3 risk groups (low, intermediate and high risk respectively) [13]. The risk category is designated as low (zero or 1 risk factor, except age), intermediate (age older than 65 years or 2 risk factors), or high (>2 risk factors) [4]. These three risk categories are associated with 5-year survival rates of 87%, 68% and 36% respectively [2]. Lactate dehydrogenase level may have a role in separating the high-risk patients into two distinct categories [9]. IPSSWM risk category is used for risk stratification in randomized clinical trials [13].
Close observation is recommended for patients who do not fulfill the criteria for WM, and for whom laboratory evidence is the only indicator of disease progression (eg, a minor decrease in hemoglobin level with asymptomatic anemia or mild increases in IgM) or mild increase of lymphadenopathy or splenomegaly without patient discomfort [12]. They can be safely observed at 3-6 monthly intervals. The risk of progression to symptomatic disease is 59% at 5 years [2]. Criteria for initiation of therapy is IgM-related complications and/orsymptoms related to direct BM involvement by tumor cells such ascytopenias, constitutional symptoms and bulky extramedullary disease [12].
Urgent therapy is needed insymptomatic hyperviscosity, moderate to severe hemolytic anemia and symptomatic cryoglobulinemia [12]. Plasma exchange may be warranted in asymptomatic individuals, such as those with multiple vascular co-morbidities and in patients with a high plasma viscosity >4cP prior to red cells transfusion [2]. Management of symptomatic, untreated WM patients Rituximab aloneis considered inperipheral neuropathy related to the IgM antimyelin-associated glycoprotein activity [9] or in frail patients who are less likely to tolerate chemoimmunotherapy [12].
Chemoimmunotherapy combinations
The combination of rituximab with chemotherapy is the first option in medically fit patients particularly, when rapid response is needed [3] because rituximab is an active non myelosuppressive agent [12]. Response rate of 70-90% have been reported in rituximab based combination [14]. The choice of chemotherapy depends on comorbidities, how fast disease control is required, and the manifestations of the disease [12]. R-CHOP is no longer considered a first-line choice [13], Dexamethasone, rituximab, and cyclophosphamide (DRC) is a primary choice in frail patients requiring combination therapy. Toxicities were mild, with only 9% of patients having grade 3 to 4 neutropenia [12].
Bendamustine-rituximab (BR) is effective in patients with high tumor bulk [12]. Bortezomib-rituximab combination may be considered in patients with specific high-risk features (i.e., high IgM levels, symptomatic hyperviscosity, cryoglobulinemia or cold agglutinemia, amyloidosis and renal impairment) or in younger patients to avoid use of alkylator or nucleoside analogtherapy [13]. Bortezomib should ideally be given once per week and possibly by a subcutaneous route. For urgent reduction of the IgM level, bortezomib can be started at twice-per-week doses for 1 or 2 cycles and then be changed to once-per-week dosing to reduce risk of neurotoxicity [12]. Bortezomib is not toxic to stem cells [13]. Rituximab plus carfilzomib are mainly used as an emerging neuropathy-sparing option. No grade ≥3 neuropathy was observed [12]. Single agent chlorambucil may still be suitable therapy for very frail patients in whom combination therapy is considered inappropriate [2].
Response criteria
CR: Absence of serum monoclonal IgM protein by immunofixation, normal serum IgM level, complete resolution of extramedullary disease, i.e., lymphadenopathy and splenomegaly if present at baseline, morphologically normal bone marrow aspirate and trephine biopsy.
VGPR: Monoclonal IgM protein is detectable, ≥90% reduction in serum IgM level from baseline, complete resolution of extramedullary disease, ie, lymphadenopathy/ splenomegaly if present at baseline, no new signs or symptoms of active disease.
Partial response: monoclonal IgM protein is detectable ≥50% but 90% reduction in serum IgM level from baseline, reduction in extramedullary disease, i.e., lymphadenopathy/ splenomegaly if present at baseline, No new signs or symptoms of active disease.
Minor response: monoclonal IgM protein is detectable ≥25% but 50% reduction in serum IgM level from baseline, no new signs or symptoms of active disease.
Stable disease: monoclonal IgM protein is detectable 25% reduction and ession in extramedullary disease, i.e., lymphadenopathy/splenomegaly, no new signs or symptoms of active disease.
Progressive disease: ≥25% increase in serum IgM level (an absolute increase of 5 g/L (0.5 g/dL) from lowest nadir (requires confirmation) and/or progression in clinical features attributable the disease [13].
Maintenance rituximab is recommended by NCCN for patients in CR for initial therapy or asymptomatic patients achieved very good, partial or minor response [14]. Maintenance rituximab increased incidence of grades 1 and 2 sinobronchial infections along with reduction of uninvolved immunoglobulins (IgA and IgG). It appeared to extend PFS and OS in comparison with observation [12]. Management of symptomatic previously treated WM patients. Re-treatment with prior regimen used for symptomatic, untreated patients may be considered if a response was achieved for 2 or more years with the priorregimen [12]. Repeat bone marrow aspirate and trephine assessment and CT scanning prior to the reintroduction of treatment [2].
BR is well tolerated in relapsed/refractory disease. Prolonged myelosuppression occurred in patients who had received prior nucleoside analog therapy [12]. Of atumumabis a fully human monoclonal antibody (IgG1) that targets a CD20 region at a different epitope than that of rituximab. It may represent a potential therapeutic option in rituximab in tolerant patients. A therapeutic test dose with appropriate prophylaxis should be considered before of atumumab administration. There is a risk of IgM flare as with rituximab [14]. Rituximab with purine analogs (rituximab and fludarabine / rituximab, fludarabine, and cyclophosphamide) remain an option for patients with highrisk of relapsing disease and adequate performance status. They have a median PFS exceeding 50 months. In patients who may be candidates for single agent oral therapy, oral fludarabine (if available) is recommended over chlorambucil [13].
Novel agents
Immunomodulatory agents:Given the potential adverse events of lenalidomide and pomalidomide, their use should be considered in the context of a clinical trial [12]. Ibrutinib is an orally administered, irreversible inhibitor of BTK. It represents a novel and effective treatment option for both treatment naive and relapsing patients not candidates for chemoimmunotherapy [12]. Extramedullary disease was affected by ibrutinib therapy [15]. It prevents binding of MYD88 to BTK in L256P cells [14]. Ibrutinib showed rapid response kinetics, with a median time to response of 4 weeks [15]. The response was highest among patients with MYD88L265Pand those with absent CXCR4 mutation [14].
The incidence of ibrutinib-triggered peripheral lymphocytosis was higher among patients with MYD88L265PCXCR4WT than among patients with MYD88L265PCXCR4WHIM [15]. Overall treatment with ibrutinib is well tolerated in WM patients [15]. Patients who progressed on first-line ibrutinib should not be retreated with ibrutinib [12]. A potential off-target effect is atrial fibrillation (5%) in patients with history of arrhythmia [15]. Ibrutinib produces a mild decrease in QT interval of unknown underlying mechanism and safety relevance [12]. The mammalian target of rapamycin (mTOR) inhibitor (everolimus) owing to the toxicities (hematologic, mouth sores and pulmonary pneumonitis) associated with everolimus, this agent is best considered in patients who are unresponsive or progressed after multiple lines of other better-tolerated therapies [12]. Discordance between serum IgM level and bone marrow disease response is commonand complicates response assessment [12].
CXCR4 antagonist (plerixafor):The feasibility of longterm use of plerixa for has been reported in patients with WHIM syndrome. It sensitizes engineered WM cells to express CXCR4WHIM receptors to undergo apoptosis in response to ibrutinib. Clinical trials of other CXCR4 inhibitors are ongoing [15]. The Akt inhibitor perifosine has shown a response rate of 35% but is associated with high levels of gastrointestinal toxicity. Histone deacetylase inhibitor panobinostat as a single agent has resulted in a minimal response or better in 47%. The median progression-free survival was 6.6 months [9]. MYD88 peptide inhibitor, MYD88L265P-directed immune activation and CD19 directed chimeric antigen receptor T cell therapy are 3 highly innovative WM specific therapies [16].
Caution
Avoid continuous oral alkylator cyclophosphamide, chlorambucil and bendamustine or nucleoside analogue (cladribine and fludarabine) therapy if SCT is considered [14].
Patients receiving purine analogues, alemtuzumab and bendamustine should receive irradiated blood products for Life [2].
Serum IgM can spike (IgM flare) during rituximabbased therapy (or other anti-CD20 monoclonal antibodies) for several weeks or months independent of tumor cell killing. This does not imply disease progression, in most cases; it will resolve [13]. On the other hand, bortezomib or everolimus can suppress IgM level [14].
Rituximab should be avoided or withheld during the first 1 or 2 courses of systemic therapy until IgM levels decrease to a safer level, or plasmapheresis should be performed before giving rituximab to patients with high IgM levels (typically >4000 mg/dL) [12] because IgM flare could prompt symptomatic hyperviscosity.
LON has been described with rituximab, mostly when it is combined with chemotherapy. An association between a specific polymorphism in the IgG Fc receptor (FcgRIIIa- V158F) and LON has been described [12].
Best response to alkylators [2], purine analogue and monoclonal antibody therapy, may not be achieved until 6 months after treatment. These agents selectively deplete CD20+ B-cell component with sparing of the CD138+ plasma cell component of the disease. There is significant B-cell depletion in the marrow but suboptimal IgM responses
Satisfactory IgM responses may be achieved after many months into treatment. Bone marrow assessment is recommended to assess response. Conversely, bortezomibcontaining regimens may demonstrate excellent IgM responses but suboptimal bone marrow responses [2].
Prophylaxis against herpes zoster is strongly recommended for WM patients receiving proteasome inhibitors [14].
Vaccinations should be avoided, if possible, 2 weeks prior to, during and for 6 months after chemoimmunotherapy [2].
Transient increases in serum IgM levels commonly occur when ibrutinib was withheld because of toxic effects or procedures. These levels decreased with reinstitution of therapy [15].
An off-target effect of ibrutinib on platelet aggregation has been described in CLL trials. Care should be taken if anticoagulant therapy or drugs that inhibit platelet function is used. Test for von Willebrand activity in patients with a history of bleeding diathesis. In case of surgery, ibrutinib should be held at least 3 to 7 days pre- and post surgery, depending upon the type of surgery and the risk of bleeding [12].
Treatment-associated morbidity:Prolonged risk of secondary infections with monoclonal antibodies and purine analogues, risk of long-lasting cytopenias, myelodysplasia and secondary malignancies from fludarabine, and worsening of peripheral neuropathy related to bortezomib [4]. Grade 2 or greater neutropenia and thrombocytopenia may occur with ibrutinib in heavily pretreated patients [12].
Stem cell transplantation
Stem cell collection should be performed pre-emptively after patients achieve first remission. ASCT is an effective treatment option for eligible patients up to 75 years. It is recommended in high risk WM with elevated lactate dehydrogenase indicating a high tumor burden. It should ideally be offered at early relapses [17]. Chemosensitivity at the time of transplant is the most important predictor of response [2]. ASCT is not as beneficial for patients exposed to more than 3 lines of therapy or with chemotherapy refractory disease. Allogeneic SCT, when appropriate, should preferably be considered investigational due to high non relapse mortality [12].
Follow-up should include history, physical examination, blood count, routine chemistry and quantification of IgM every 3 months for 2 years, every 4-6 months for an additional 3 years, and subsequently once a year with special attention to transformation and secondary malignancies, including secondary leukemia. Radiological or ultrasound examinations every 6 months for 2 years are recommended, and annually thereafter only in cases of initial splenomegaly or lymph node enlargement. Regular CT scans are not necessary outside clinical trials [3].
Future options
Trials with ibrutinib and other BCR inhibitors are needed to assess their efficacy and tolerability in treatment-naive patients. BCR inhibitors combined with proteasome inhibitors in relapsed/refractory setting would be of interest to overcome resistance by interfering with the 2 key pathways that are affected by MYD88. Combination of CXCR4 antagonists with ibrutinib in patients with CXCR4WHIM mutation as well as Obinutuzumab, as a combination partner in WM are of interest [12].
Conclusion
The long survival and advanced age of presentation in WM must be considered when selecting the most appropriate treatment.
To Know More About Orthopedics and Rheumatology Open Access Journal Please click on: https://juniperpublishers.com/oroaj/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers
#Juniper publishers#open access journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Global Health, Cancer Challenges and Control in African Settings-Juniper Publishers

Abstract
The problem of cancer in Africa is so dire that international institutions are predicting “ a new scourge on a huge scale, one which, both directly and through its enomic impact, will increase poverty, misery and the death rate”. A comprehensive policy response is therefore urgently needed and it’s a duty to unravel the assertion that theses pathologies are restricted to high income nations. A global and coherent health program must be built and implemented at the national, regional and continental levels. To address this epidemiologic challenge, one the first steps will be the international cooperation and coordination based on resolute actions and programmes.
Keywords: Cancer, Africa, developing countries, Non Communicable Diseases, Global health
Introduction
Even in developing countries such as African countries non communicable diseases (NCDs) are among leading causes of death. It’s a duty to unravel the assertion that theses pathologies are restricted to high income nations. Admittedly, Human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS), Malaria, Tuberculosis, Ebola virus disease (EVD) and other endemic tropical sicknesses have had the priority in health’s political agenda. Nevertheless, it will be an unrecoverable error to underestimate the seriousness of this scourge [1-3].
Abbreviations:
HIV/AIDS: Human Immunodeficiency Virus and Acquired Immune Deficiency Syndrome; NCDs: Non Communicable Diseases; EVD: Ebola Virus Disease; IARC: International Agency for Research on Cancer; WHO: World Health Organization; UN: United Nations; AU: African Union; NTD: Neglected Tropical Diseases; SCCA: Stop Cervical, Breast and Prostate Cancer in Africa; MDGs: Millennium Development Goals; SDGs: Sustainable Development Goal; SSA: Sub-Saharan Africa; GDP: Gross Domestic Products
Cancer Statistics
Instead of the lack of reliable cancer registries in many African regions, the International Agency for Research on Cancer (IARC) latest statistics on cancer trends worldwide exhibit a pessimistic and critical outlook: 8 million of new cancer cases (57%), 5.3 million of the cancer deaths (65 %) and 15.6 million (48%) of the 5-year prevalent cancer cases occurred in developing countries [4,5]. In Africa, the cancer incidence has been estimated to 847.000 new cancer cases and 591.000 patients died from cancer. In women, the three most common cancers are breast, cervical and liver malignant tumours. In men, prostate, liver cancers and Kaposi sarcoma are the three most frequent malignancies [4,5]. Cancer causes worries, suffering and millions of deaths across the globe and is a great concern to modern society: “There were 14.1 million new cancer cases, 8.2 million cancer deaths and 32.6 million people living with cancer (within 5 years of diagnosis) in 2012 worldwide” [4]. For reasons that are both complex and multi factorial, ranging from chronic infections, the change in lifestyles and living conditions to environmental pollutions and from structural changes in the population (demography and aging) to heredity, cancer has grown in every continent [1,4,5] (Table 1) (Figure 1).
Risk factors, NCDs and tropical sicknesses
The prevalence and characteristics of cancers vary, particularly in terms of social and economic development of a given country. Thus, developed and developing nations have their own specificities. In northern countries, exposure to the sun, smoking, eating habits, lack of physical activity, excessive consumption of alcohol and, to a lesser degree, heredity and viral infections, are the principal causes of cancer. Furthermore, urban development and frenetic industrialization are responsible for the increase in cancer risks. Indeed, physical carcinogens (radioactivity) or chemical ones (asbestos, hydrocarbons, nitrates) and phytosanitary products are deleterious environmental pollutants resulting from anthropogenic activities whose breadth of impact on human health has not yet been measured. Besides these risk factors, in Africa, there are strong demonstrated links between cancers and tropical diseases contributing to lower the life expectancy of populations.
Then, African populations are suffering from both types of illnesses: communicable and non communicable diseases. Health matters are going from bad to worse. When superimposed on parasitic and communicable diseases which chronically affect millions of individuals, the tremendous health pressure contributes to reducing quality of life and life expectancy, which could decrease by 20 years in some countries according to World Health Organization (WHO) [1,4,5]. Schistosomiasis, malaria, viral hepatitis (B and C) and other tropical diseases are objective allies of cancer, feeding a vicious circle which reduces to nothing all of the tireless efforts and attempts to establish better public health for the benefit of social and economic development. These parasitoses and viral infections spread endemically in this part of the world and interact with other factors to lay the ground for the emergence or promotion of certain types of cancer such as Burkitt’s lymphoma, bladder tumors, malignant hepatomas, and cervical cancer. Stomach cancer is associated with a bacterial infection due to helicobacter pylori and a mycotoxin (Aflatoxin B1), secreted by a filamentous fungus, Aspergillus flavus is involved in the oncogenesis of hepatic cancers affecting Africans. These demonstrated and admitted scientific facts are against some arguments that malignancies are not a health priority in African Sub-Saharan countries [1,3-5].
Global health and sustainable development goals
This paper also wanted to debunk this argument or myth that is always put forward. The fundamental roots and the crucial determinants of the cancer burden in Africa and less developed countries have to be attacked.
As said the Assistant Director-General of Non Communicable Diseases and Mental Health at World Health Organization “in the developed western countries the situation remains under control, as things stand, but for the poor, less developed countries it might turn into a new scourge on a huge scale, one which, both directly and through its economic impact, will increase poverty, misery and the death rate.” [6]. To address this epidemiologic challenge, one the first steps will be the international cooperation and coordination based on resolute actions and programmes. To achieve these goals, the international political will, determination and implication is undoubtedly required. At the international level, encouraging efforts and decisions began with the Moscow Declaration on NCDs, endorsed by Ministers of Health in May 2011 and the United Nations (UN) Political Declaration on NCDs endorsed by Heads of State and Government in September 2011. In order to respect these engagements, the World Health Assembly endorsed the WHO Global Action Plan for the Prevention and Control of NCDs 2013-2020 in May 2013 [7].
At the African level, the African Union (AU) reaffirmed its political will and commitments to fight the spread of cancers and other NDCs by taking several resolutions as at the Sixth Conference of AU Ministers of Health held in Addis Ababa in Ethiopia from 22nd to 26nd April 2013 with the following theme “The Impact of Non-Communicable Diseases (NCDs) and Neglected Tropical Diseases (NTD) on Development in Africa” or more recently at The 10th Stop Cervical, Breast and Prostate Cancer in Africa (SCCA) Conference [8]. Globally, health issues have been taken into consideration in the eight Millennium Development Goals (MDGs). Four out of eight items were child mortality and maternal health, hunger, malaria and HIV/ AIDS [9]. However, the MDGs of the last decade didn’t pay any attention to cancers and other non communicable diseases. In the post- 2015 Sustainable Development Goal (SDGs), it’s recognized that health is an important pillar of sustainable development as are climate change and food security. In each country, a wide range of 17 goals must be implemented and achieved in fourteen years (2016-2030) [10].
Even so, the achievability of these goals in low and middle income countries has to be questioned. We have to keep in mind that most countries in sub-Saharan Africa (SSA) have not achieved or partially achieved the millennium development goals. The main reasons of such failures are poverty, political instability, mismanagement, corruption, illiteracy, analphabetism [10,11]. For instance, in 2002, in the Abuja declaration, African governments have committed to spending 15% of their gross domestic products (GDP) or their national budgets on health. Since then, the vast majority of the African Union members have not fulfilled their commitment [12]. In addition, the weakness of health systems (human resources, drug availability, technical platform, health inequities, social insurance, and health funds) is an enormous impediment to the route for better health outcomes from cancer and NCDs. A successful completion of such goals for sustainable development remains, obviously, a high priority especially in social and health fields.
Cancer economics
If the social impact and the morbidity of cancer are clear-cut, the economics of this pathology are also alarming. In 2010, the overall annual financial statement of cancer was estimated to 1.16 trillion USD [4,13]. The cost of the 13.3 millions new cancer cases, in 2010, was evaluated to 290 billion US Dollars. The medical cost was estimated to 154 billion and the non medical costs accounting are assessed to 67 billion US Dollars. As regards the income losses, it has reached 69 billion US Dollars. In the near future, according to the WHO, by year 2030, the global cost will rise by 458 billion US Dollars given the population aging trends worldwide [13]. Once again, the burden of cancer is significantly supported by poor and emerging countries: 47 % of the incidence and 55% of the mortality are occurring in these countries that are facing a huge challenge to fight this scourge [14]. In the meanwhile, the basic package of cost effective strategies to target and to notably reduce the common cancer risk factors in those nations should require a financial effort as small as 2 billion US Dollars per year [15]. Nevertheless, up to now, surprisingly and strangely, only 3% of the aid for health development from developed nations is spent in cancer and other NCDs control [14].
Integrated actions with other NCDs and transversal programs
In short, cancer has a tremendous negative impact in the social and economic situations of emerging and developing countries. As a matter of fact, a comprehensive and coherent health program must be built and implemented at the national, regional and continental levels. Since NCDs (cancers, cardiovascular diseases, chronic respiratory diseases diabetes) share many common risk factors (obesity, harmful use of alcohol, tobacco consumption, physical inactivity, malnutrition) an integrated program would be the appropriate and relevant approach to combat cancer in African countries [6,16]. Furthermore, cancer determinants are behavioral, cultural, social, cultural, environmental and political. The health policies and strategies wouldn’t be only technical although improving access to essential medicines, radiotherapy, vaccines, biomedical and imaging technologies are vital. As a result, some tentative responses have to be local and specific, adapted to the continent realities and must include as much as possible local human resources [16-20]. Vertical programs designed to tackle a specific disease with a specific risk factor are nowadays obsolete or irrelevant to uproot the cancer and NCDs outbreak and expansion [17-20]. The Epidemiologic transition is factual in the African landscape and certainty leaves no room for doubt.
Conclusion
Despite many and various internal obstacles, there‘s a growing associational network (local voluntary sector, civil society, non-governmental organizations (NGOs), patients and their families ,community leaders, health professionals, African diasporas) involved in the fight against cancer throughout the continent which is striving to be a great part of the solutions [1,16,18,19,21,22]. Hope lies there too. As said the German philosopher and poet Friedrich Hölderlin (1770- 1843), “Where increases the danger grows also what saves” [23]. In any case, with the existing knowledge (public awareness, early diagnosis and treatment, drug affordability, capacity building, scientific and technical infrastructures improvements) it’s utterly possible to drastically, technically and humanely scale down the cancer disaster in African developing countries and save thousands of human lives.
To Know More About Orthopedics and Rheumatology Open Access Journal Please click on: https://juniperpublishers.com/oroaj/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers
0 notes
Text
FDA 510 (k) Process- How To Get It Right The First Time?

Abstract
The medical devices that are designated to be marketed need to go through a clearance process set forth by FDA. The Premarket notification (PMN) or 510(k) is the most common regulatory pathway in US but poses many challenges to medical device manufacturers. FDA has cleared more than 1, 40,000 medical devices since 1976. This is a clearance process, and not an approval, for medical devices. 510(K) submission has a purpose, a process and should be well understood in order to avoid unnecessary delays and failures.
Keywords: Medical device; Regulation; FDA; 510(K); Substantially Equivalent
Abbreviations: FDA: Food and Drug Administration, PMA: Premarket Approval; SE: Substantially Equivalent;
Introduction
Getting a clearance letter from FDA on 510(K) for a medical device is a milestone and the ultimate goal for any medical device manufacturer, be it a small or a large company. This demonstrates that the medical device so produced has demonstrated a “substantially equivalent” (SE) status to a predicate device: a medical device cleared by FDA prior to 1976. This implies that the current medical device is deemed fit to be acceptable based on the difference in the device since first clearance. Any alterations in the devices or any changes to indications, contraindications or operations require a new 510(K) submission. Lab data is almost always mandatory to be included in the submission. Reducing unnecessary waste from a system and getting most effective medical devices by quality processes such as lean is desired [2].
What is a medical device?
As per FDA:
A medical device is “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
Recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them.
Intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
Intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes” [2].
Classification of Medical Devices
Based on the level of risk [3], in 1976, Medical Device Amendments to the Federal Food, Drugs and Cosmetics Act, classified all medical devices in three categories:
Class I: Simple devices posing no or minimal risk to the user and exempt from FDA clearance, like bedpan, elastic bandages, stethoscope etc. These are about 40% of all devices manufactured.
Class II: Moderate risk to the user and generally require FDA clearance before getting marketed, like sutures, condoms, X-Ray systems etc. Some are FDA exempt and some require a PMA. These are about 50% of all devices manufactured [4,5].
Class III: These life- supporting or life-sustaining devices pose a serious risk to the user and require a PMA and FDA clearance before marketed like, stents, implants, heart pumps etc. These require a PMA, as well [6-9].
What is PMA?
Premarket Approval (PMA) is the demonstration by the medical device manufacturer of the safety of the intended use and the usefulness of a new medical device to the FDA, of which FDA has no prior knowledge or experience. This would always include human and lab trial data. Class III devices always require a PMA and other such devices that do not qualify under 510(k) submission.
What are 510 (k)?
It is section 510-360 subsection k-Registration of producers of drugs or devices. As per this submission, the producer of the medical device must demonstrate that this medical device is substantially equivalent to a predicate device that has been cleared previously by FDA. There is a clearance given by FDA and not an approval. If there is a change in the use, contraindication or operation of use as compared to a previous cleared medical device, then this new device would require a 510(k) submission.
K number from FDA
All medical Devices submitted for 510(K), at least ninety days before introduction of a device, are assigned a K number followed by six digits. These six digits have significance to it: the first two digits being the year of submission and the last four digits begin with a zero in the order it was received for a review. Zimmer was the one which has 50(K) number K760001 indicating it to be the first submission since FDA in 1976. Boston Scientific Scimed Inc. has K0000, being having this number allotted in a new millennium on January 3, 2000.
How to format a 510(k)?
A 510(k) submission, not only requires a sound understanding of the process of submission, but also requires a thorough formatting with substantial inclusions. Some of the material that needs to be included is:
Conclusion
FDA is there to safeguard the interest of the public and make sure the efficacy and safety of a medical device [5,8]. The classification of a medical device, predicate device, premarket approval, and 510 (k) are important concepts in regulation of a medical device. The new medical device should be innovative and support public health. Some people argue that 510(k) is inefficient and time consuming for newer devices to come into the market and some also have doubts on the ability of 510(k) on the safety and efficacy of a medical device. Some companies have been seen to defraud FDA on the medical devices. Whatever it may be, 510(k) is a process in place that may be eventually see more improvements [1,4].
#Juniper publishers#open access journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Brachial Plexus Neurofibroma Treated with Volumetrically Modulated Arc Therapy (VMAT): A Case Report

Abstract
Neurofibromatosis was first described in 1882 by Friedrich Daniel von Reckling hausen, a German pathologist. Neurofibroma is a benign peripheral nerve sheath tumor that consists of Schwann cells, associated or unassociated with axons, perineural cells, and fibroblasts. Whenever possible, the treatment of choice should be surgical, but the management depends on the location and growth pattern. We present the case of a patient with left axillary neurofibroma without neurofibromatosis (NF) in whom surgery was delayed due to involvement of the brachial plexus, so was sent to radiotherapy, planned and treated with volumetrically modulated arc therapy (VMAT).
Keywords: Neurofibroma; VMAT; Radiotherapy; Axillary Tumor; INCART
Abbreviations: NF: Neurofibromatosis; VMAT: Volumetrically Modulated Arc Therapy; CRO: Radiation Oncology Center; INCART: Institute Rosa Emilia Sánchez Pérez de Tavares; CW: Clockwise; NTO: Normal Tissue Objective; OAR: Organ at Risk
Introduction
Neurofibromas are benign tumors that arise from nerves and are composed of Schwann cells, perineural cells, and fibroblasts [1]; typically arise in the setting of neurofibromatosis type I (NF I) [2], but may also occur independently, when it comes to multiple tumors is the main clinical manifestation of the different types of neurofibromatosis and are processes that are transmitted with autosomal dominant inheritance with variable penetrance. In most cases are treated by surgery, especially when they are symptomatic or with evident anatomical changes [3,4], but when a neurofibroma involves a particularly long segment of nerve or nerves, are generally impossible to remove without removing the entire nerve, causing a major neurological deficit, therefore, these variants are not usually subjected to surgery. In this report, we describe an axillar neurofibroma in a patient without NF I, which was treated with radiotherapy using volumetrically modulated arc therapy (VMAT).
Case Summary
Female patient, 55 years old, who had previously presented to an external surgery department was remitted to the Radiation Oncology Center (CRO) at National Cancer Institute Rosa Emilia Sánchez Pérez de Tavares (INCART), in Santo Domingo, Dominican Republic, with history of pain and volume increase in left armpit, diagnosed as non-surgical axillar neurofibroma. Medical record, including MRI, CT and pathologic specimen, were reviewed. The patient was considered initially for surgery like standard treatment, but during process evidenced brachial plexus commitment, because of this was omitted the surgery and was referred to radiation oncology. The Physical exam reported: Karnofsky (KPS) 100%, ECOG 0, in the left axillary region was felt a mass about 10 cm in diameter (Figure 1), mobile, painful, hard consistency with left arm weakness. A shoulder MRI with gadolinium reported: oval image 8.5 x 5.3 cm anteroposterior, anterior to the subclavian neurovascular bundle located in the anatomical path of the brachial plexus, highly suggestive of neural image type neurofibroma. Described image shows a bottom center 1.9 cm cystic degeneration, in turn shows an intense post gadolinium enhancement and heterogeneous accordance with important vascularization (Figure 2).
Simulation Ct and delineation
The patient was positioned in supine using a table for breast carbon fiber of the CIVCO® brand. Package tumor localization (TumorLOC®) Brilliance CT was used for the location of tumor tomography simulation. This tool allows you to place the isocenter manually depending on the selected organs and can create orthogonal beams associated with that default position of isocenter. Tomographic scanning technique was 120kV, axial cut, over 200,3 mm apart.
Field arrangement and treatment plan
The treatment technique corresponded to volumetrically modulated arc therapy (VMAT) for this, two semi-arches were used. The first from 0° to 179° towards the direction of clockwise (CW) and the second reverse direction arc (179° to 0°). They were established for each arc 98 control points with maximum speed of gantry 4.8 deg / s. Each semi-arch lasted 48 seconds (0.8min) (Figure 3 & 4). The bodies’ risks were considered for planning spinal cord, left lung, left humeral head and left mammary gland. As establishment of an NTO (normal tissue objective) use the automatic option. Resolutions (mm) and priorities (%) planning for OAR (organ at risk) were, respectively: spinal cord 2 mm and 35%, humeral head 2.65 mm and 25%, left lung 3 mm and 30%, left breast 3 mm and 25%.
Assessment treatment plan
Evaluating different plane cuts ensure that 100% of the prescribe dose is within the volume of total dose planning 5940 cGy. The bodies assessed risks are below the limits set by Quantec / RTOG (Figure 5).
One of the benefits of this proposal lies among other things in the runtime patient treatment compared to some other CRT3D or IMRT technique. The maxim and average doses to OAR were: 27.3 Gy / 10.5 Gy (VMAT) and 36 Gy / 9.12 Gy (IMRT)for left humeral head (green); 60.0 Gy / 5.2 Gy (VMAT) and 60.5 Gy / 5.7 Gy (IMRT) for left breast (yellow); 8.5 Gy / 2.7 Gy (VMAT) and 9.6 Gy / 2.9 Gy (IMRT)for spinal cord(purple) and finally 17.3 Gy / 1.77 Gy (VMAT) and 35.5 Gy / 2.5 Gy (IMRT) for left lung (blue) (Figure 6)
QA
The two semi-arches were evaluated for creep refers using an amorphous silicon system to perform dosimetry portal. Descriptors parameters for evaluation were the index gamma 3% and distance agreement (DTA) 3mm. Predicted doses vs. the portals doses obtained successfully passed the evaluation criteria (Figure 7).
Diagnosis
Left axillary neurofibroma. The microscopic biopsy description reported: sections showed mesenchymal neoplasm, partially encapsulated, constituted by the proliferation of spindle cells, elongated nuclei arranged in swirling pattern and fibrillar collagenous stroma storiform on congestive vessels with thickened walls and areas of bleeding. Differential diagnoses included: schwannoma, malignant peripheral nerve sheath tumor and desmoid tumor [5].
Discussion
Neurofibromatosis was first described in 1882 by Friedrich Daniel von Recklinghausen, a German pathologist. It is a disease caused by an abnormality in a gene on chromosome 17.Thediagnosis of Neurofibromatosis type I (NF1) is confirmed If 2 or more of these criteria are met in an individual: six or more stains coffee with milk greater than 5 mm in pre pubertal patients and over 15 mm in post pubertal patients; two or more neurofibromas of any type or one plexiform neurofibroma; sign Crowe (axillary or inguinal freckling); [6-9] Glioma optic nerve; two or more Lisch nodules (iris harmartomas); typical bone lesions (dysplasia of the sphenoid dysplasia or thinning of long bone cortex with or without pseudoarthrosis); history of neurofibromatosis type I in parents or siblings [10]. Meanwhile, to make the diagnosis of NF 2 is necessary to have the following criteria: bilateral masses in the eighth cranial demonstrated techniques by appropriate images or first-degree relative with NF2 and one of the following: unilateral mass the eighth cranial nerve, or two of the following: neurofibroma, meningioma, glioma, schwannoma, subcapsular lenticular opacity youth back [10].
In the case of our patient, it has none of the criteria for NF1or 2. Neurofibroma is a benign peripheral nerve sheath tumor that consists of Schwann cells, associated or unassociated with axons, perineural cells, and fibroblasts. The tumor routinely expresses S-100 protein. Benign neurofibromas most often occur in association with neurofibromatosis type 1 (NF1) but may also occur sporadically [1,2], like in our case report.
The biological behavior of neurofibromas is usually benign, but it is not rare sarcomatous degeneration in plexiform. Neurofibromas occur in multiple locations, exhibit different growth patterns and morphologies at highly variable rates, a classification system stratifies these tumors into 5 types: localized cutaneous, diffuse cutaneous, localized intraneural, plexiform, and massive soft tissue neurofibromas [4].
The management depends on the location and growth pattern. Whenever possible, the treatment of choice should be surgical, especially when they produce functional or cosmetic déficits, ideally with complete removal of the tumor, but some tumors diffusely involve adjacent normal tissues such as nerves and blood vessels, making complete remove of the tumoral most impossible [1,3,4,6,8,9]. Medical therapies have been investigated, most are still investigational [4] and Radiotherapy has been rarely used like initial treatment, but by default in patients with incomplete resection [1,4]. Needle et al. [6] reported 121 children with plexiform neurofibromas who underwent resection and were followed for over 20 years. Those that were entirely removed were less likely to recur and had a longer median time to failure. Near-total and subtotal resections demonstrated recurrence rates of 39.5% and 44%, respectively.
Conclusion
We must emphasize team work and multi disciplinary approach to tackling the disease. The commitment caused by neurofibromas depends in much of their location, skin lesions usually cause deformity, while more injuries shallow tend to generate functional compromise. Despite being mostly benign, some cause destruction secondary to the pressure exerted, so the symptoms are depend on the size of the lesion. The therapeutic approach should be multidisciplinary, with the initial treatment recommended surgery, but in case of not being able to perform or partial resection, radiotherapy with doses between 54-60 Gy can achieve adequate local control. In reviewing the literature an extensive description of the disease and epidemiological data was found are scarce, so studies to know the disease more accurately are needed.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on:
https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on:
https://juniperpublishers0.wixsite.com/juniperpublishers
#Juniper publishers#Open access Journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Encouraging Debut of Immunotherapy In Myeloma

Abstract
Multiple myeloma (MM) is a malignant disorder of antibody-producing clonal plasma cells. It is the second most common hematologic neoplasia worldwide [1]. Despite recent advances in myeloma treatment (high-dose chemotherapy followed by autologous stem cell transplantation, novel immunomodulatory drugs, and proteasome inhibitors); MM remains largely incurable with chemotherapy [2]. This is mostly due to the persistence of minimal residual disease (MRD), which leads to high relapse rates [2]. Furthermore, the prognosis of MM patients who become refractory to recently developed novel agents is very poor [1].
Abbreviations: MM: Multiple Myeloma; MRD: Minimal Residual Disease; PD-L1: Programmed Cell Death Ligand; MDSCs: Myeloid-Derived Suppressor Cells; ACT: Adoptive Cellular Therapy; SLAMF7: Signaling Lymphocyte Activating-Molecule Family Member 7; ADCC: Antibody-Dependent Cellular Cytotoxicity; RRMM: Relapsed / Refractory Myeloma; DC: Dendritic Cells; NK: Natural Killer; GM-CSF: Granulocyte- Monocyte Colony-Stimulating factor; C/T: Cancer Testis Antigens; IMiDs: Immunomodulatory Drug; ACT: Adoptive T Cell Therapy; CAR: Chimeric Antigen Receptors; BCMA: B-Cell Maturation Antigen
Introduction
Myeloma is associated with profound immune dysfunction affecting the innate and adaptive immune system. Myeloma cells play also an important role in maintaining immunosuppression [3]. In MM, there is reduced expression of HLA molecules, reduced tumor antigen peptides expression, enhanced expression of inhibitory ligands such as programmed cell death ligand 1(PD-L1) and PD-L2, and recruitment of counter-regulatory cells such as T regulatory cells (Tregs) and myeloid-derived suppressor cells (MDSCs) [4].
The importance of host antitumor immunity is evidenced by long-term molecular CR observed post allogeneic stem cell transplantation [5] due to a graft-versus-myeloma effect exerted by donor-derived T lymphocytes [6]. An overdue era of immune therapy in MM has begun [7].
So far, the only established immuno therapeutic approach for MM is allogeneic stem cell transplantation. However, it is associated with high morbidity and mortality and remains an option for only a fraction of patients [2].
MM immunotherapy can be divided into several categories:
Monoclonal antibodies targeting surface molecules present on myeloma cells;
Monoclonal antibodies targeting checkpoint inhibitors on immune cells;
Pharmacologic immunomodulation;
Cancer vaccines; and
Adoptive cellular therapy (ACT) [3].
Monoclonal antibodies targeting surface molecules present on the myeloma cells
In MM, several surface molecules have been explored in preclinical and clinical studies as potential targets of monoclonal antibodies. They include SLAMF7 (signaling lymphocyte activating-molecule family member 7), CD38, CD40, CD138, CD56, CD54 (also known as intercellular adhesion molecule 1), IL-6, PD1, CD74, CD162, B2-macroglobulin and GM-2 ganglioside. In 2015, FDA approved daratumumab (anti-CD38) and elotuzumab (anti- SLAMF7) for treatment of myeloma [5].
Monoclonal antibodies targeting CD38: CD38 is a type II transmembrane glycoprotein, expressed at high and uniform levels in most, if not all, plasma cells in all stages of the disease. It is not expressed on primitive hematopoietic precursors (CD34+CD38-), suggesting that hematopoietic recovery would occur following CD38-targeted cytotoxic agents [1]. Both Daratumumab and isatuximab are anti-CD38 monoclonal antibodies that trigger antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity and antibodydependent phagocytosis, as well as inhibition of the enzymatic activity of CD38. Moreover, even in the absence of Fc-receptorexpressing effector cells, both monoclonal antibodies can induce direct apoptosis and lysosome-mediated cell death in MM cells harboring p53 mutations. However, CD38 levels alone may not be the sole determinant of sensitivity to daratumumab, because CD38-negative Tregs were also reduced in responsive patients [7].
Daratumumab was approved for use in patients who have failed at least 3 prior lines of therapy [8]. Isatuximab shows single-agent activity in relapsed / refractory myeloma (RRMM) [9]. Daratumumab was administered for prolonged periods at moderate to high doses with little or no toxicity, despite the fact that the CD38 molecule is also expressed at lower levels, on a fraction of hematopoietic cells, cerebellar Purkinje cells, liver and lung smooth muscle cells, and insulin-secreting cells of pancreas [1]. SAR650984 is another anti-CD38 antibody which is under investigation in clinical trials [9].
Monoclonal antibodies targeting SLAMF-7: SLAMF7 is a cell surface glycoprotein receptor highly expressed on MM cells mediating family member 7 adhesion to BM stromal cells. It is selectively expressed on plasma and natural killer (NK) cells and lacks expression on other tissues. Elotuzumab is a fully humanized monoclonal antibody directed against SLAMF-7. It triggers ADCC and enhances NK cell function against MMCs [3]. Elotuzumab was approved in combination with revlimid and dexamethasone for treatment of myeloma who received 1-3 prior therapies [8].
Compared with chemotherapeutic agents, monoclonal antibody therapies appear to be associated with fewer side effects and have distinct mechanisms of action that make them more suitable and effective when combined with other antimyeloma therapies, especially in patients with chemotherapyresistant disease [10].
Monoclonal antibodies targeting checkpoint inhibitors on immune cells: The engagement of T cell with its target is regulated by a complex balance of costimulatory and coinhibitory bidirectional signals (checkpoints) [3] which allow the cells to turn on and off as needed. Checkpoint inhibitors take the brakes off the immune system [8]. Anti-PD-1 is a checkpoint inhibitor that blocks the inhibitory interaction of PD-1 on T or NK cells with its ligand PD-L1 on tumor cells or tumor-promoting accessory cells [3]. Blockade of PD-1/PD-L1 inhibits accessory cell (plasmacytoid dendritic cells, pDC or MDSCs)-induced MM proliferation and survival while triggering host T- and NK-cell anti-MM cytotoxicity [5].
Recently, the anti-PD-1, pembrolizumab, in combination with Rd (PRd), showed 38% response rate in lenalidomide refractory patients with an acceptable toxicity profile. The overall response rate was 50% in a phase ½ study combining pembrolizumab with pomalidomide in patients with a median number of 3 prior therapies. Seventy-five percent of patients were double refractory to IMIDs and PIs [3]. These studies demonstrate a role for checkpoint inhibition in MM treatment paradigm and underscore the need for IMIDs immunomodulation to achieve this response [3].
Cancer Vaccines
Cancer vaccine aims to increase the precursor frequency of antigen-specific T cells or antibodies through in vivo priming [3]. Vaccination against cancer-specific antigens represents a promising strategy to modulate patient antitumor immune response, particularly in the settings of early-stage or minimal residual disease [5]. Vaccines, in combination with immunomodulatory agents, may serve to achieve and/ or maintain MRD hoping to prolong PFS (and possibly OS) [3]. Several vaccination approaches have been used for myeloma: idiotype vaccines, GM-CSF-based cellular vaccines and dendritic cells (DC)-based vaccines [3].
Idiotype Vaccines
Idiotype refers to the unique immunological properties of any individual immunoglobulin [6]. The Id is expressed and presented in an HLA-restricted manner on the surface of malignant plasma cells [3]. The secreted idiotype was up taken and presented by dendritic cells and may act as T cell antigens [6]. However, idiotype vaccines were weakly immunogenic and failed to elicit a measurable clinical benefit even when combined with strong adjuvants such as granulocyte-monocyte colonystimulating factor (GM-CSF), IL-12, alum or keyhole limpet hemocyanin [3].
GM-CSF-based cellular vaccines
GM-CSF-based vaccine consists of 2 allogeneic cell lines: H929 and U266, coupled to a GM-CSF-secreting by stander cell line, K562/GM. This vaccine formulation has been tested in patients with a near complete remission i.e. patients having absent an M-spike and persistence of detectable immuno fixation for 6months. This study suggested that the generation of tumorspecific immunity in a low disease burden state can significantly delay relapse [3].
DC-based vaccines
Idiotype-loaded DCs have been used as vaccines in MM patients, mostly in the setting of clinical remission after auto transplant [6]. Decreased regulatory-T cell function and minimal disease state post transplant suggest that this is an optimal setting for vaccination [5]. Cancer testis antigens (C/T) are physiologically expressed in testis and placenta trophoblast only and have a potential role in cell cycle and apoptosis. C/T antigens are up regulated in MM [6]. C/T antigens are considered ideal antigens to target and are the best studied examples within this category. They fulfill several parameters, including their low expression on normal tissues and their expression in more aggressive disease and in advanced stage disease [3]. However; their manipulation requires profiling of the C/T antigen expression pattern in every MM individual patient [3]. An alternative approach is fusion of DCs with patient-derived tumor cell lysates or apoptotic tumor cells. It optimizes antigen presentation and immune priming against the entire antigenic repertoire of each unique patients’ tumor. Presentation of antigens from myeloma cell lines by dendritic cells is greatly enhanced by coating myeloma cells with a specific antibody such as anti-CD138 or use of myeloma cells lysed by repetitive freezethaw cycles [6].
Immunomodulatory drug (IMiDs)
IMiDs (thalidomide, lenalidomide and pomalidomide) are incorporated into therapies for relapsed / refractory myeloma (RRMM) and newly diagnosed MM. Moreover, maintenance therapy with lenalidomide in both transplant-eligible and -ineligible patients has markedly improved patient outcome [5]. They targets the tumor microenvironment through caspase-8- mediated apoptosis; abrogation of MMC binding to BM stromal cells; modulation of cytokine secretion; up regulation of T, NK, and NK-Tcells; and down regulation of T regulatory cells. Recently, the E3 ubiquitin ligase cereblon has been identified as the molecular target of lenalidomide. Predicated on their immuno stimulatory effects, IMiDs may enhance activities of monoclonal antibodies, checkpoint inhibitors, and vaccines [5].
Adoptive T cell therapy (ACT)
ACT aims to enhance T-cell antitumor activity through ex vivo manipulations [3]. Myeloma is an ideal disease to treat with adoptive T cell therapy [11]. This can be achieved through T cells engineered to express large numbers of affinity-enhanced TCRs, activated marrow infiltrating T cells and more recently with cytotoxic T cells endowed with tumor-reactive chimeric antigen receptors (CAR). Among these strategies, CAR-based therapies are perhaps the most appealing, as CART cells recognize their target antigens in an MHC-independent manner [1].
CAR T-cell therapy is the adoptive transfer of cytotoxic T cells that are genetically engineered to express CAR. CAR expressed on the cell surface redirect the cytotoxic T cells toward the original target of the antibody in a non- HLA-restricted manner [11]. CAR T cells provide a new and currently uncovered spectrum of effector mechanisms against myeloma [12]. There is a fully loaded pipeline of novel CARs targeting myeloma antigens, including CD38 [1] and CD44v6 as well as SLAMF7-CARs [12,13].
CD38-CART cells are powerful immunotherapeutic tools since they appeared capable in vitro of eliminating primary CD38+ MM cells from patients who had become resistant to various chemotherapies. This suggests that CD38-CAR therapy could be a viable choice for patients with few or no further chemotherapy options. Further studies are warranted to diminish the undesired effects of CD38-CART cells against normal CD38+ cells [1].
B-cell maturation antigen (BCMA)-CART cells BCMA is a target for immunotherapy because it is uniformly expressed on myeloma cells. BCMA has no known expression on normal solid tissue and only very restricted expression on normal hematopoietic cells (normal plasma cells, some memory B cells, and pDC). The observed toxicity was cytokine release syndrome with fever, hypotension and hematologic toxicity. The latter was quite significant in some patients, but within the range that can be explained by the lymphodepleting conditioning regimen and inflammation mediated by BCMA-CART cells in the bone marrow. B-cell depletion is not anticipated when targeting BCMA, as the majority of normal B cells are BCMA negative. The persistence of BCMA-CAR T cells after administration to patients in this trial was relatively short (3 months) [12].
The opportunity for using CAR is for dramatic tumor cell reduction, even in high-risk, refractory MM [5]. It can be used also in the setting of fulminant relapsed disease when there is a need for a rapid reduction in disease burden that would justify the associated toxicity [3]. Moreover, use of lenalidomide and/ or checkpoint inhibitors post-CAR-T cell therapy may allow for persistence of cancer immune surveillance by avoiding T-cell exhaustion [5]. Its efficacy could be improved if combined with immune checkpoint inhibitors and/or with survivin and/or MCL-1 inhibitors which are effective modifiers of cell adhesion-mediated immune resistance induced by the tumor microenvironment [1].
Conclusion
It is likely that the most effective immunotherapeutic approach for MM will include strategies for expanding the repertoire, function, and persistence of myeloma-directed T cells as well as enhancing the sensitivity of MM cells to immune attack [4].
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on:
https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on:
https://juniperpublishers0.wixsite.com/juniperpublishers
#Juniper publishers#Open access Journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Pharmacists Perspective on Management of GBM

Abstract
Management of patients with glioblastoma multiform requires the concerted efforts of multiple healthcare professionals, including pharmacists. Pharmacists across different practice settings may encounter patients being treated for glioblastoma multiforme. The oral chemotherapy temozolomide is a standard of care forglioblastoma multiforme, with dosing regimens and cycles that require dispensing considerations by the pharmacist as well as careful patient monitoring. Pharmacists should be able to direct the patient with glioblastoma multiforme to payment assistance for this drug as well. Bevacizumab is used to increase progression-free survival in recurrent glioblastoma multiforme, and pharmacists can also aid in the dosing and monitoring of this agent. Sequelae of glioblastoma multiforme include brain edema, seizures, venous thromboembolism, depression, and fatigue. Management of sequelae may include the use of corticosteroids, antiepileptic drugs, anticoagulants, and antidepressants, which can result in complex medication regimens. Toxicities and drug-drug interactions can be prevented and managed by pharmacists in both the outpatient and inpatient setting. Glioblastoma multiforme often leads to some degree of cognitive dysfunction, sorepeated education on medication regimens, adverse effects, and expectations of therapy are important for the patient and caregiver(s). Pharmacists are uniquely positioned to aid in the care of patient with glioblastoma multiforme.
Keywords: Glioblastoma multiforme glioblastoma; Brain tumor; Pharmacist; Temozolomide; Bevacizumab
Abbreviations: GBM: Glioblastoma Multiforme; MGMT: O6-Methylguanine Methyltransferase; IDH1: Isocitrate Dehydrogenase 1; RCTs: Randomized Controlled Trials; RT: Radiation Therapy; TMZ: Temozolomide; HR: Unadjusted Hazard Ratio; CI: Confidence Interval; NCCN: National Comprehensive Cancer Network; FDA: Food and Drug Administration; ANC: Absolute Neutrophil Count; CTC: Common Toxicity Criteria; PCP: Pneumocystis Cariniipneumonia; TMP/SMX: Trimethoprim/Sulfamethoxazole; ISMP: Institute for Safe Medication Practices; VEGF: Vascular Endothelial Growth Factor; BCNU: Biodegradable Carmustine; AEDs: Antiepileptic Drugs; VTE: Thromboembolism; ICH: Intracranial Hemorrhage; UFH: Unfractionated Heparin; SSRIs: Selective Serotonin Reuptake Inhibitors
Introduction
Glioblastoma multiforme (GBM), the most common malignant primary brain tumor in adults, is a devastating malignancy with a rapid clinical course [1]. The disease course and psychological impact to patients and their families requires a multidisciplinary approach to treatment. Multiple review articles have stressed the importance of the roles of neurosurgeons, neurooncologists, radiologists, nurses, rehabilitation therapists, as well as psychological support teams and general practitioners, but the role of the pharmacist in the management of GBM has yet to be delineated in the published literature [1-3]. Issues pertinent to pharmacists in the management of GBM include chemotherapy, drug interactions, pharmacologic management of sequelae, and supportive care. The objective of this article is to provide an overview of the treatment of GBM, with a focus on the pharmacologic agent’s temozolomide and bevacizumab, as well as highlight the areas where pharmacists can be a part of the GBM management team.
Glioblastoma multiforme is classified as a grade IV malignant cancer originating from astrocytic glial cells of the brain [4]. Glioblastoma multiforme can occur as either primary (de novo) or secondary (a lower grade glioma that progresses to GBM), with over 90% occurring as primary [1,2]. Overall, GBM accounts for 50% of glial cell tumors, with an incidence rate of 3.2 per 100,000 person-years [4,5]. Glioblastoma multiforme occurs more commonly in persons between the ages of 45 and 65 years, and affects whites at an incidence twice that of blacks [6]. The presentation of GBM varies, with common signs and symptoms of headaches, seizures, memory loss, and changes in behavior [4]. The highly aggressive nature of GBM makes treatment difficult, with an expected 5-year survival rate of <5% [5,6]. Only one third of patients with GBM are expected to survive 1 year after diagnosis [7]. Important prognostic factors include histologic diagnosis, age, and performance status, as measured by Karnofsky Performance Scale (KPS) [5,7].
Also, recent understanding of molecular genetics variables suggests that O6-methylguanine methyltransferase (MGMT) promoter methylation and isocitrate dehydrogenase 1 (IDH1) mutationshave prognostic and therapeutic response implications as well [8]. Despite advances in research and pharmacologic treatment methods, the median survival of 9 to 14 months after diagnosis is similar to what it was 20 years ago [5]. Unfortunately, GBM recurs in nearly 100% of patients, and is more difficult to treat [2]. Recently, literature has been published offering additional therapeutic options for recurrent disease.
Treatment overview - non-pharmacologic
Surgery and radiation: The cornerstone of treatment of GBM is surgery, despite its lack of validation in phase III randomized controlled trials (RCTs) [2]. Tumor resection allows for an accurate diagnosis, a decrease in tumor mass (which alleviates associated symptoms), and a more favorable response to postsurgical radiation therapy (RT) [9]. Surgery only increases survival when it achieves>98% resection of the tumor. However, this is difficult due to the infiltrative nature of the tumor. If submaximal tumor reduction occurs, residual tumor may behave more aggressively and result in worsening edema and mass tumor effect [2].
Postsurgical RT, the first postsurgical adjuvant therapy used historically, is still a part of the standard of care today [2]. In the 1960s to 1970s RCTs consistently showed postsurgical RT to be efficacious in the treatment of GBM, extending survival from 4 to 6 months to 10 to 11 months. Variations of RT regimens as well as RT with radiosensitizers have been studied, but no improvements in survival have been made. Today, localized RT is initiated at least 2 weeks after surgery and administered as fractionated external beam RT at a 60 Gy dosein 30 fractions over 6 weeks [7,9]. Abbreviated courses may be used in those with poor performance statuses (KPS <60) or in elderly patients (≥65 years) [2,7,9].
Treatment overview - pharmacologic
Temozolomide - evidence: Temozolomide (TMZ) is available orally as well as a powder for injection [10]. It is an alkylating agent indicated for adjuvant treatment for newly diagnosed GBM, and is now the standard for those who have a good performance status (KPS ≥60) [7,10]. The methylation status of the DNA repair enzyme, MGMT, is thought to be a predictor of response to TMZ [7,11]. Overexpression of the MGMT protein confers resistance to TMZ, and methylation of the MGMT gene can inactivate the production of the MGMT protein. Those patients with a methylated MGMT promoter region are likely to respond to therapy. Obtaining MGMT methylation status before treatment is not currently a standard of care because there are no strategies to overcome TMZ resistance. Treatment with TMZ is administered regardless of MGMT status.
A key trial included 573 patients with glioblastoma, and randomized them to treatment with postoperative RT and concomitant TMZ, followed by 6cycles of adjuvant TMZ, or to RT alone given 5 days weekly for 6 weeks [12]. The results were published by Stupp et al in 2005, showing that TMZ combined with RT reduced the risk of death by 37% compared to RT alone [unadjusted hazard ratio (HR), 0.63, 95% confidence interval (CI) 0.52 to 0.75; P<0.001]. After 28 months the median survival was 14.6 months for TMZ combined with RT, and 12.1 months with RT alone. The 2-year survival rate was 26.5% in the combination group and 10.4% in the RT alone group. The main adverse effects of TMZ were hematologic, with 16% of patients in the combination group experiencing grades 3 or 4 hematologic adverse effects (leukopenia, neutropenia, thrombocytopenia, and anemia).
No patients in the RT alone group experienced grades 3 or 4 hematologic adverse effects. A later analysis of the trial revealed that during the 5-year follow-up period, approximately 10% of patients in the combination group were still alive, compared to 2% in the RT alone group [13]. The regimen used in the trial is described in (Table 1), and has since been referred to as the “Stupp regimen.” Unfortunately, given the trial design, it is unclear which part of the Stupp regimen - concomitant TMZ, adjuvant TMZ, or the combination of both - is responsible for the benefits that are seen. A myriad of alternative dosing regimens and schedules of TMZ have also been studied, but the National Comprehensive Cancer Network (NCCN) guidelines still recommend the use of the regimen used in the study by Stupp and colleagues [7,12].
Temozolomide - implications for the pharmacist:
Dosing regimens: The standard dosing scheme and alternative dosing schemes for TMZ can be viewed in (Table 1). Although TMZ does not have US Food and Drug Administration (FDA) approval for use in recurrent GBM, smaller studies of dose-dense regimens have shown some efficacy with its use for this indication [14-16]. The theory behind dose-dense TMZ regimens is that they more effectively deplete MGMT, so that the affected DNA cannot be repaired, allowing the tumor cells to be more susceptible to the antitumor activity of the alkylating agent [11,17]. It is thought that patients with GBM who are refractory to initial therapy have an unmethylated MGMT gene, resulting in overexpression of MGMT, and could therefore benefit from dosedense TMZ regimens.
Toxicities and dose reductions: The most common adverse effects of TMZ during the concomitant phase with RT include thrombocytopenia, nausea, vomiting, anorexia, and constipation [10,12]. At any point during therapy, common adverse effects may include alopecia, nausea, vomiting, anorexia, headache, and constipation. Temozolomide is classified as moderately to highly emetogenic when doses exceed 75 mg/m2/day, andit is low to minimally emetogenic when doses are <75 mg/m2/ day [18]. When combined with RT at a dose of ≤75 mg/m2, it is considered moderately emetogenic. For emesis prophylaxis, the NCCN Antiemesis Guidelines recommend an oral 5-HT3 antagonist with or without lorazepam and with or without an acid reducer (such as a histamine-2 receptor antagonist or proton pump inhibitor). When TMZ is given at doses rendering it lowly emetogenic, metoclopramide or prochlorperazine may be used. Temozolomide may be taken on an empty stomach should nausea/vomiting occur; some find bedtime is most convenient [10]. (Table 2) reviews antiemesis options for temozolomide.
Hematologic toxicities are a concern with TMZ and can occur early in treatment [10]. Prior to dispensing TMZ, the pharmacist should ensure the patient meets the following criteria: absolute neutrophil count (ANC) >1.5 × 109/L, platelet count >100 × 109/L, and common toxicity criteria (CTC) nonhematological toxicity < grade 1 (except for alopecia, nausea, and vomiting). Complete blood counts should be obtained each week. The doselimiting toxicity for TMZ is myelosuppression (neutropenia and thrombocytopenia) with grade 3 or 4 neutrophil abnormalities occurring in approximately 8% of patients and grade 3 or 4 platelet abnormalities in 14% of patients. Some toxicity may require TMZ dose reduction/discontinuation, especially during the concomitant phase with RT (Table 3). Dose reductions based on hematologic toxicities during the maintenance phase can be seen in (Tables 4 & 5). A recent review of FDA MedWatch data from 1997 to 2008 found 76 cases of aplastic anemia or aplasia were reported in 3490 patients taking TMZ [19]. These reports were from patients on TMZ for any indication, not just GBM. Patients are most at risk for hematologic toxicities when receiving concomitant RT and/or other myelosuppressive chemotherapy.
Given the severe lymphocytopenia that patients experience during the concomitant phase when TMZ is combined with RT, Pneumocystis carinii pneumonia (PCP) prophylaxis needs to be administered during concomitant therapy [10]. During concomitant therapy, prophylaxis for PCP should be continued regardless of ANC, and continued after the concomitant phase until lymphocytopenia is resolved or no worse than CTC grade 1. Continuation of PCP prophylaxis should be considered in the maintenance phase if the patient is also receiving steroids. The pharmacist should collaborate with the patient’s care team to discuss when PCP prophylaxis is no longer necessary. The NCCN listsoral trimethoprim/sulfamethoxazole (TMP/SMX) as the preferred agent for PCP prophylaxis [20]. It can be administered as single strength or double strength daily or double strength 3 times per week. Alternative agents for PCP prophylaxis in patients who are allergic or intolerant to TMP/SMX include dapsone, inhaled pentamidine, oratovaquone.
Dispensing: Since TMZ is supplied as 6 different capsule strengths, pharmacists will often have to dispense varying quantities of different strengths in order to obtain the correct dosage for each patient [10]. The package insert has a chart that suggests capsule combinations based on a patient’s daily dose. Each strength should be dispensed in a separate bottle, or the original container, and labeled with the number of capsules that should be taken from each container each day. An error reported to the Institute for Safe Medication Practices (ISMP) involved a pharmacist who misinterpreted the quantity on the temozolomide bottle as the strength [21]. The temozolomide script was written for a dose of 60 mg (3 of the 20 mg capsules for each dose), and the pharmacist inadvertently filled the prescription with 100 mg capsules after mistaking the quantity of 20 listed on the bottle for the strength. If taken as filled, the patient could have experienced severe hematologic toxicities [21]. The ISMP suggests highlighting the strength of the TMZ prescription to draw attention to it.
Pharmacists should also counsel patients on the directions and taking it at the same time every day, and confirm understanding with the patient. Impaired cognitive function may be a result of the disease, so patients may struggle with reading or comprehending instructions. The use of calendars and pillboxes may be helpful as well. Pharmacists should also be cognizant that dosages will likely change depending on which cycle patients are in, whether they are receiving concomitant RT, and/or if they experience toxicities. The pharmacist will need to order TMZ based on if the patient is following a 5-day or 42- day regimen, or perhaps a continuous or dose-dense regimen. It is important that the pharmacist document (or has access to documentation of) the patient’s ANC and platelet values for that cycle. Refills should not be dispensed until hematologic criteria are met, as dictated by the prescribing physician and the TMZ package insert.
Some patients with GBM may have swallowing difficulties or dysphagia, making it difficult to ingest the TMZ capsules. The capsules are on the ISMP list of medications that should not be crushed or opened, due to the slow-release formulation and safety issues of inhalation and/or skin exposure [22]. Swallowing difficulties are especially concerning to patients when combinations of different strengths of capsules must be taken in order to achieve the prescribed daily dose [10]. As such, patients can have their pharmacist compound a TMZ solution following the recipe found in (Table 6) [23]. Temozolomide is also available as an injection [10]. This may be especially useful for patients with refractory nausea due to tumor progression, dysphagia due to a brainstem tumor, or younger patients reluctant to swallow capsules [24]. When infused over 90 minutes, the intravenous form of temozolomide displays similar pharmacokinetics as the oral formulation, which is also 100% bioavailable.
Calculate the required quantity of each ingredient for the total amount to be prepared.
Weigh and/or measure each ingredient accurately.
Empty the contents of the temozolomide capsules into a suitable mortar.
Add the povidone K-30 to the mortar and blend the powders well.
Dissolve the citric acid in the purified water and add to the powder mixture.
Add a small amount of Ora-Plus to the mixture and mix well.
Add the remainder of the Ora-Plus geometrically and mix well.
Transfer to a calibrated container.
Rinse the mortar with either the Ora-Sweet or Ora-Sweet SF, add to the calibrated container, and mix well.
Repeat until the final volume has been obtained.
Mix the solution well.
Package in a tight, light-resistant container.
Label with “keep out of reach of children,” and “use only as directed.”
Expiration: 60 days from time of preparation if stored in a refrigerator.
Payment assistance: The pharmacist (both community and ambulatory care) can also play an important role in helping patients and their families obtain financial assistance with paying for TMZ treatment. The cost of TMZ is thousands of dollars per month, regardless of use of brand or generic. Pharmacists can help patients enroll in financial assistance programs by the manufacturer. Merck & Company, Inc. offers the ACT Program and the Co-Pay Assistance Program, both of which provide financial assistance to patients whose income is below a certain level. The ACT Program is for those who do not have prescription coverage and the Co-Pay Assistance Program is for those with private insurance who cannot afford their co-pay [25]. The website rxassist.org provides an overview of each program and links to application forms. If pharmacists receive rejection messages from a patient’s insurance company or the co-pay is unaffordable to the patient, the pharmacist should notify the prescriber immediately so that the necessary steps can be taken to avoid a delay in therapy. Recently, generic formulations have been made available; however, drug acquisition can still be a challenge.
Bevacizumab- evidence
The tumor characterization of glioblastomas such as intense vascular proliferation and increased expression of angiogenic factors such as vascular endothelial growth factor (VEGF) have made VEGF an attractive therapeutic target for management of glioblastomas [26]. Bevacizumab is a humanized monoclonal antibody designed to target VEGF and studies have demonstrated improved progression free survival in recurrent GBM [27]. In 2009, bevacizumab received accelerated approval from the FDA for use as monotherapy in progressive glioblastoma, based on improved radiologic response rates observed with bevacizumab monotherapy in 2 single-arm/noncomparative phase II trials compared with historical data [26,28].
The first phase II trial included 167 patients with recurrent glioblastoma who were randomly assigned to receive bevacizumab 10mg/kg alone or in combination with irinotecan 340mg/m2 or 125mg/m2 (with or without concomitant enzymeinducing antiepileptic drugs) once every 2 weeks [26]. The results of the trial demonstrated an estimated 6-month progression free survival rate of 42.6% in the bevacizumab monotherapy arm versus 50.3% in the combination arm. The median overall survival times were 9.2 months and 8.7 months, respectively. In terms of safety, approximately 46.4% of patients experienced ≥ grade 3 adverse events in the bevacizumab arm versus 65.8% in the combination arm. The most common toxicities reported in the monotherapy arm included hypertension and convulsion versus convulsion, neutropenia, and fatigue in the combination arm.
The second trial the FDA based its accelerated approval on was a phase II trial with 48 heavily pretreated patients who received single agent bevacizumab 10mg/kg every 2 weeks and after tumor progression irinotecan was added similar to the previous study [28]. Median progression free survival was 16 weeks and median 6 month overall survival was 57%. The most common adverse events reported included thromboembolic events, hypertension, hypophosphatemia, and thrombocytopenia. The results of these two studies demonstrate the activity of bevacizumab in GBM with acceptable toxicity profile and also suggests the limited benefit with the combination with irinotecan [26,28].
However, combining bevacizumab with chemotherapy is not a foregone conclusion. A Dutch study explored combining bevacizumab with lomustine [29]. The BELOB study trial randomly assigned patients to bevacizumab, lomustine, or bevacizumab plus lomustine at first recurrence of GBM. Progression free survival at 6 months was 41% with the combination of bevacizumab and lomustine versus 18% with bevacizumab alone and 11% withlomustinealone. The overall survival at 9 months was 59% in the combination group versus 38% and 43% for the bevacizumab and lomustine-alone groups, respectively. Recently, a study was published evaluating the addition of bevacizumab to radiotherapy plus temozolomide in newly diagnosed glioblastoma [30]. In this study, the addition of bevacizumab did not improve patient survival. Bevacizumab’s role in the management of GBM continues to evolve in the recurrent setting and data is not matured in the newly diagnosed setting.
Bevacizumab - implications for the pharmacist:
Dosin: Bevacizumab dosing in the aforementioned clinical trials is 10mg/kg every 2 weeks. Wong, et al performed a meta-analysis of 15 studies involving over 500 patients treated with bevacizumab for recurrent GBM [31]. The meta-analysis found no difference in bevacizumab doseresponse between 5mg/kg and 10 to 15mg/kg. However, a prospective analysis needs to be conducted to confirm the findings.
Toxicities: In general, bevacizumab is well tolerated in patients with recurrent GBM, and the toxicities observed in clinical trials are comparable to those seen in other cancers [27]. Low-grade bleeding, hypertension, impaired wound healing, and proteinuria are the most common adverse events attributable to bevacizumab in recurrent GBM studies. Blood pressure readings should be documented at baseline and prior to bevacizumab administration. Adverse events such as life-threatening intracranial bleeding have occurred in a ≤3% of patients and prevalence of thromboembolism is approximately 1.6% to 12.5% [32]. No special precautions or additional monitoring are necessary when treating GBM patients with bevacizumab.
Other treatment options: Another initial treatment option for GBM is local administration of chemotherapy [7]. The biodegradable carmustine (BCNU) wafer is implanted during surgery, and may be administered regardless of performance status. The wafers do not result in systemic absorption of the nitrosoureacarmustine, thus minimizing systemic side effects. Irinotecan has been studied in combination with bevacizumab for recurrent glioblastoma multiforme [26,28,33]. Parameters such as complete blood cell count with differential and liver function tests should be monitored at baseline and regularly prior to irinotecan administration. As irinotecan is metabolized hepatically, caution should be taken when administering with enzymeinducing antiepileptic drugs (AEDs) such as phenytoin. Patients receiving concurrent irinotecan and phenytoin will have reduced exposure to irinotecan and its active metabolite, SN-38 [34]. To avoid decreased chemotherapeutic efficacy, irinotecan doses are significantly increased if patients are on concomitant enzyme-inducing AEDs [33].
Sequelae and treatment-related adverse effects
Brain edema: Dexamethasone is commonly used for the management of brain edema in patients with GBM or with other cancers that have metastasized to the brain [1,35]. Brain edema associated with GBM is usually of the vasogenic extracellular type, which is plasma leakage into the brain parenchyma due to increased brain capillary permeability and a pressure gradient. Tight junctions on endothelial cells of the blood-brain barrier can become dysfunctional, leading to capillary permeability. Vascular endothelial growth factor has been implicated in this process, as it mediates proteins involved in tight junction formation. Corticosteroids, such as dexamethasone, reduce expression of VEGF and therefore reduce formation of cerebral edema. Other mechanisms for edema reduction by corticosteroids have also been proposed. Overall, reductions in capillary permeability can be seen as soon as 1 hour after corticosteroid administration. With dexamethasone, full effect is reached within 24 to 72 hours of administration.
Management of brain edema with corticosteroids - implications for the pharmacist: Patients with GBM often receive dexamethasone for cerebral edema at diagnosis, postoperatively, at the time of radiation, and if the tumor progresses [1,35,36]. Management of edema can help alleviate associated headache, cognitive deficits, nausea/vomiting, and seizures. The dose of dexamethasone can range from 4 mg to 100 mg daily, with typical doses in the range of 12 mg to 16 mg daily. It can also be administered intravenously. One study found similar improvement in neurologic function with a 4 mg dose compared to 16 mg, but a higher incidence of muscle weakness and cushingoid faces were seen with the 16 mg dose [37]. Other adverse effects that pharmacists should be able to counsel patients on include hyperglycemia, drug interactions (Table 7), and the possibility of corticosteroid-induced ulcers [35]. It may be advisable for patients to receive concomitant histamine-2 receptor antagonists or a proton pump inhibitor as ulcer prevention. Infection and psychiatric complications are also risks. Upon discontinuation, corticosteroids should be tapered over 2 to 3 weeks. It is recommended that this be achieved by decreasing the dose by 50% every 4 days. Patients who have a poorer performance status may be tapered by 25% every 8 days to avoid deterioration associated with rapid tapering.
Seizures: At the time of GBM diagnosis, up to 40% of patients have experienced seizures [1,38]. Seizures may often be the presenting symptom of GBM. Because edema and tumor size contribute to seizure development, a reduction in tumor size and a decrease in edema may partially or fully control seizures [5]. However, a majority of patients will still require treatment with AEDs during their disease course. Meta-analyses and reviews have found that seizure prophylaxis with AEDs is ineffective, even when patients with subtherapeutic AED levels were excluded [1,38]. Also, adverse effects of AEDs are documented to be 20% to 40% higher in patients with brain tumors as compared to those without brain tumors. Thus, use of AEDs is not warranted when there are likely no benefits and only risks involved. In patients with brain tumors who have not yet experienced seizures, The American Academy of Neurology does not recommend prophylactic AED use due to lack of benefit and the propensity of these medications to interact with chemotherapy and corticosteroids [38].
For patients with GBM who do experience seizures, standard epilepsy therapy applies [1,39,40]. Monotherapy is often used given its lower incidence of adverse effects and better compliance rates. There is disagreement on preferred therapy/regimens for these patients, as little evidence exists to support the use of one agent or combination of agents over another. Seizure control, adverse effects, and drug interactions are major factors that dictate choice of therapy. Many of the AEDs are well-known for their induction of hepatic cytochrome P-450 enzymes, especially 3A4 [5]. This has implications for the patient with GBM since medications they commonly take, including some chemotherapy, steroids, antibiotics, warfarin, and antidepressants, are metabolized by and/or also induce or inhibit these hepatic enzymes as well [39]. (Table 7) lists drug interactions that may occur between chemotherapy for GBM and agents (both AED and non-AED) used for management of its sequelae.
Valproic acid and levetiracetamare agents commonly used as first-line monotherapy for seizures in patients with GBM [40]. A retrospective analysis of the patients enrolled in the trial by Stupp, et al, analyzed the use of AEDs with radiation and temozolomide, adjusting for known prognostic variables [41]. While overall survival did not differ between patients taking AEDs and those who were not, there appeared to be a survival advantage from the chemoradiation (temozolomide/ radiation) in patients taking valproic acid as compared to those taking enzyme-inducing AEDs or no AEDs. The correlating median survival times were 17.3 months, 14.4 months, and 14.0 months, respectively. It has been hypothesized that valproic acid enhances antitumor effects of chemoradiation through inhibition of histone deacetylase, inducing autophagy [42,43].
Regardless of possible antitumor effects, valproic acid has shown to be effective alone and in combination with other AEDs for seizures in some patients with brain tumors, including GBM. Van Breemen and colleagues found valproic acid alone and in combination with levetiracetam resulted in a 79.3% and 81.5% seizure response rate, respectively [43]. Considerations for use of valproic acid include thrombocytopenia, drug interactions due to inhibition of CYP2C9, the need for add-on agents for further seizure control in some patients, and therapeutic drug monitoring [40,44]. Levetiracetam may also be effective monotherapy, with the benefits of good tolerance, lack of drug interactions and no need for therapeutic drug monitoring [40]. While drug interactions may be avoided with levetiracetam, dose adjustments are required for renal dysfunction, whereas this is not the case with valproic acid [34,40].
Phenytoin is another AED commonly used as monotherapy due to its efficacy, but has disadvantages of drug interactions, required therapeutic drug monitoring, and dose-related adverse effects, including drowsiness and dizziness [40,44]. The drug interaction between phenytoin and dexamethasone is especially concerning. Both drugs induce the metabolism of the other, resulting in increasing dosage requirements of both for seizure and edema control [45]. Therapeutic doses of phenytoin when used concomitantly with dexamethasone have been reported as high as 600 to 1000 mg/day. Other AEDs used as monotherapy in patients with GBM include topiramate, zonisamide, and lamotrigine [40,44].
Seizure Control - implications for the pharmacist: Seizure control in patients with GBM is difficult due to disease progression, worsening edema, and drug interactions [5]. Pharmacists can assist in seizure control and toxicity management through therapeutic drug monitoring, especially in the case of highly protein-bound AEDs such as phenytoin and valproic acid. Dexamethasone is administered periodically to patients with GBM, and it is not uncommon for patients to have multiple dose adjustments to effectively reduce brain edema. Because of the ability of dexamethasone to induce CYP3A4, dose adjustments in AEDs metabolized through this pathway are often warranted, and can be recognized and managed by pharmacists.
Venous thromboembolism: Like many cancers, GBM is associated with an increased risk for venous thromboembolism (VTE) [46-48]. It is estimated that symptomatic VTE occurs in 19% to 29% of patients with gliomas. Identified risk factors for VTE in patients with malignant glioma include larger tumor size, leg paresis, operation time >4 hours, and use of chemotherapy [46]. A retrospective cohort study of 9489 patients diagnosed with malignant glioma in the 1990s was followed for 2 years [47]. Over half of these patients had GBM. The investigators found that over 6 months, 1 year, and 2 years, the cumulative incidences of VTE in the entire cohort were 6.1%, 7.0%, and 7.5%, respectively. Among those who experienced a VTE, 70% had a deep vein thrombosis and 30% had a pulmonary embolism. Approximately half of these patients had undergone neurosurgery in the preceding 2 months. Similar to other findings, patients with GBM were at an increased risk for VTE compared to other histologies (HR, 1.7; 95% CI, 1.3 to 2.3). Patients with ≥3 comorbidities were at a 3.5-fold greater risk for VTE than those without comorbidities (HR, 3.5; 95% CI, 2.8 to 4.3). Another risk factor was age ≥65 years. The greatest limitation to these findings is that the standard regimen of temozolomide and radiation had not been instituted as a standard of care during the time of this study, so these data do not account for any effects this regimen would have on VTE development.
Data are limited on safety and efficacy of VTE prophylaxis in patients with brain tumors [48]. Because of this and the increased risk for intracranial hemorrhage (ICH), it is recommended that prophylaxis be reserved for surgical and hospitalized patients. Both unfractionated heparin (UFH) and low molecular weight heparin have been studied in this patient population and are effective at VTE reduction. As for the treatment of VTE in patients with brain tumors, most trials on pharmacologic agents have excluded patients with brain tumors. Use of pharmacologic agents is limited by clinicians’ concerns for ICH, patient compliance, and drug interactions. While inferior vena cava filters have been used historically, data in the last decade have shown UFH displays superior efficacy and acceptable safety. An initial UFH bolus should be considered carefully, as it is associated with a transient state of overanticoagulation, which can lead to hemorrhagic complications.
Low molecular weight heparin is not ideal for initial treatment because it has a longer half-life and is not as easy to monitor as UFH. Its role in this patient population may be better suited as bridging therapy to long-term treatment with warfarin in a patient who has successfully tolerated UFH. Long-term use of warfarin has shown to be effective and safe for patients with brain tumors. Management of these patients is the same as the general population, but they may require more frequent monitoring.
Venous thromboembolism - implications for the pharmacist: Because patients with GBM are in a persistent hypercoaguable state, pharmacists can play in important role in counseling patients on the signs and symptoms of VTE, as well as managing maintenance medications of those patients being treated for VTE. Signs and symptoms of bleeding should be discussed as well. In all cases, management of drug interactions should involve a thorough review of pertinent labs, medication and medical history, and future management plans, as well as collaboration with the patient’s care team. (Table 8) provides general tips for patient counseling for patients with GBM.
Depression, cognitive impairment, and fatigue: Patients with GBM experience a great emotional (and physical) burden from the time of diagnosis through treatment. Many patients experience mood disturbances, cognitive problems, fatigue, and existential distress throughout the course of their disease [49]. Tumor location has been implicated in mood disturbances, with depression being associated with left-sided and frontal tumors. Depression has been estimated to occur in 15% to 28% of patients with GBM, but results from patient selfreporting are often higher. However, there are little data on the use of antidepressants in this patient population. In order to investigate selective serotonin reuptake inhibitors (SSRIs) and their impact on toxicities and survival in patients with GBM, a review was performed on data from 160 patients from 1999 to 2008 [50]. A majority of the patients received temozolomide and radiation, and 21.8% of patients overall received SSRIs during initial therapy. The most common SSRIs were sertraline and citalopram. No difference in toxicities was observed between patients taking SSRIs and those who were not. Interestingly, 2-year survival in patients taking SSRIs was 31.8% and 17.4% in those not taking SSRIs, although this difference was not statistically significant (P=0.18).
Cognitive impairment and fatigue are not uncommon in patients with GBM [49]. A left-sided tumor can affect verbal fluency and learning, attention, and executive functioning. More progressive, or high-grade tumors, have been associated with rapid decline in cognition, but long-term survivors (over 3 years from diagnosis) may only have moderate cognitive impairment, allowing them to continue activities of daily living [49,51]. Fatigue is commonly reported in patients with all grades of GBM, and may be most prominent after radiation. Along with non-pharmacologic behaviors (activity enhancement, cognitive behavioral therapy, etc), psychostimulants such as methylphenidate are options for those who suffer from extreme fatigue [52].
Depression, cognitive impairment, and fatigue - implications for the pharmacist: Because of the cognitive decline that many patients with GBM experience, frequent and repeated verbal and written communication with patients and their caregivers is of utmost importance [49]. Pharmacists are in the unique position to empower patients and their caregivers with the information they need to understand and adhere to their treatment plan, and devise solutions for how to implement changes. Pharmacists can help manage treatment-related adverse effects, and aid in the identification and avoidance of drug-drug interactions, which have potential to result in further adverse effects and toxicities.
Conclusion
Glioblastoma multiforme is the most common form of malignant brain tumors in the adult population. New therapies such as bevacizumab have emerged in the treatment of newly diagnosed GBM and recurrent disease. As such, pharmacists are integral in the management of these patients. There are numerous challenges regarding dispensing, dosing, administration of medication, and patient understanding of disease. It is imperative that pharmacists obtain medication histories at all encounters since patients are commonly taking concomitant therapy for management of sequelae (eg, anticonvulsant therapy) which can result in clinically relevant drug-drug interactions. As patients with GBM often have neurocognitive complications, pharmacists should ensure comprehension of oral medication counseling.Compounding of TMZ in the community practice setting may be necessary for patients who are unable to take numerous capsules [53,54].
It is important that detailed medication calendars are provided and caregivers are actively involved in the management of these patients. Also, in the community practice setting, laboratory parameters such as ANC and platelet counts should be documented prior to temozolomide dispensing to prevent hematological toxicities. Drug acquisition is often a problem with temozolomide resulting in pharmacists applying for the drug assistant program such as the ACT Program to obtain free product, samples, coupons, and vouchers for patients. In the hospital setting, laboratory values and vitals should be documented when administering bevacizumab to prevent hypertension, impaired wound healing, and proteinuria. Improved knowledge of GBM and its sequelae, and an understanding of patient comprehension issues will greatly assist pharmacists in both institutional and community practice settings in the management of patients with GBM.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on:
https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on:
https://juniperpublishers0.wixsite.com/juniperpublishers
#Juniper publishers#Open access Journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Young Patients and Robotic Radical Prostatectomy: The Only Way is Up

Abstract
Prostate cancer within a younger cohort of men continues to be an increasing problem. With a younger population, greater expectations are present, from erectile function, to continence to oncological outcomes. Significant prostate cancer is increasingly diagnosed in younger men, less than 55 years [1]. In a non-screened population young patients are choosing radical surgery for intermediate and high risk disease [1]. This patient group have high expectations with regards to oncological and functional outcomes. The next question arises, what can we do to improve outcomes?
Keywords: robotic radical prostatectomy, outcomes
Abbreviations: ERSPC: European Randomized Study of Screening for Prostate Cancer; AUA: American Urological Association
Mini Review
Early-onset prostate cancer (<55 years), differs from prostate cancer diagnosed at an older age in several ways [2]. Firstly, among men with high-grade and advanced-stage prostate cancer, those diagnosed at a young age have a higher cause-specific mortality than men diagnosed at an older age [2]. This highlights biological differences between early and late-onset disease [2]. Early-onset prostate cancer also has a strong genetic component. This indicates that young men with prostate cancer could benefit from evaluation of genetic risk [2].When the clinico-pathologic features of men 55 years old or less were examined, it was determined this cohort are more likely to have favourable pathologic characteristics [3]. Yet, screening is not standard.
The results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial showed a statistically significant decrease (29%) in prostate cancer for screened patients, and 23% negative impact on the life-years gained because of quality of life [4]. This was examined further in young patients [4]. As a result Prostate cancer screening can be cost-effective when it is limited to two or three screens between ages 55 to 59 years [4]. American Urological Association (AUA) now no longer recommends testing in men < 55 years of age without significant risk factors [5]. The argument for this is that harms of PSA-testing including over diagnosis and overtreatment outweigh the benefits [5]. Further supporting this is the fact that aggressive prostate cancer with prostate cancer mortality occurs frequently in this cohort. Failure to screen these men may lead to delay in diagnosis and loss of opportunity for curative treatment.
When examined further, there was no significant difference between the rates of insignificant and high-risk prostate cancer between men < 55 years and > 55 years, in either the prostate biopsies or prostatectomy specimens [5].In contrast to this, as part of another cohort, the majority of men < 55 years of age had Gleason 8-10 prostate cancer on biopsy with a PSA > 4.0 [6]. As these men are at the highest risk for PCSM, failure to screen younger men may result in a missed opportunity for treatment with curative intent [6]. Additionally, this study demonstrated younger men treated with radiotherapy had worse prostate cancer mortality compared to those treated with radical prostatectomy [6]. Although younger patients may have low-risk disease, the extended life expectancy of these patients exposes them to long-term effects of treatment-related morbidities. Due to their life expectancy, the long-term risk of disease progression leading to death from prostate cancer is increased [2].
There are different methods of treating these patients with radical therapy. Low dose brachytherapy, is one way of treating this cohort. This was reviewed in men aged less than 55 years with localised prostate cancer [7]. Effective tumour control, with minimal toxicity was demonstrated [7]. The 80 month PSA free survival was 98% [7]. The median 5-year IPSS and IPSS QoL were 7.5 and 1 respectively [7]. The median IIEF at 5 years was 19 [7]. No secondary malignancies have been reported [7].
When outcomes have been reviewed, results have been encouraging [1]. Despite significant disease in these patients, excellent oncological and functional outcomes are achieved for younger patients [1]. Both erectile function and continence outcomes are acceptable, highlighting the suitability of robotic radical prostatectomy in the management of younger men with prostate cancer [1].
The three long-term goals of radical prostatectomy are cancer control, recovery of urinary continence and sexual function. RARP offers excellent short-term trifecta outcomes when performed by an experienced surgeon [8]. Younger patients demonstrated a shorter time to achieving the trifecta and higher overall trifecta rates when compared to older patients at 6 weeks, 3 months and 6 months after RARP [9,10]. However, younger men were more likely to undergo prostatectomy, have lower grade cancer, and equivalent cancer-specific survival at 10 years compared with older men [10]. However, if high risk disease, younger patients had a worse prognosis [10].
Conclusion
In conclusion, both LDR brachytherapy and robotic radical prostatectomy provide improved outcomes for younger patients diagnosed with prostate cancer.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more Open Access Journals in Juniper Publishers please click on:
https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on:
https://juniperpublishers0.wixsite.com/juniperpublishers
#Juniper publishers#Open access Journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Pleural Malignant Solitary Fibrous Tumor Mimicking a Nerve Sheath Tumor in a Patient with Neurofibromatosis Type 1

Abstract
Neurofibromatosis type 1 (NF1), formerly known as von Recklinghausen’s disease is a nervous system tumor disorder of variable expressivity. Malignant solitary fibrous tumors (SFT) are rare spindle cell soft tissue tumors. We report a case of a 35-year-old male who presented clinically with NF1 and a mass that mimicked a nerve sheath tumor radiologically, but pathologically was, in fact, a malignant solitary fibrous tumor. Based on a literature review, the association between NF1 and malignant SFT has never been published.
Keywords: Neurofibromatosis type 1; Malignant solitary fibrous tumor
Abbrevations: SFT: Solitary Fibrous Tumors; NF1: Neurofibromatosis type 1
Mini Review
Neurofibromatosis type 1 (NF1) is a multisystem disease characterized by cutaneous findings, as well as benign and malignant tumors of the nervous system. NF1 occurs in about 1 in 3,000 individuals, and about half of the affected individuals are first cases in their family [1].
A solitary fibrous tumor (SFT) is a rare spindle cell tumor that most commonly arises in the pleura. These tumors comprise only 1-2% of all soft tissue tumors, and of those, between 12 to 22% are malignant [2-3].
No link has ever been made between NF1 and a malignant SFT Conzo et al. [4-5] first described SFT in an NF1 patient, and Yamamoto, et al later reported another case, but neither case dealt with malignant SFT.
Here, we describe a patient with clinical NF1, who presented with a mass that mimicked a nerve sheath tumor, but biopsy and immunohistochemical assays showed that the correct diagnosis was of a malignant solitary fibrous tumor.
Case Presentation
In August 2015, a 35-year-old man presented from an outside hospital with chest pain, recurrent left lower lobe pneumonia and a pleural mass seen on chest radiograph. Physical exam revealed multiple café-au-lait spots on his face, back, chest, abdomen, and legs that ranged from 5mm to 15mm, as well as nodules present on the patient’s right flank, as seen in (Figure 1), and lateral to his left eyebrow. Ophthalmologic exam revealed bilateral Lisch nodules. Due to the array of clinical findings, a biopsy of the right flank nodule was performed and revealed a neurofibroma with plexiform features (Figure 2).
The chest x-ray from the outside hospital was not available at the time of writing, but, as a surrogate, scout images from the initial CT scan performed in our institution may be seen in (Figure 3), displaying the thoracic mass initially appreciated at the outside hospital. A CT scan of the thorax with contrast was performed in our institution, which showed a left posterior intrathoracic soft tissue mass with areas of enhancement, neural foraminal widening and rib splaying (Figures 4a & 5a). An MRI of the same area was performed (Figure 4b & 5b) to better show the mass in question, and a biopsy was recommended as the mass was more suggestive of a nerve sheath tumor, or, less likely, a pleural fibroma.
Ultrasound-guided fine needle aspiration and biopsy of the left pleural mass showed bland spindle cells without defined architectural pattern within a collagenous background without necrosis or mitotic activity. Immunohistochemical staining was positive for CD34, supporting a diagnosis of solitary fibrous tumor (Figure 6).
Subsequently, the pleural mass was resected and histologic evaluation showed a spindle cell lesion with biphasic morphology. The majority of the tumor showed classic solitary fibrous tumor with benign features (Figures 7a & b) with a portion of the tumor showing markedly increased cellularity, cytologic atypia and increased mitotic activity (>4/10 high power fields) diagnostic of malignant transformation (Figures 7c & d). Immunohistochemical stain for CD34 (Figure 7e) was positive, confirming the findings.
Discussion
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen’s disease, is caused by an alteration of the NF1 gene, which is a tumor suppressor gene located on the long arm of chromosome 17 (17q11.2). While genetic testing is not required, it is helpful in confirming the diagnosis. The NF1 gene encodes a protein called neurofibromin, which is a member of the GTPase activating protein family. A loss of function mutation to the NF1 gene results in increased cellular proliferation that manifests as multisystemic tumorous growth. Neurofibromatosis can be diagnosed clinically if at least 2 of the 7 following criteria are fulfilled: 6 or more hyperpigmented macules, axillary or inguinal freckles, two or more neurofibromas or one plexiform neurofibroma, optic nerve glioma, two or more iris hamartomas, body dysplasia, or a first degree relative with NF1 [6].
On the other hand, SFT classically presents as a pleural mass. Klemperer and Rabin first described the tumor in the pleura in 1931 [7]. Most of the tumors are benign neoplasms that are diagnosed in adults with an age range between 50-70 years-old and a male/female ratio of approximately 1 [8-10]. Of the pleural-based SFTs, most arise from visceral pleura (80%) with the remainder arising from parietal pleura [9]. Gross examination of an SFT typically shows a smooth glistening capsule surface [9]. Histologically, most tumors are composed of collagen-forming spindle-cells, arranged in interlacing fascicles with a classic “patternless” pattern [2]. The morphologic findings are typical, though not always demonstrable in small biopsy sections; therefore the characteristic immunohistochemical positivity for CD34 is significantly helpful in separating SFT from other spindle cell neoplasms [11]. Criteria for malignancy include the presence of necrosis, increased mitotic activity, greater cellularity, and cellular polymorphism [10]. Malignant SFT is diagnosed with presence of at least one of these criteria [10]. Approximately 15-35% of SFT are malignant at the time of diagnosis with clinical characteristics of higher recurrences and lower overall survival [2]. Nonetheless, regardless of benign.
Or malignant presentation of SFT, the hallmark of treatment is large en-bloc resection of the primary tumor with close clinical surveillance for recurrences. Mediastinal lymph node dissection and adjuvant treatments such as radiotherapy and chemotherapy are not usually employed for treatment purposes, but rather as palliative options for cases of distant metastasis and locally advanced recurrence that are not amenable to surgery [2].
Historically, NF1 has been associated with various tumors, most notable of which are malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and gastrointestinal stromal tumor (GIST), with an overall occurrence rate that reaches up to 5-13%, 0.5%, and 6% respectively [12-14]. However, there are no systematic reviews that link NF1 with development of SFT. Only two case reports have been published with anecdotal evidences where SFT were found concurrently in NF1 patients. Of these two reports, both patients had pathologic diagnoses of benign SFTs. Specifically, Conzo et all described a patient who had an encapsulated tumor arising from the upper pole of the right kidney that mimicked an adrenal gland or renal tumor, but later proved to be a SFT [4]. Yamamoto et al described a patient who underwent Tc-99m MIBI parathyroid scinitigraphy, which showed uptake highly suggestive of an ectopic parathyroid adenoma, but also later proved to be a SFT [5]. Our case appears to be the first documented case of malignant pleural SFT in an NF1 patient, which also showed signs of mimicry, as seen in the first two cases. Initial radiologic evaluation of the pleural mass suggested nerve sheath tumor, especially in the setting of other clinical findings of neurofibromatosis such as plexiform neurofibroma, as well as presence of a para-orbital mass. Nonetheless, other neoplastic causes such as pleural lipoma, pleural fibrosarcoma, mesothelioma, and metastatic pleural disease could not be ruled out by imaging alone without a definitive biopsy of the mass. Therefore, fine needle aspiration of the mass was performed and showed diagnostic features of SFT and subsequent excision showed areas of malignant transformation.
While NF1 patients have an increased risk of developing various tumors and malignancies, there is no definitive link between NF1 and SFT due to lack of published data [15]. Hence, it is difficult to comment on the exact relationship between these two disease processes. Whether or not NF1 is associated with the development of SFT and the relationship between such, as well as possible malignant transformation of SFT would need to be investigated further.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
To Know More About Open Access Journals Please click on:
https://juniperpublishers.com/index.php
#Juniper publishers#Open access Journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Robotic Radical Partial Nephrectomy: Keep Pushing the Boundaries

Abstract
The purpose of robotic radical partial nephrectomy is to ensure oncological clearance, whilst maintaining renal function. Main renal artery clamping during robotic partial nephrectomy (RPN) may compromise post-operative renal function [1]. Recent technologic advances mean resectional technique and outcomes can be improved. Specifically, robotic platforms, with robotic-assisted instrumentation, have helped overcome previous barriers to widespread adoption of laparoscopic surgery [2]. Image guidance can improve surgeon ease, accuracy, and comfort with complex procedures [2]. How can urologists make use of these advances to improve oncological and renal function outcomes during and after robotic radical partial nephrectomy?
Keywords: Robotic radical Nephrectomy; Resection; Fluorescence
Abbreviations: RPN: Robotic Partial Nephrectomy; NIR: Near Infrared; ICG: Indocyanine Green; NIRF: Near-Infrared Fluorescence; ICG: Indocyanine Green
Mini Review
Modern technologies such as near infrared (NIR) indocyanine green (ICG) fluorescence imaging represent potentially useful tools to facilitate intraoperative assessment of vascularization and tissue perfusion [3]. Recently, the use of a near-infrared fluorescence (NIRF) imaging system using indocyanine green (ICG) dye has been described during robotic partial nephrectomy as an adjunctive means of identifying renal vasculature and tumour clearance. ICG is a water-soluble dye that fluoresces bright green when viewed under near-infrared light (700-1000 nm) [4]. This technology has been applied to robotic partial nephrectomy, to look for the differentiation of renal tumor from normal parenchyma [4]. In this application, it has been hypothesized that normal kidney tissue fluoresces green, while the tumor commonly remains hypofluorescent, thereby aiding tumor excision [4]. Secondly, NIRF imaging with ICG has been employed to facilitate selective arterial clamping during robotic partial nephrectomy, allowing for a regional perfusion deficit in the kidney to be readily identified and therefore targeted at a given tumor [4].
The technique described is one of a standard lateral position with transperitoneal approach [5]. Two 8 mm robotic working ports were triangulated with the robotic camera port. 5 mm and 10 mm assistant ports are usually used. The main renal artery and segmental renal arteries would be isolated [5]. The renal tumour can then be exposed by opening a window in Gerota’s fascia [5]. With the aid of intraoperative ultrasound, the margins of the tumour can be identified, and the planned resection margin scored with diathermy. A bulldog vascular clamp was applied to selected renal arteries, followed immediately by administration of 2 ml (5 mg) of intravenous ICG [5]. NIRF demonstrated no fluorescence, meaning, selective ischaemia of the selected zone [5]. The tumour showed no fluorescence compared to the normal renal parenchyma [5]. Excision can be performed by switching between white light and fluorescence modes [5].
A layer of green fluorescing normal renal parenchyma covering the base of the resected specimen provided intraoperative reassurance of a negative margin. NIRF using intravenously injected ICG has emerged as a useful adjunct to robotic partial nephrectomy. It provides the surgeon with a visual contrast between normal parenchyma and the tumour, and allows demonstration of regional perfusion deficit after segmental clamping of renal vasculature, facilitating a bloodless surgical field [5]. Additionally this tool can also be used to check the perfusion of renal parenchyma was also checked with ICG fluorescence after completion of the renorrhaphy [3]. Intraoperative NIR ICG fluorescence imaging represents a useful tool to support surgical decisions during RAPN. This can give good results [3]. This study used a test of selective clamping of the tumour-feeding arteries after injection of 10 mg of ICG to assess the adequacy of tumor ischemia [3].
The perfusion of renal parenchyma was checked with ICG fluorescence after completion of the renorrhaphy [3]. This demonstrated intraoperative NIR ICG fluorescence imaging represents a useful tool to support surgical decisions during RAPN [3]. Additionally, recent studies have shown the associated decrease in global ischemia to minimize resultant loss of renal function at certain time endpoints [4]. This system can also be used to confirm the definite border between normal and tumor kidney tissues [6].ICG-based systems have been helpful for confirming negative margin status in even the most complex cases [6]. Intraoperative imaging of ICG with NIRF is a safe and effective method to accurately identify the renal vasculature and to differentiate renal tumors from surrounding normal parenchyma [7]. The capacity for multimodal imaging within the surgical console further facilitates this imaging [7].
Due to the growing possibilities especially in robot-assisted partial nephrectomy (RPN) even large, hilar or intrarenal tumours can be removed [8]. Nevertheless a short ischemia time should be a major goal also in complex tumours [9]. This can be achieved using super-selective clamping of the tumour feeding vessels based on fluorescence imaging [9]. Robotic partial nephrectomy with selective clamping of specific tumour feeding arterial branches using indocyanine green can be performed safely even in complex tumour constellations [9]. The minimized ischemic trauma to the remaining parenchyma may lead to superior renal function preservation and significant reduced eGFR decrease.
However, this method should be used with caution for visualization of renal vasculature that is not at the surface of the kidney or complex anatomy [10]. Robotic partial nephrectomy with selective arterial clamping, when used with nearinfrared fluorescence imaging and indocyanine green, can be a safe approach to resecting renal masses if the patient’s vascular anatomy is favourable [10]. A major drawback is that those vessels not at the surface of the kidney cannot be visualized with this technique and may result in excess bleeding during tumor excision [10]. Tumour complexity and perioperative variables are not associated with successful selective clamping, which may be related to complexity of renal vascular anatomy [11]. Further studies are warranted to identify anatomic characteristics associated with successful arterial clamping [12].
Its use has also been examined amongst various histologic subtypes [12]. This technique is safe and effective during robotic partial nephrectomy of various histologic subtypes [12]. Indocyanine Green (ICG) is emerging as a potential adjunct to robotic partial nephrectomy by its ability to help in the realtime identification of renal vasculature and the neoplasmparenchymal margin [13]. ICG dye does not appear to significantly alter distance of renal margin [13]. The mild increase in distance is likely attributable to ensuring equal fluorescent appearance on both sides of the resected tissue [13]. Facilitation of tumor excision may require proper dosing of indocyanine green [14]. Under dosing causes inadequate fluorescence of adjacent tumour parenchyma [14]. Overdosing causes tumours to fluoresce inappropriately. Two doses of indocyanine green have proven highly reliable in achieving differential fluorescence of kidney and renal cell carcinomas [14]. However, important points to note are the fluorescence patterns of renal masses have not been adequately described according to histology, and it remains unknown if fluorescence pattern can reliably predict histology or malignancy [14]. A three-grade classification of renal mass ICG fluorescence pattern is correlated with some histologic findings but unable to reliably predict malignant vs benign lesions [15].
This is especially important to consider when considering staged bilateral robotic partial nephrectomies are a safe and feasible method of managing bilateral renal masses [16]. An approach involving the initial management of the kidney at highest-risk of becoming a solitary kidney can successfully control malignancy while preventing progression to dialysis [16]. In conclusion, near infrared imaging using fluorescence properties of indocyanine green appears useful for identification of arterial branches that can be clamped selectively and in order to reduce the amount of renal parenchyma exposed to warm ischemia. Very short temporary test-clamping of various branches can also be performed to identify the correct supplying vessel before partial nephrectomy. With this technique, the majority of key steps from the hands of the first assistant and utilizes current immunofluorescence technology to allow for safe selective arterial clamping for amenable tumours. Special equipment for NIR imaging (light source, camera with filter and NIR optimized scope) is necessary.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
To Know More About Open Access Journals Please click on:
https://juniperpublishers.com/index.php
#Juniper publishers#Open access Journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Biphosphonate in Locally Advanced Breast Cancer

Abstract
Introduction and Objective: Clinical studies have demonstrated synergistic antitumor effects of chemotherapy (CT) and zoledronic acid (AZ). In the essay Neo - AZURE, to determine whether the addition of AZ to neoadjuvant chemotherapy gives compléte histological responses. We report a prospective evaluation comparing compléte pathological response between different subs - biomolecular groups.
Methods: From 2012 to 2014, 432 patients received neoadjuvant chemotherapy + AZ. The main objective is the complete histologic response. Secondary endpoints were clinical response according to RECIST criteria, estimate the overall survival of patients targeted by the study, assess bone density before and at the end of chemotherapy, the side effects associated with the treatment protocol, and Quality life
Results: histologic complete response with zoledronic acid was 40.13% .the higher in the subgroup Her2 / luminal (RH ± Her2 +) and under Her2 + (HR-Her2 +) and the lowest rate was observed in the triple negative group as classified by Sataloff, overall survival was 45.77 months for subgroups (Her2 / luminal and in Her2 + group) vs 44.11 months for triple negative group.
Conclusion: These data suggest a possible direct antitumor effect of AZ in combination with CT. The studies were recently published in the Proceedings of the American Academy of Sciences (PNAS) shows that bisphosphonates namely zoledronic acid the ability to block the abnormal growth of signals transmitted via the HER receptors, these studies demonstrated that the same can inhibit zoledronic acid tyrosine kinases in case of secondary transfer and thereby potentiate and treat breast cancer became resistant primary treatment.
Keywords: Antitumor activity; Breast cancer; Neoadjuvant chemotherapy; PCR; Zoledronic acid
Abbreviations: CT: Chemotherapy; AZ: zoledronic acid; PNAS: Proceedings of the American Academy of Sciences; OPG: Osteoprotegerin; BMI: Body Mass Index; pCR: Pathological Complete Response
Introduction
Bisphosphonate (zoledronic acid) are pharmacological agents that inhibit the activity of osteoclasts . These are desanaloguesstructurelsdu pyrophosphate. Sur terms of their chemical structure , the bisphosphonate are all characterized by the existence of two phosphate groups bound to a central carbon atom, thereby forming a complex p- c- p. Chaînes latérales two (R1 and R2) are bonded to carbon p -c -p structure by covalent bonds. P -c -p structure and the chain (generally a hydroxyl group) allow the formation of a complex with hydroxyapatite crystals, giving bisphosphonates very high affinity for bone mineral. R2 is the side chain responsible for the inhibitory activity of bisphosphonates on osteoclast (Figure 1).
Zoledronic acid (AZ) (bisphosphonate) acts directly on the tumor cells by blocking the prenylation of G proteins (Ras, Rho) and accumulation in the IPP cells of APPPI. Ras proteins and Rho involved in different signaling pathways that regulate adhesion, migration, invasion and cell proliferation. Inhibition of these cellular functions by AZ has been described in breast cancer.
In vitro it exerts antitumor activity by altering the expression of TRAIL (TNF-related apoptosis-inducing ligand) and that of osteoprotegerin (OPG) in human breast cancer cells (MCF-7, MDA-MB 231), which makes these tumor cells more susceptible to undergo apoptosis. Another function inhibits endothelial cells by blocking adhesion, migration and survival of these cells. This inhibition is due to the fact that the AZ interferes with the prenylation of different GTPases (Ras, Rho) and phosphorylation of various kinases (FAK, INK, ROCK) (Figure 2).
Zoledronic acid inhibits the function of endothelial cells in vitro by blocking adhesion, migration and survival of these cells. This inhibition of endothelial cell functions by zoledronic acid is due to the fact that it interferes with the prenylation of different GTPases (Ras, RhoA) with the phosphorylation of various kinases (FAK, INK, ROCK). In vivo, zoledronic acid blocking tumor angiogenesis (Figure 3) déprivant in the tumor in an essential growth factor for endothelial cells: VEGF. This deprivation is due to the fact that the AZ inhibits infiltration of tumors by macrophages, thereby limiting the degradation of extracellular matrix by proteases secreted macrophages and, thereby, blocking the release of VEGF matrix of Moreover AZ VEGF significantly reduces circulating levels in patients with metastatic breast cancer. in addition, patients with metastatic breast cancer who have circulating VEGF levels decreased in response to a treatment with AZ have a significantly decreased risk of relapse in comparison with patients whose circulating VEGF levels remain unchanged after treatment with bisphosphonate. These clinical results therefore suggest that AZ could exert antitumor activity through their anti-angiogenic.
Several preclinical and clinical studies [1-3] (Figure 4) demonstrate that the AZ stimulates the expansion and activation of γδ T human lymphocytes when administered in the presence of low doses of IL-2. AZ also stimulates the cytotoxic activity of human T cells γδ to a wide variety of tumor cells in vitro. In vivo, AZ enhances the antitumor activity of lymphocytes T human γδ a recent study also showed a long T. la activation potentiation of antitumour properties lymphocytes T lymphocytes vγ9vδ2 by using a pharmacological approach seems AZ be promising and further studies are ongoing to further characterize the clinical implications of this activation. studies recently published in the Proceedings of the American Academy of Sciences (PNAS) shows that bisphosphonates zoledronic acid namely the ability to block the abnormal growth signals transmitted via the Her receptor (Her1, Her2, Her3, Her4) through which passes an abnormal growth signal, these studies showed that zoledronic acid can inhibit the tyrosine kinase even in case of secondary mutation and thus potentiate and treat breast cancer become resistant to primary treatment.
Methods
From 2012 to 2014, 438 patients were included respondents the inclusion criteria. This is a prospective study over a period of 3 years. Of all patients (N = 438), 432 received neoadjuvant chemotherapy and zoledronic acid. Six of them have progressed after chemotherapy treatments and 04 are excluded from the study, at the end of neoadjuvant chemotherapy, a patient refused surgery therefore no assessment of pCR in this patient who is the main objective of our study, a total of 7 patients study outputs, so it remains 431 who have had a mastectomy with lymph node dissection seen the very advanced stage of the tumor (IIIA, IIIB, IIIC) and the appointment of delay Involved in the period radiotherapy (7 - 15 month). No conservative treatment has been practiced. The median follow-up of patients was 42 months.
L’analyse Was done with SPSS 14 software, Patient characteristics are presented with the classical methods of descriptive statistics: frequencies and percentages for categorical variables, medians, extreme values for continuous variables. The association between pCR and the characteristics of the tumor is assessed using a chi-square test 2. Overall survival was estimated by the Kaplan-Meier method, taking into account the time from the date of chemotherapy, it is defined as the time from the first cycle of chemotherapy and death from any Cause. It is compared among groups using the log-rank test with significance limit of 0.05.
Primary objective
Determine the pathological complete response (pCR) in women with locally advanced breast cancer, neoadjuvant chemotherapy placed under Type 04 (doxorubicin + cyclophosphamide) and 04 (Docetaxel ± Trastuzumab) associated with zoledronic acid.
Secondary objectives
To evaluate the clinical response according to RECIST criteria. Estimate the overall survival of patients targeted by the study. Evaluate bone density before and at the end of chemotherapy as well as side effects associated with the therapeutic protocol and the quality of life.
Result
The pathologic complete response rate was 40.13% according to classification Sataloff (1995) .We found that the pathologic complete response rate (Table 1) was the highest in the subgroup (Her2 / luminal) and (Her2 +) and the lowest rate in the triple negative group as classified by Sataloff. These results are consistent with a single study of literature is the study Rouzier [4] where the pathological complete response to neoadjuvant chemotherapy in patients who overexpressed Her2 + is obtained even in the absence of trastuzumab. This leaves suggest that the answer is guided by specific biological factors in Her2 + tumors and zoledronic acid may play a role in inhibiting tyrosine kinases with its potentiation of treatment. Thus the triple negative patients are insensitive to zoledronic acid neoadjuvant.
The Objective response rate was 97% after (C4) with 3% stabilizations and 99, 3% of which 0.7% C8 after stabilization. The clinical complete response was 28% after C4 respectively, and 46.8% after C8. Total 3456 neoadjuvant chemotherapy cycles associated with zoledronic acid was administered to patients in neoadjuvant. treatment postponements occurred in 29 cycles (due to febrile neutropenia + hypocalcemia) no decrease doses was performed. Hematological toxicity of the most common grade I and II was leukopenia. It represents 31, 48% of cycles. Neutropenia was observed in 28.7% of cycles and febrile neutropenia (Table 2,3) was observed in 0.5% of cycles. Hypocalcemia was around de 7,8% more compared to the literature study (Neozotac) [5] of the order of 0.6%.One patient experienced renal failure grade II with dose reduction of 3.5 mg (Table 4) 5172 adjuvant zoledronic acid cures were administered to patients over 03 years.
Of Processing reports related to hypocalcemia occurred in 5 cures; no dose reduction was performed. Zoledronic acid was well tolerated with no renal failure and osteonecrosis of the jaw in adjuvant (Table 5). Among the predictors studied we find as a predictor of pathologic complete response without estrogen receptors, the SBR grade III, and overexpression of Her2 (Table 7-9). These results are consistent with the literature [5]. Tumor size has been described as a predictor, after the analysis there was a nonsignificant trend. Another predictor is clinical, is that of the body mass index (BMI) was correlated with a better pCR with 68.2% in normal patients (p = 0.006), and zero in obese patients. This has been described in the literature [5]. Among the prognostic factors, we found that the pathologic complete response was correlated with better survival without relapse according to the classification of Sataloff.
We found that the pCR is much higher in the age group which is between (35-50 years) with 53.17% (Table 6). Those over 50 years in 2nd position with 27.7%> from the young woman <35 years of pCR is 19%, not statistically significant. The pCR was also in favor of the menopausal group in 51, 4%, and 48, 55% in premenopausal women, statistically insignificant This could be explained by the mechanism that zoledronic acid could interact on a poor background estrogen stabilizing microenvironment with decreased tumor cell proliferation. The average duration of progression-free survival was also significantly in the subgroup (Luminal -Her2, Her2) to the triple negative. It is 45.18 months in the group (luminal - Her2 +) vs 38.95 in the triple negative group, As against the overall survival has improved in the 4 groups with 45.77 months for (luminal - Her2), 45.32 months for Her2 group, 44.37 months for luminal and 44.11 months for the TN group higher compared to the literature or overall survival of 30 months TN (Figures 5-10).
We found that there’s been fewer events compared to the literature [6] 34 vs 53. Local recurrence was 5% vs 15%, against not by isolated bone recurrence when compared with Neoazure the study [6] 17 cases of bone recurrence.
The Patients who developed brain metastases had together bone in secondary locations 2.08% vs 11% in the literature [6].
The 2.08% of patients who have developed brain and bone metastases, had hormonal status be (ER +, PR +, Her2 +) in 1 case and negative hormonal status.
(ER - PR, Her2 -) in 8 cases, his patients did not complete histological response.
The Brain and bone were more relapses in the triple negative group.
The deaths were due to brain metastases.
Was observed in our study a difference in quality of life between (C1) to the admission of the patient, and after the (C8), we found general signs and a deterioration in the psychological condition to C1 in contrast to these general signs C8 and mental condition improves, up to 12, and 24 months.
In our study there’s a osteoprotecteur effect of zoledronic acid to neoadjuvant chemotherapy combined proven on BMD at the C1 and C8, with a gain of 26.39%. Patients who were osteopenic and osteoporotic has the (C1) have switched to a normal BMD (C8) with effect protector since followed after 48 months, no patient has developed fracture it with or without hormone therapy with a better quality of life.
Our results are correlated to literature associated with adjuvant hormone therapy and neoadjuvant, zoledronic acid may provide osteoprotecteur benefit by preventing the decrease in BMD and maintain a good quality of life for survival.
A New meta-analysis published by EBCTCG group in the journal The Lancet [7], on bisphosphonates (zoledronic acid) in the adjuvant setting have improved overall survival with decreased risk of metastatic relapse, especially bone, death linked to cancer and death from any cause.
Conclusion
Neoadjuvant chemotherapy with zoledronic acid in recent years has shown interesting properties in terms of clinical response, with increased pathologic complete response. PCR with zoledronic acid is increased according to the higher tumor grade and negative hormone status, but also molecular subtypes of breast cancer Her2. The importance of identifying predictors of pCR and therefore responder patients and not early responders to chemotherapy and zoledronic acid. With our study zoledronic acid showed a synergistic antitumor effect with doxorubicin situation neoadjuvant zoledronic. L’acide a good model ad hoc to evaluate in vivo efficacy in reducing tumor volume of an associated treatment chemotherapy.
The overexpression of Her2, provides better pathologic complete response after the addition of zoledronic acid, is consistent with the literature where the benefit of chemotherapy with anthracyclines seems mainly and only in Her2 positive patients, versus negative Her2, and zoledronic acid potentiates this. So the identification of specific biological factors in Her2 + tumors is important for complete response to neoadjuvant chemotherapy. The studies that have been published recently in the Proceedings of the American Academy of Sciences (PNAS) [8- 11] demonstrating that bisphosphonates zoledronic acid namely the ability to block the abnormal growth signals transmitted via the HER receptors, these studies have shown that zoledronic acid may inhibit the tyrosine kinase even in case of secondary transfer and thereby potentiate and treat the breast cancer has become resistant to primary treatment.
These Clinical results underline again the existence of a possible bone tumor effect and extra-bone zoledronic acid, independent of its bone antiresorptive activity.
This new understanding and confirmation of this mechanism lays the opportunity and promise of zoledronic acid for the prevention of relapse (metastases) and the treatment of many cancers characterized by these oncogenes (HER) in the occurrence of breast cancer.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more about Juniper Publishers Please click on:
https://juniperpublishers0.wixsite.com/juniperpublishers
#Juniper publishers#Open access Journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes
Text
Quality Of Life and Satisfaction with Care among Breast Cancer Survivors Receiving Different Treatments Strategies in Sri Lanka

Abstract
Introduction and Objective: This study was aimed to evaluate the QOL among BCS who receiving various treatment strategies in Teaching Hospital, Karapitiya (THK).
Method: This cross sectional study was performed with conveniently selected 142 BCS (Mean age (SD) - 55 (10.29) years) at oncology unit of THK during July 2016 using WHOQOL-BREFF questionnaire.
Results: Among the studied BCS, 32.4% were studied up to O/L and 61.3% were married. 76.8% were unemployed and 38.7% had monthly income between Rs 10,000-30,000. 51.4% were living with their husband and children and 93% were Buddhist. 34.5% had undergone to CT. Mean (SD) of physical (PD), psychological (PsD), social (SD) and environmental (ED) domains were 47.79 (11.20), 42.17 (15.19), 48.86 (15.68) and 51.83 (13.61) respectively. Age, marital status, level of education and monthly income and current treatments were significantly affected to their QOL. But CFs had no significant impact on QOL. Generalized body pain, insomnia, vomiting, fatigue, tingling sensation or numbness of legs or hands, problem of testy with food were significantly affected to their QOL. Majority of BCS satisfied with their family support and support provided by HCW. Monthly income, level of education, marital statuses were significant with their satisfaction on family support and monthly income was significant with their satisfaction on the support provided by the health care HCW. Satisfaction with health care and family support had significant impact on QOL of BCS.
Conclusion: Physical and psychological domains were impaired mostly among BCS. SDF, current treatment strategies, side effect of treatments, satisfaction with health care provided and family care significantly affected BCS’s QOL. SDF had impact on BCS’s satisfaction with health care provided and family care. Modifiable factors such as improvement of side effects and immense care for their satisfaction will be enabling to empower the QOL of BCS.
Keywords: Quality of Life; Breast cancer survivors; Socio-demographic factors; Clinical factors; Health Care Workers
Abbreviations: BCS: Breast Cancer Survivors; WHO: World Health Organization; QOL: Quality of Life; HCW: Health Care Workers; THK: Teaching Hospital Karapitiya
Introduction
Now a day, cancers have become a critical health issue worldwide which create numerous problems to the health domains of individuals. Cancer Research UK [1]. Among these cancers, breast cancer is the most common cancer in women Cdc.gov [2]. According to most recent statistics available in Sri Lanka, the incidence of breast cancer among female population is 18.8 per 100,000 populations and 60-64 years old female population is the most vulnerable age group for breast cancer (cancer incidence data Sri Lankan year [3] During the last decade, survival rates for breast cancer have increased as a result of earlier detection and increased use of adjuvant therapy Macmillan.org.uk [4]. Women receive radiation therapy and/or chemotherapy along with systemic hormonal therapy for breast cancer treatment depending on the stage and estrogen receptor status at diagnosis.
Diagnosis of disease and starting treatments, its side effects are directly affected to their QOL. Therefore, it is important for health care professionals to become familiar with QOL of Breast Cancer Survivors (BCS). QOL is a broad, multidimensional concept that lacks a precise definition in the medical literature. According to the World Health Organization (WHO), quality of life is individuals‟ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns” (WHO, 1993). With the diminished QOL among the cancer patients, their level of satisfaction has a greater effect. The way health care workers (HCW) caring them and family members caring to them directly affected to the prognosis and also to spend their remaining life in a satisfactory manner, if they are suffering in critical type of cancer. The aim of this study was to assess QOL and satisfaction with health care and family care among BCS receiving different treatments strategies.
Material and Methods
This study was a cross-sectional study of 142 convenient samples of BCS that were admitted for any type of treatment in the oncology unit of Teaching Hospital Karapitiya between May to August 2016. It is a major oncology unit in the southern province of Sri Lanka. Any patients with breast cancer, age between 18 yrs to 80 yrs, under any type of treatment were eligible to enter the study.
The exclusion criteria were; patients had chronic diseases, hearing and speaking disabilities previous and concurrent malignancies.
Interviewer administered, semi structured questionnaire with both closed ended and open ended questions were used to collect the data on the current topic. The questionnaire includes seven sections as, socio-demographic information, status of cancer, treatments modality evaluation, assessments of side effect of treatments, assessments of QOL and satisfaction with care.
Socio-demographic data included age, marital status, level of education, monthly income, occupational and living status and clinical data including age of diagnosis, duration of disease, stage of carcinoma, type of treatments, its side effects.
WHO-BREEF Sinhala version was used to assess the QOL of BCS by Somasiri KG, Gunawardhana S, et al. [5] QOL measurement instrument is a short version of a QOL assessment instrument WHOQOL-100 (WHO, 1997). WHOQOL-BREF which provides a fast profile of 4 areas (domains). From those 26 questions, 2 questions were related to general health and overall QOL; and the following 24 questions provided a broad and comprehensive assessment of the QOL of a patient each question of the WHOQoLBREFF instrument had five of the answer choice on an ordinal likert scale. All of them produced a profile of four domains; physical, psychological, social relationship and the environment (WHO, 1997). Investigator developed questions were used to assess BCS’s satisfaction with health and family care [6].
The study was approved by the ethical committee of the Faculty of Medicine, University of Ruhuna. Before interview survey, the interviewer explained the purpose of these questions to all eligible individuals and requested their participation.
Data were presented by percentages and tables (Tables 1-4). One way –ANOVA test was used to assess the difference between categorical variables. Statistical significance was set at p < 0.05. All calculation performed using SPSS 20 version.
Results
The study sample compromised 142 BCS who were receiving different treatment strategies in THK. The mean (SD) age of the BCS was 55 (10.29). 32.4% (46) of them were studied up to O/L and 87 (61.3%) of them were married. 103 (76.8%) participant were unemployed (housewives) and 55 (38.7%) participants had a monthly income ranging from Rs 10,000-30,000. 51.4% of them are living with their husband and children 93% were Buddhist. Majority of the study participants diagnosed their condition when they 5th decade of their lives (45, 31.7%) and 37.3% (53) were in the 2nd 6 months of their disease duration. 54.2% had spread to adjacent lymph nodes and 26.1% had distance metastasis of their breast cancer. During study period, 34.5% women were receiving chemotherapy. The most severely affected side effects after different treatment modalities were generalized body pain (40.8%), anorexia (58.5%), fatigue (38.7%), hot flushes and/or sweat (32.4%), problem with food’s tasty or spicy food (52.1%) and hair loss (55.6%).
Mean (SD) of physical domain was 47.79 (11.20), psychological domain was 42.17 (15.19), social domain was 48.86 (15.68) and environmental score 51.83 (13.61) respectively. Environmental and social domains had higher mean score than physical and psychological domains.
Physical domain had no significant differences among the different socio-demographic characteristics (p>0.05). However, psychological domain was significantly differ among different age categories (p=0.03) and environmental domain was significantly differ among different groups of marital status (p=0.002), level of education (p=0.001) and monthly income (p=0.001) and social domain was significantly differ among different groups of monthly income (p=0.04). Other hand, there was no significant difference between QOL domains and status of cancer. Physical domain was significantly different among the ongoing treatment strategies (p=0.002) and there were no significant differences between current treatment options and other QOL domains among the study participants (p>0.05).
Generalized body pain (p=0.018), insomnia (p=0.005), vomiting (p=0.005), fatigue (p=0.008), tingling sensation or numbness of legs or hands (p=0.024) and problem with tasty of food (p=0.006) were significantly different with physical domain. Anorexia (p=0.019), tingling sensation or numbness of legs or hand (p=0.001), problem with testy of food (p=0.036) were significantly different with psychological domain. Problem with tasty with food and spicy food (p=0.013) and anorexia (p=0.01) had statically significant difference with social domain. Environmental domain were significantly different with tingling or numbness sensation of hand and/or foot (p=0.03).
Participants were categorized in to three groups using their scores for satisfactions with family support and health care provided.
Generally, most of the participants were satisfied about health care services and their family support. 68 (47.9%) participant were satisfied with their family support and 52 (36.6%) patients were satisfied with support given by the health care workers.
Monthly income (p=0.008), level of education (p=0.001) and marital status (p=0.02) had significant differences with family support. Monthly income (p=0.001) had significant difference with satisfaction with the support provided by the HCW.
There was significant association between satisfaction with family care and physical, psychological, social and environmental domains. When considering health care services, there was significant association between satisfaction with health services and physical and environmental domain.
Discussion
Quality of life (QOL) assessments have found acceptability among physicians, nurses, and psychosocial staff for the several reasons. Assessments of QOL benefit for Breast Cancer Survivors (BCS) as it provide some insight into their life. They provide insights into life domains affected by breast cancer that are usually unaddressed, including a patient’s mental health, emotional well-being, family and social relations, and abilities to maintain a career, uphold finances, and pursue leisure activities. (Perry, Kowalski and Chang, 2007) According to the current study results, the four QOL domains analyzed on this study, environmental domain showed the highest score and the psychological domain had the lowest score. Barrios et al. [7] reported poor QOL in BCS in panama in all four domains Barrios [7]. Similar findings were observed in a study done in Jordan as well where they showed poor QOL among patients who are receiving adjuvant treatments Alzabaidey [8]. When considering the social and physical domain, physical score showed a lower score than the social domain. In this study most of the participants were physically and psychologically get affected in their quality of life. QOL on social and environmental domains were high in the current study. The reasons behind this finding may be the cultural background, where females are more dependent on the others in the family, females’ worrying more about the family and other issues in the family. Also, different socio-economic backgrounds may affect in these variations.
According to the finding of this study, age was significantly affected to the psychological score domain (p=0.03) in women. Where younger BCS had badly affected psychologically due to their disease. Similar finding were reported by a study done by Anderson et al, with younger BCS (<50years) had worse outcome of depressive symptoms. They found the reasons for this result were as pre mature menopause, menopausal symptoms, and infertility. The latter was common in younger women and had a role in the level of distress after treatments.
In our settings, Income was a major factor affecting the BCS. As Sri Lanka is a developing country, most of the participants had poor income level. Brest cancer is a life threating illness and after diagnosis and starting of treatments it would directly affect to their income. Most of the participants came from rural areas. Therefore, they have to spend a lot of money for their transport facilities. In addition to that, all medications and investigations were not available in our setting. Therefore, they have to spend a lot of money for investigation and medication. As this study revealed, lower income level had directly affected to their QOL.
Among four domains of quality of life, social (p=0.04) and environmental (p=0.001) domains showed statistically significant with monthly income. Earlier studies also revealed that monthly income was statistically significant with QOL domains Safaee, Tatar & Somunoğlu [9], zou et al. [10]. In this current study, marital status (p=0.002) and level of education (p=0.001) showed a statistically significant with the environmental score. This study finding is supported by another study where the marital and educational statuses were significantly affected to their QOL (Awadalla et al. 2007). According to this, occupational (P>0.05) and living statuses (p<0.05) were not affected to their quality of life. Having a partner did not produce significant difference to their QOL. This finding was supported by a study done in Panama; where no significant deference was found between QOL domains and status of living of women Barrios [7].
When comparing the score of quality of life domain and clinical variables (age of onset the illness, stage of cancer, and duration of the illness) no statistically significant difference was found. A study done in Iran has shown the duration of disease and grade of tumor had a significant difference with the QOL score. Those with duration of disease less than four months reported significantly lesser global quality of life scores Safaee et al. [9]. But instrument which was used for the data collection was QLQ-C30. Similar study done by Awadalla et al. [11] showed the duration of illness was statistically significant with the QOL.
Among side effects, more severely affected side effects after treatments were the generalized body pain (40.8%), insomnia (26.1%), anorexia (58.5%), fatigue (38.7%), and pain in lower back (16.9%), tingling or numbness in hands or feet (40.1%), hot flushes and/or sweats (32.4%), problems with food’s tasty or spicy food (52.1%), hair lose (55.6%). Fatigue was the commonest side effect among BCS of this study. According to the previous study, more common problems were the anorexia and fatigue in patients receiving chemotherapy Alzabaidey [7]. Similarly in our study, most of the participant had a currently receiving chemotherapy and complained fatigue and anorexia. Results indicate, anorexia (p=0.02), tingling or numbness of legs or hand (p=0.001) and problem with tasty of food (p=0.03) had a statistically significant difference with the psychological domain and generalized body pain (p=0.02), insomnia (p=0.005), vomiting (p=0.005), fatigue (p=0.008), tingling or numbness of legs or hands (p=0.001), problem with testy of foods(p=0.006) had significant difference with physical score domains. Even although the most of the participants complained about hair loss during their chemotherapy, there was no statistically significant difference with QOL domains. Anorexia (p=0.01) and problem with tasty of food (p=0.013) had a significant difference with social score domain and tingling or numbness of hands or legs (p=0.03) had a significant with environmental score domain.
In this study, ongoing treatments (p=0.002) were statistically significant with their physical score domain. This study finding is similar to a study by, (Kyei et al. 2014), where patients who had radiotherapy had a highest QOL score than patients who had chemotherapy. A study done by Gokgoz et al. [12], they showed that the patients who were currently receiving chemotherapy had a lower global health QOL than from those who receiving hormone therapy. Furthermore, a study done in Jordan, assessing QOL of BCS receiving adjuvant therapy reported that a significant difference found between physical score (p=0.001) and treatments and but there was no significant difference psychological score (p=0.621) and treatments strategies Alzabaidey [5].
During this study, we assessed patients’ level of satisfaction with the family care and the health care provided. Participants divided to three groups using their satisfaction score. Most of the participants were satisfied with their family support (47.9%) and health care provided (36.6%). Otherwise, 52(36.6%) were very dissatisfied with their family support and 46(32.4%) were very dissatisfied with their health care services provided. Regarding family support for the BCS, it was affected to their four domains of quality of life. But health care provided affected only on their physical and social domain. Regarding sociodemographic data, marital status affected on satisfaction with health care provided and marital status, level of education and monthly income affected on the satisfaction with family support. A study done in Sri Lanka by Mudduwa and Punchihewa [13] showed a high proportion of patients receiving satisfactory family support to cope with the illness. Similarly, study done in panama, satisfaction with family support and health care provided had a statistically significant difference with four domain of quality of life Barrios [7]. Also a study done in Taiwan, reported that the, patients had the most dissatisfaction with the health factors HL et al. [14]. It could be due to the difference between family backgrounds, bond with other family members and the caring behavior of family members in Sri Lanka [15-17].
Conclusion
This study, mostly affected QOL domains were the physical and psychological domains among BCS. Age was associated with psychological domain. Income of the participant was associated with the environmental and social domain. Marital status and level of education was affected on environmental domain. None of the CF was associated with QOL domains. Fatigue, generalized body pain, insomnia, vomiting, tingling or numbness sensation of legs or/and hands, problem with tasty of food were affected mostly on QOL mainly on physical domain. Anorexia, tingling or numbness of legs or/and hands and problem with tasty of food were affected on psychological domain. Anorexia and problem with testy of food were affected on social domain and tingling and numbness sensation of legs or/and hands affected environmental domain. Current treatments were affected on physical domain. Most of the participant satisfied with their family support and support provided by the HCW. Satisfaction with family support had significant impact on the four QOL domains and satisfaction with HCW was impacted on both physical and social domains of QOL.
To Know More About Cancer Therapy & Oncology International Journal Please click on:
https://juniperpublishers.com/ctoij/index.php
For more about Juniper Publishers Please click on:
https://juniperpublishers0.wixsite.com/juniperpublishers
#Juniper publishers#Open access Journals#Peer review journal#Juniper publisher reviews#Juniper publisher journals
0 notes