#along with differential expression of hox genes
Text
Ya know sometimes it’s funny to me
I’m like why do I always write the boys as humans and specifically recently mermaids, why not write them as robots?
And then I look at my backlog of human bio and fish anatomy stuff and I’m like-
Yeah okay I could world-build for hours using this
And on the other hand I am completely in the dark about most programming and robotics things
#this is specific to Luca au#like it’s so funny I have soooooo much random worldbuilding for Luca au#but only like 10% will make it into the fic#like y’all don’t need to know the boys dna is all sorts of fucked up#or how the disguise magic works through a mechanism of heterochromatin formation on specific points#or that the way the transformation works is by a change in shh and wnt signaling along the spinal chord#along with differential expression of hox genes#which btw is total sci-fi#could never happen#or the exact way their digestive system works#or how the cartilage in the jaw breaks down into two main bones allowing for larger bite#or how their reproductive system works#or their entire family lineage from 500 years back#or how their writing system works#well actually that last one might come back#Luca au#I’ve put toooooo much thought into this#lol
33 notes
·
View notes
Text
Properties, Removing Approaches, and also Shipping Programs pertaining to Dihydrotestosterone as a Normal Method to obtain Beneficial Wellness Results
We all present a clear case of any 48-year-old lady together with Web page complex by simply congestive heart failure together with still left ventricular ejection portion involving 25%, who was initially addressed with Linsitinib order lengthy span of high-dose steroid drugs without the specialized medical as well as echocardiographic enhancement. The girl was described each of our clinic 1 yr later and was begun together with cyclophosphamide 2 mg/kg per day along with prednisone 60 mg/d followed by slow taper. Eventually, the sufferer acquired outstanding advancement. Affected person was then transitioned to azathioprine pertaining to 1.A few years together with continual illness remission. It may be hard to figure out selleck screening library myocardial condition activity position compared to damaged tissues throughout Web page along with prolonged duration of coronary heart failing symptoms. Here is the 1st case statement displaying that will Cascading stylesheet heart condition may possibly stay energetic regardless of 1 12 months involving corticosteroid therapy, and also important improvement or remission may still be accomplished by simply applying far more intense cytotoxic immunosuppressive remedy.Porcine reproductive along with the respiratory system affliction computer virus (PRRSV) is definitely an appearing dog virus that has triggered high economic cutbacks for your swine sector globally. Previous within vitro plus vivo reports established that PRRSV an infection induces significant manufacture of interleukin 15 (IL-10), a new pleiotropic cytokine with immuno-modulatory characteristics involved with sponsor security. However, the underlying regulating mechanisms in the course of PRRSV remain mostly not known. With the current economic research, we analyzed the term kinetics regarding IL-10 throughout PRRSV-infected principal porcine alveolar macrophages (PAMs) and indicated that PRRSV disease activated IL-10 mRNA along with health proteins appearance within a time- as well as dose-dependent fashion. Hang-up of numerous substances in the Toll-like receptor (TLR) or RIG-I-like receptor (RLR) signaling paths indicated that your TLR adaptor Dupracetam myeloid differentiation main result gene Eighty eight (MyD88) has an important role inside IL-10 induction during PRRSV infection. In addition, therapy using particular inhibitors or perhaps siRNA knockdown assays demonstrated that NF-kappa N and also p38 MAPK (mitogen-activated health proteins kinase) are required with regard to PRRSV-induced IL-10. Consumed with each other, PRRSV an infection drastically induced IL-10 term this also induction depends on NF-kappa W account activation and also p38 MAPK inside PAMs. (C) This year Elsevier Limited. All protection under the law set aside.HOX healthy proteins are usually broadly associated with hematopoietic development. These kinds of transcription aspects blend any protected DNA-binding homeobox which has a divergent N-terminus in which mediates connection with varying cofactors. The causing combinatorial variety is understood to become to blame for mammalian HOX nature. Diverse this suggested mechanism for normal HOX purpose, ideas show that, while hematopoietic immortalization as well as leukemogenesis, personal HOX qualities tend to be ruled nearly solely through the homeodomain. Change studies in between HOXA1 and also HOXA9, A couple of individuals nonrelated paralog organizations, revealed that gene expression designs regarding HOX changed tissues within vitro are generally based on the nature in the homeodomain. Similar effects were noticed in vivo during HOX-mediated leukemogenesis. An swap of the homeodomains had been ample to change your slow, low-penetrance phenotype involving HOXA1-induced leukemia on the intense fast-acting disease elicited simply by HOXA9 and the other way round.
1 note
·
View note
Text
« In the fruit fly Drosophila there is a gene called eyeless. Geneticists have the perverse habit of naming genes by what goes wrong when they mutate. The eyeless gene normally negates its name by making eyes. When it mutates and fails to have its normal effect on development, the fly has no eyes, hence the name. It is a ludicrously confusing convention. To avoid it, I shall not refer to the eyeless gene, but will use the comprehensible abbreviation ey. The ey gene normally makes eyes, and we know this because when it goes wrong the flies are eyeless. Now the story starts to get interesting. There is a very similar gene in mammals, called Pax6, also known as small eye in mice and aniridia (no iris) in humans (again named for the negative effect of its mutant form).
The DNA sequence of the human aniridia gene is more similar to the fruit fly's ey gene than it is to other human genes. They must be inherited from [our] shared ancestor. Again, I shall call it ey. Walter Gehring and his colleagues in Switzerland did an utterly fascinating experiment. They introduced the mouse equivalent of the ey gene into fruit fly embryos, with astounding results. When introduced into the part of a fruit fly embryo that was destined to make a leg, it caused the eventual adult fly to grow an extra 'ectopic' eye on its leg. It was a fly eye, by the way: a compound eye, not a mouse eye. I don't think there is any evidence that the fly could see through it, but it had the unmistakable properties of a respectable compound eye. The instruction given by the ey gene seems to be 'grow an eye here, of the kind that you would normally grow'. The fact that the gene is not only similar in mice and flies, but induces the development of eyes in both, is very strong evidence that it was present in [our common ancestor]; and moderately strong evidence that [our common ancestor] could see, even if only the presence versus the absence of light. [...]
Drosophila ('dew lover') has long been the geneticists' favourite animal. Embryology should never be confused with genetics, but recently Drosophila has assumed a starring role in embryology as well as genetics, and this is a tale of embryology. Embryonic development is controlled by genes, but there are two very different ways in which this might theoretically happen. [...] Textbooks of biology are wrong when they describe DNA as a blueprint. Embryos do nothing remotely like following a blueprint. DNA is not a description, in any language, of what the finished body should look like. Maybe on some other planet living things develop by blueprint embryology, but I find it hard to imagine how it would work. It would have to be a very different kind of life. On this planet, embryos follow recipes. Or, to change to another equally un-blueprint-like analogy, which is in some ways more apt than the recipe: embryos construct themselves by following a sequence of origami folding instructions.
The origami analogy fits early embryology better than late. The main organisation of the body is initially laid down by a series of foldings and invaginations of layers of cells. Once the main body plan is safely in place, later stages in development consist largely of growth, as if the embryo were being inflated, in all its parts, like a balloon. It is a very special kind of balloon, however, because different parts of the body inflate at different rates, the rates being carefully controlled. This is the important phenomenon known as allometry.
The Fruit Fly's Tale is concerned mostly with the earlier, origami phase of development, not the later, inflationary one. Cells are not laid like bricks to a blueprint, but it is the behaviour of cells that determines embryonic development. Cells attract, or repel, other cells. They change shape in various ways. They secrete chemicals, which may diffuse outwards and influence other cells, even some distance away. Sometimes they die selectively, carving out shapes by subtraction, as if a sculptor were at work. Like termites co-operating to build a mound, cells 'know' what to do by reference to the neighbouring cells with whom they find themselves in contact, and in response to chemicals in gradients of concentration. All cells in the embryo contain the same genes, so it can't be their genes that distinguish one cell's behaviour from another's. What does distinguish a cell is which of the genes are turned on, which usually is reflected in the gene products — proteins — that it contains.
In the very early embryo, a cell needs to 'know' where it lies along two main dimensions: fore and aft (anterior/posterior) and up-down (dorsal/ventral). What does 'know' mean? It initially means that a cell's behaviour is determined by its position along chemical gradients in each of the two axes [anterior/posterior and dorsal/ventral]. Such gradients necessarily start in the egg itself, and are therefore under the control of the mother's genes, not the egg's own nuclear genes. [...]
These labelling concentrations persist in the substance of the cells that are produced as the egg subsequently divides. The first few divisions occur without any addition of new material, and the divisions are incomplete: lots of separate nuclei are made, but they are not completely separated by cellular partitions. This multinucleate 'cell' is called a syncitium. Later, partitions form, and the embryo becomes properly cellular. Through all this, as I say, the original chemical gradients persist. It follows that cell nuclei in different parts of the embryo will be bathed in different concentrations of key substances, corresponding to the original two-dimensional gradients, and this will cause different genes to be turned on in different cells (we are now, of course, talking about the embryo's own genes, no longer the mother's). This is how differentiation of cells begins, and projections of the principle lead to further differentiation at later stages of development. The original gradients set up by maternal genes give way to new and more complex gradients set up by the embryo's own genes. Consequent forkings in the lineages of embryonic cells recursively generate further differentiations.
In arthropods there is a larger-scale partitioning of the body, not into cells but into segments. The segments are arrayed in line, from front of head to tip of abdomen. Insects have six head segments, of which the antennae are on segment 2, followed on other segments by the mandibles and then other mouthparts. [...] The three thoracic segments (Ti, T2 andT3) [each] bear a pair of legs. T2 and T3 normally bear wings, but in Drosophila and other flies only T2 has wings. [...] Cells 'know' (in the sense already excused) which segment they are in, and they behave accordingly. Each cell is told which segment it is in through the mediation of special control genes called Hox genes, which turn themselves on inside the cell. The Fruit Fly's Tale is mostly a tale of Hox genes.
It would make things neat and easy to explain if I could now tell you that there is one Hox gene for each segment, with all the cells of a given segment having only its own numbered Hox gene turned on. It would be even tidier if the Hox genes were arrayed along the length of a chromosome, in the same order as the segments they influence. Well, it isn't quite as tidy as that, but it very nearly is. The Hox genes are indeed arranged in the right order along one chromosome, and that is wonderful — gratuitously so, given what we know of how genes work. But there aren't enough Hox genes for the segments — only eight. And there's a more messy complication that I must get out of the way. The segments of the adult don't exactly correspond to the so-called parasegments of the larva. Don't ask me why (perhaps the Designer was having an off day), but each adult segment is made up of the back half of one larval parasegment plus the front half of the next. Unless otherwise stated, I'll use the word segment to mean larval (para) segment. As for the question of how eight Hox genes in a row take charge of some 17 segments in a row, it is partly done by resorting to the chemical gradient trick again. Each Hox gene is mainly expressed in one segment, but it is also expressed, in decreasing concentration as you go backwards, in more posterior segments. A cell knows which segment it is in by comparing the chemical outputs of more than one upstream Hox gene. It is a bit more complicated than that, but there is no need to go into such detail here. [...]
A Hox gene, then, is a gene whose mission in life is to know whereabouts in the body it is, and so inform other genes in the same cell. We are now armed to understand homeotic mutations. When things go wrong with a Hox gene, the cells in a segment are misinformed about which segment they are in, and they make the segment they 'think' they are in. So, for instance, we see a leg growing in the segment that would normally grow an antenna. This makes perfect sense. The cells in any segment are perfectly capable of assembling the anatomy of any other segment. Why should they not? The instructions for making any segment lurk in the cells of every segment. It is the Hox genes, under normal conditions, that call forth the 'correct' instructions for making the anatomy appropriate to each segment. As William Bateson rightly suspected, homeotic abnormality opens a revealing window on how the system normally works.
This brings us to the most wonderful part of the Fruit Fly's Tale. After they had been discovered in Drosophila, Hox genes started turning up all over the place: not only in other insects such as beetles, but in almost all other animals that have been looked at, including ourselves. And — this really is almost too good to be true — they very often turn out to be doing the same kind of thing, even down to informing cells which segment they are in and (better still) being arrayed in the same order along chromosomes. [...] Hox genes have now been found in every animal that has been looked at except ctenophores and sponges, including sea urchins, Limulus, shrimps, molluscs, annelid worms, acorn worms, sea squirts, nematode worms and flatworms. »
— The Ancestor’s Tale: A Pilgrimage to the Dawn of Life, Richard Dawkins and Yan Wong
2 notes
·
View notes
Text
Biomed Grid | Vitamin A and its Derivatives- Retinoic Acid and Retinoid Pharmacology
Introduction
In vivo, the fat soluble Vitamin A (retinol) can be reversibly metabolised to the aldehyde (retinal) which can in turn, be further oxidised in a non-reversible manner to retinoic acid (RA). Enzymes that oxidize retinol to retinaldehyde belong to two classes: the cytosolic alcohol dehydrogenases (ADHs) belonging to the mediumchain dehydrogenases/ reductase family; and microsomal shortchain dehydrogenases/reductases (retinol dehydrogenases, RDHs [1]. The next step in RA synthesis is the oxidation of retinaldehyde to RA, which is carried out by three retinaldehyde dehydrogenases (RALDHs): RALDH1, RALDH4 and RALDH3 [1,2]. The orange pigment of carrots (beta-carotene) can be represented as two connected retinyl groups, which are used in the body to contribute to vitamin A levels [3]. The physiological and biological actions of this class of substances centre on vision, embryonic development and production, cellular growth and differentiation, skin health, and maintenance of immune function.
Initial studies had focused on vitamin A deficiency and its major consequences: night blindness and Xerophtalmia. Fridericia and Holm [4] investigated the influence of dietary A in the rhodopsin of the retina. Clearly, the rats lacking the fat-soluble vitamin A had a defect in the function of visual purple. Yudkin [5] achieved one of the earliest identifications of vitamin A as a component of the retina. Subsequently, Wald [6] determined the amount of vitamin A present in pig retinas. Wald G [7,8] was well established the visual cycle: light decomposed rhodopsin to retinal and opsin. Retinal could either recombine with opsin to reform rhodopsin or it converted to free retinol. Retinol could reform rhodopsin, but only in the presence of the RPE(Kuhne). The further structure and metabolism of retinoids implicated that retinaldehyde was the visual pigment.
Biochemistry of Vitamin A
There is now a well-developed medicinal chemistry of RA (Figure 1 & 3) [9]. The group of pharmacologically used retinoids include vitamin A (all-trans retinol), tretinoin (all-trans retinoic acid), isotretinoin (13-cis retinoic acid) and alitretinoin (9-cis retinoic acid). The monoaromatic retinoids include acitretin and etretinate. The third generation polyaromatic retinoids include bexarotene and tazarotene. In view of this broad spectrum of pharmacological activity, these substances provide useful to treat multifactorial dermatological disorders and other hematological disorders such as acute promyelocytic leukemias (APL) (Figure 1 & Figure 2) [8,10-12].
Figure 1
Figure 1 & 2: Discovery of the molecular basis of vitamin A derivative retinoic acid action (Figure data adapted from Zhu G,January1991,2013,at the top);and vitamin A in vision cycle (Figure data adapted from Wald G,1935;Wolf G,2001,at the bottom).
Function of Vitamin A and its Physiological Role Vision
Vitamin A is needed by the eye retina,11-cis-retinal (a derivative of vitamin A) is bound to the protein “opsin” to form rhodopsin (visual purple) in rods cells [8], the molecule necessary for both low light (scotopic vision). As light enters the eye, the 11-cis-retinal is isomerized to all-trans retinal in photoreceptor cells of the retina. This isomerization induces a nervous signal (a type of G regulatory protein) along the optic nerve to the visual center of the brain. After separating from opsin, the all-transretinal is recycled and converted back to the 11-cis-retinal form via a series of enzymatic reactions. The all-trans- retinal dissociates from opsin in a series of steps called photo-bleaching. The final stage is conversion of 11-cis-retinal rebind to opsin to reform rhodopsin in the retina [6-8] (Figure 2) vision cycle. Kuhne showed that rhodopsin in the retina is only regenerated when the retina is attached to retinal pimented epithelium (RPE) [8]. As the retinal component of rhodopsin is derived from vitamin A, a deficiency of vitamin A inhibit the reformation of rhodopsin and lead to night blindness. Within this cycle, all-trans retinal is reduced to all-trans retinol in photoreceptors via RDH8 and possible RDH12 in rods and transported to RPE. In the RPE, all-trans retinol is converted to 11- cis retinol, then 11-cis retinol is oxidized to 11-cis-retinal via RDH5 with possible RDH11 and RDH11 [1]. This represent each RDH for the roles in the visual cycle (Figure 2) .
Figure 3: A well developed medical chemistry of retinoic acid (RA). The all-trans and 13-cis forms of retinoic acid,two isomers of RA,are equally effective inhibiting proliferation. Retinyl acetate,and retinal(Vitamin A) are less potent inhibitor. Am80(Tamibarotene) is more potent inhibitor. The chemical structures of more potent analogues involved from labile flexible polyene structures to aromatic stable moieties are shown[9,10].
Embryonic Development
More recent, vitamin A and its metabolites play a key importance in embryo morphogenesis, development and differentiation in normal tissues. Retinoic acid (RA) is lipophilic molecule that act as ligand for nuclear RA receptors (RARs), converting them from transcriptional repressor to activators [2,11,12] in RA signaling pathway. It has been demonstrated that retinoic acid was identified as a morphogen(teratogen) responsible for the determination of the orientation of the limb outgrowth in chicken [13,14] and its retinoic acid receptors (RARs) appear at early stage of human embryonic development in certain types of tissues [15]. Vitamin A play a role in the differentiation of this cerebral nerve system in Xenopus laevi. The other molecules that interact with RA are FGF-8, Cdx and Hox genes, all participating in the development of various structures within fetus. For instance, this molecule plays an important role in hindbrain development. Both too little or too much vitamin A results in the embryo:defect in the central nervous system, various abnormalities in head and neck, the heart, the limb, and the urogenital system [15]. With an accumulation of these malformations, an individual can be diagnosed with DeGeorge syndrome [2].
Dermatology
Vitamin A, in the retinoic acid form, plays an important role in maintaining normal skin health through differentiating keratinocytes (immature skin cells) into immature epidermal cells. In earlier studies, Frazier and Hu (1931) [16] made the observation that both hypovitaminosis A and hypervitaminosis A provokes epithelial alterations together with decreased keratinization and hair loss. At present,13-cis retinoic acid (Isotretinoin) in clinical used to acne treatment. The mechanism was shown to reducing secretion of the sebaceous glands, triggering NGAL (neutrophil gelatinase-associated lipocalin) and other gene expression and selectively inducing apoptosis [17]. But precise action of retinoid therapeutic agents in dermatological diseases are being researched.
Hematopoiesis
vitamin A is important for the regulation of hematopoietic stem cell dormancy [18]. Mice maintained on a vitamin A-free diet loss HSCs (hematopoietic stem cells), showing a disrupted re-entry into dormancy after exposure to inflammatory stress stimuli. This condition highlight the impact of dietary vitamin A on the regulation of cell-cycle mediated stem cell plasticity [19]. In vitro, all-trans retinoic acid (ATRA) stimulates at least two-fold the clonal growth of normal human CFU-GM and early erythroid precursor BFU-E [20]. Cis-RA stimulates clonal growth of some myeloid leukemia cells. In suspension culture, there was an increase in cell number at day 5 in the presence of RA in half of 31 samples, which suggest that RA may play a role in the proliferation and survival of certain leukemia clones in vitro [21,22].
In contrast to the enhancement of normal hematopoietic proliferation, RA (10-6 - 10-9 mol/l) is capable of inducing differentiation of the F9 mouse teratocarcinoma, HL-60 cells [23,24] and some blasts from patients with promyelocytic leukemia [23]. Maximum HL-60 differentiation (90% of cells) occurs after a 6 day exposure to 10-6mol/l retinoic acid. Further in vitro studies found that retinoic acid induced differentiation of leukemic blast cells in only 2 of 21 patients with AML, both of these patients had promyelocytic variant [24]. These data suggest that retinoids may induce maturation of promyelocytes. Retinoic acid also inhibits the proliferation of other dermatological malignant cells (Myger,1975; Peck,1975).
Maintenance of Immune Homeostasis
There is a link between retinoid and immune homeostasis. RA is crucial for maintaining homeostasis at the intestinal barrier and equilibrating immunity and tolerance. de Mendonca Oliveira LM and colleagues [25] have in detail illustrated the impact of retinoic acid on immune cells and inflammatory diseases. After the absorption and metabolism of vitamin A and its precursor(β-carotene) into RA by alcohol dehydrogenase(ADH) and retinal dehydrogenase(RALDH) in CD103+ DC cells in gut, RA plays an important roles in mucosal immune response by promoting differentiation of Foxp3+ inducible regulatory T (Treg) cell and immunoglobulin(Ig) A production. In this process, RA promote dendritic cells to express CD103 and to produce RA. Vitamin A and zinc deficiency (VAD) lead to a decrease of serum IgA. Oral administration of RA in VAD mice can efficiently be reestablishing IgA production. These effects are mediated by an increase of the early B cell factor 1(EBF1) and paired box protein-5(pan-5) transcription factors, which are critical for B cell development. RA accelerates the maturation of human B cells and their differentiation into antibody-secreting plasma cells.
In addition, RA induces the homing of innate immune cells, such as innate lymphoid cells (ILCs) besides regulatory and effector T and B cells, to the gut. Among three ILCs, ILC3 depend on the transcription factor retinoic acid receptor-related orphan nuclear receptor gamma (RORrt) and secrete IL-17 and IL-22. During infections, RA can induce the production of proinflammatory cytokines by dendritic cells (DCs), promoting the generation of effector T cells and restoring the balance of Th17/Treg cells in the GALT (gut-associated lymphoid tissue), and the protection of the mucosa. Moreover, vitamin A is capable of inducing the IL-6- driven induction of proinflammatory T(H) 17 cells, promoting antiinflammatory T reg cells differentiation, regulating the balance between pro- and anti-inflammatory immunity [26,27].
Retinoid Acids in MDS Treatment
The geometric isomer of the naturally occurring retinoic acid is 13-cis retinoic acid (13-CRA). Based on in vitro and in vivo antineoplastic activity, this agent has entered clinical trials for a variety of neoplasms including MDS. Retinoic acid is one of the biological inducers of differentiation that has been preliminarily tested in patients with preleukemia. Myelodysplastic syndrome (MDS) are a group of hematopoietic disorders characterized by ui- or multilineage maturation defects of the bone marrow [28]. Differentiation induction therapy is used in MDS to improve this maturation defects and induce a multilineage clinical response in a subgroup of MDS patients.13-CRA may have moderate effect on 20-30% of patients with MDS [29]. A various of combination therapy with 13-cis RA and growth factors G-CSF or erythropoietin (EPO) improve impaired cytokine secretion (IL-1beta, IL-6, IL-8) from monocytes [30]. In a prospective multicenter study, EPO-beta- ATRA [31] or EPO-13-cis RA [32] combination appears to erythroid response reaching about 36%-60% of therapeutic efficacy in anemia of low/intermediate risk MDS(LDMDS) (marrow blasts < 10% or excluding RARBt). More data analysis, erythroid response maintained an independent positive impact on survival, particularly in non-RARE patients in the first 3 years from diagnosis (90% survival in EPO responders compared to 50% of non-responders) [33]. Zhu [34] successfully conducted a CR patient with refractory anemia with multilineage megaloblastic dysplasia following traditional medicine and erythropoiesis-stimulating agent vitamin B12 and folate growth factor. His peripheral parameters presented pancytopenia (hemoglobin 59g/l, red blood cell count 1.9x1012/l, leukocyte count 2.6x109/l, platelet value 11.8x109/l).
He remained well over 10 years. While another MDS had its unequivocal evidence of disease progression in response to phytohemagglutinin (PHA), inducing the generation of interleukin-2, accelerating the number recovery of CFU-S and initiating DNA synthesis of cells. She had 2.5% blast plus promyelocytes in ~70% cellular marrow before beginning PHA, and 20.7% blast plus promyelocytes in a 90% cellular marrow after ten days (total dosage 250mg) of PHA. Venditt etal [35] conduct that 23 patients with high-risk myelodysplastic syndrome (HRMDS) were treated with a 10 days course of oral ATRA (45mg/m2) and subcutaneous low-dose cytosine arabinoside (LDARAc) given at the dose of 20mg twice a day. In all cases (RAEB9, RAEBt9 and CMML4) [36] bone marrow blasts infiltration was greater than 10% (12-30%). Overall, 5(23%) of 22 patients achieved complete responder and 2(9%) as partial responders. The overall median survival was 8 months (range 1-27months), whereas the median survival of responders was 16months(8-27months), the median duration of response was 11months(2-21months). It seems that the combination of ATRA and LDARA-c may be effective in approximately 30% of HRMDS patients [35].
Valproic acid (VPA) has been used as an anticovulsant for decades. VPA is a potent inhibitor of histone deacetylases (HDAc). It can modify the structure of chromatin allowing recruitment of transcription factors to restore epigenetically suppressed genes. VPA has been shown to posses antiproliferative activity and to overcome the differentiation block in leukemia blast cells [37]. Some clinical trials with VPA monotherapy or in combination with ATRA have been reported in MDS. In a piloty study of Kuendgen and colleagues [38- 40] patients with MDS or AML secondary to MDS were treated with VPA monotherapy or with ATRA later resulting in a 44% of response rate. In the follow-up study of 43 patients, an even higher response rate of 52% was observed in those low-risk MDS patients, while for the patients with excess blasts (RAEB) and CMML response rates were 6% and 0% respectively, which implicate the difference of MDS subtypes. In another trials, Siitonen etal [41] reported that according to IWG criteria,3 patients(16%) of 19 MDS responded to treatment following VPA,13-cis RA and 1,25(OH)2D3 combination. All the responses were hematological improvement. One patient responded to the treatment with an increase in platelet value from 67x109/l to 105x109/l. His peripheral blood and bone marrow blast cells decreased from 4% to 0% and from 19% to 7%, respectively. Furthermore, the disease remained stable in 11 patients but progressed in 5 during treatment. This is encouraging results.
Table 1: Results of Retinoic acid therapy in MDS.
A series of these studies are summaried in (Table 1). While some patients experienced improvement in peripheral blood counts, complete responses were reported in only a small proportion of these studies [42-43]. The sole exception was a patient who presented with 29% marrow blasts and 90% abnormal metaphases with 13-cis RA. He obtained a complete clinical and cytogenetic remission therapy [44-49]. This clinical response to 13-cis RA drug was due to in vivo growth inhibition of malignant monocytoid clone [50]. Continued follow-up of this study in this field will be of interest [51-54] (Table 1).
Retinoic Acids in Skin Disease
Vitamin A is necessary for normal epithelial cell differentiation and maturation [55-57]. Retinoids influence on skin keratocyte proliferation, epidermal differentiation and kerintinisation. Those retinoids including natural and chemically synthesized vitamin A derivatives are common used as systemic and topical treatment of various skin disorders. At present there have well developed three generations: the naturally occurring retinoids (all-trans retinol, Aretinoin, Isotretinoin, Alitretinoin) the monoaromatic retinoid and the polyaromatic retinoid derivatives [58].
Table 2: Results of 13-cis RA in severe acne treatment.
Isotretinoin is an orally active retinoic acid derivative for the treatment of acne (papulo- pustular,nodulo-cystic, conglobata) [59],since it shows an excellent efficacy against severe refractory nodulocystic acne. Peck’s [60] original observation in 1978-79 of the effectives of 13-cis RA in cystic acne has been well supported. In double-blind studies using small doses of 13-cis RA regimen, Farrell [61] in 15 patients, Jones [62] in 76 patients, Plewig [63] in 79 patients and Rapini [64] 150 patients reporting have confirmed this results. A summary study of limited review on 365 affected persons are presented in (Table 2). The drug action involves an inhibition of sebum excretion rate(SER) in sebaceous glands and production rate of free fatty acids[60,61,65-68] through trigerring NGAL (neutrophil gelatinase-associated lipocalin) expression [17] normalise follicular keratinisation [69] and the decrease in colonisation of propionibacterium acnes and associated inflammation in skin surface microflora [70].This response, mediated by toll-like-receptor 2(TLR2), is increased in acne patients due to high expression of TLR2 [71] (Table 2).
Figure 4: An advanced squamous cell carcinoma of skin before(left) and after(right) isotretinoin [57].
Encouraging results have also been used 13-cis RA in small numbers of patients with rosacea, Gram-negative folliculitis, Darier’s disease, ichthyosis and pityriasis rubra pilaris [72,73]. In the treatment of rosacea, isotretinoins led to a significant reduction of erythemia, papules and pustules in several studies [72,73]. During treatment of rosacea,13-cis RA act as a potent anti-inflammatory and sebum-suppressive agent. Long-lasting remission can be reported for first patient over 12 months [72]. The use of low dose isotretinoin (0.15-0.3mg/kg bw daily) showed high efficacy and was well tolerated. Isotretinoin is only partially effective in psoriasis, in contrast etretinate which is effective in psoriasis but ineffective in severe acne. Promising, some trials have reported with isotretinoin in patients with squamous and basal cell carcinomas [74,75] cutaneous T-cell lymphoma [56] recurrent malignant glioma [76] malignant eccrine poroma [77] and keratoacanthomas [78,79] and xeroderma pigmentosum with squamous cell carcinoma [79]. In literature, there were at least 10 CR patients with squamous cell carcinoma (SCC). Skroza etal [74] reported a CR patient with well-differentiated SCC following the daily dosage of 0.5mg/kg/day for 5 months. Dring 1-year follow up, he remained all in normal range. Using combination chemotherapy and isotretinoin for 4 months, Zaman [80] reported a complete clinical remission of tumors in a case of 15 year old female of xeroderma pigmentosum with SCC. Another collection of four SCC of skin obtained CR through isotretinoin at daily dose of 1mg/ kg/day twice a day for 4 months (Figure 4) [57]. The mechanism may involve the modification of epidermal growth factor receptor (EGFR) and certain protein kinase. At present, It has clearly known the results that amplified (50-fold EGF receptor in SCC relative to normal skin keratinocytes) or mutant EGFR is oncogenic in origin of some SCC [81]. This oncogenic receptor EGFRvIII has also been found in malignant glioma and invasive breast carcinoma [82-89]. Zhu [90] conduct a short CR using chemotherapy and topical 5% Fu of retinoic acid ointment in a 75-year old patient with SCC. She had a 8x5cm rodent ulcer in her left ear and facial area. A shrinkage of irregular and harden marginal valgus converted to flat and superficial red and scar noted after one month treatment. These findings suggest that retinoids may be effective and well-tolerated therapy for advanced epidermoid SCCs in some studies [91-95] (Figure 4) .
ATRA in patients with gastric cancer (GC)
Recently [96], two cohorts of group presented ATRA trials on patients with GC. Jin etal presented a better benefits of gastric dysplasia with omeprazole and sucralfate and the addition of ATRA (68% vs 37%) compared to patients treated with omeprazole and sucralfate alone. Hu etal also showed that ATRA significantly prolong overall survival following the combination of conventional chemotherapy. ATRA anticancer mechanisms of action against GC cells included cell cycle blocking and differentiation initiation(p21WAF1/CIP1 induction, decreased ERK/MAPK pathway), decreased expression of HER2 oncogenic receptor in patient’s gastric mucosa, apoptosis initiation and inhibiting CSC(cancer stem cell) properties such as tumorspheres formation and patient derived xenografts(PDX) growth in mice. In GC cells, CD44+ stem/progenitor cells and a high ALDH (aldehyde dehydrogenase, R-ALDH, ALDH1A1 and ALDH1A3) activity could be considered as putative targets to inhibit tumor growth, to overcome resistance to cancer therapy and to improve GC prognosis.
The-Structure-of-Retinoic-Acid-Receptors-Molecular-Basis-of-Retinoic-Acid-Action-and-the-RAR-Gene-Transcription RARs structure
The retinoic acid receptors (RAR) belong to the large family of ligand responsive gene regulatory proteins that includes receptors for steroid and thyroid hormones [97]. There are three retinoic acid receptors (RAR), RARα, RARβ and RARγ which are conserved throughout vetebrates encoded by their different RAR (chr 17q21, chr 3p24 and chr12q13) gene, respectively. The RARA contains 462 amino acids(aa) [98,99] RARB consists of 455aa [100] and RARG contains 454aa [101] respectively. The RAR is a type of nuclear receptor which act as a transcription factor that is activated by both all-trans RA and 9-cis RA. The RARs have different functions and may activate distinct target genes. The RARa is expressed in a wide variety of different hematopoietic cells [98,99] the RARβ in a variety of epithelial cells [100] and the RARr in differentiation of squamous epithelia and human skin tissue [101,102]
All RARs contain a variable N-terminal region(A/B), a highly conserved cysteine-rich central domain(C) responsible for the DNA binding activity, and a relatively well-conserved C-terminal half(E) functionally its role in ligand binding and nuclear translocation. These three main domain are separated by a hinge region(D) [12,97,102].The central DNA binding domain(88-153aa) exhibits an array of cysteine residues compatible with the formation of two so-called zinc finger(Miller,1985).Each of them a zinc atom tetrahedrically coordinated to four cysteine and each of the hypothetical zinc finger is encoded by a separate exon of the receptor gene (Figure 5) Zinc finger 1, 88-108aa, Zinc finger 2, 124- 148aa] [97-103].The N-terminal zinc finger of the DNA binding domain confers hormone responsiveness to HREs, determing target gene specificity and responsible for functional discrimination between HREs whereas the C-terminal finger contains the sugarphosphamide backbone of the flanking sequences [103,104] (Figure 5) .
Figure 5:Amino acid sequence of the DNA binding domain of the hRARa into two putative zinc–binding finger (Figure from George Zhu a feeling for scientific drawing based on Evans RM, Science, 1988, 240:899- 895; Beato M,Cell,1989, 56: 335-344; Giguere V,Nature,1987;330:624-29; Petkovich M, Nature, 1987,330: 444).
The molecular basis of retinoic acid action and the RAR gene transcription
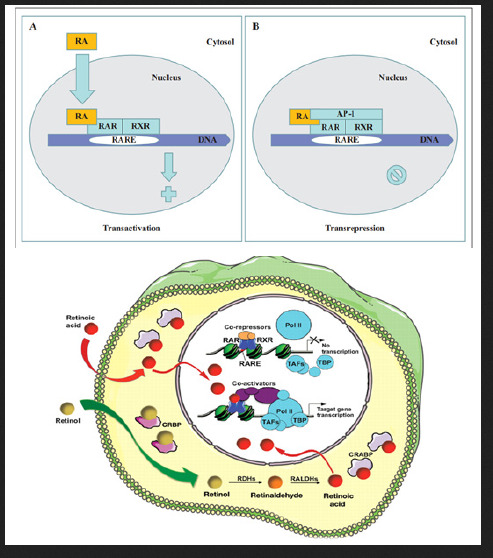
Retinoic acid (RA) is a lipophilic signal molecule which is able to induce acute and direct activation of the expression of specific genes supports its molecular model of action that resembles that of steroid hormones [105]. The cellular retinoic acid-binding protein (CRABP) may be involved in this transfer [9,10]. In the nucleus, RA receptors (RAR) function as a heterodimer with retinoid X receptors (RXRs) [106-109]. RAR/RXR can bind to DNA motif at RA-response elements (RAREs, also HRE) in the regulatory sequences of target genes in the absence of ligand, thereby interacting with multiple protein complexes that include co-repressors N-CoR [110] SMRT [111] and histone deacetylases (HDACs), and maintaining gene repression. Here, RAREs consist of a direct repeat of a core hexameric sequence 5’ (A/G)G(G/T)TCA-3’ [112] or of the more relaxed 5’-(A/G)G(G/T) (G/T)(G/C)A-3’ motif, separated by 1,2,5 bp [113]. A corepressor represses expression of genes by binding to and activating a repressor transcription factor, the repressor in turn bind to target gene’s operator including RARE sequence, then blocking transcription of that gene (see corepressor-wikipedia). Transcriptional regulation thus drives from the binding of hormone-receptor complexes to RARE sites on target DNA [12,97,103]. In the presence of RA(all-trans RA,9- cis RA),binding of the RA ligand to RAR alter the conformation of the RAR, a conformational change in the DNA-bound receptor leads to the release of co-repressor complexes associated with the RAR/RXR dimer and the recruitment of co-acitivator complexes. These induce chromatin remodeling and facilitate assembly of the transcription pre-initiation complex including RNA polymerase II (Pol II) [114], TATA-binding protein (TBP) and TBP-associated factors (TAFs) [2,12,103,115,116] (Figure 6). Subsequently, transcription of target genes is initiated. This also represent liganddependent transcriptional activation which mediated by nuclear receptors. Like thyroid hormone receptor (THR) [117,118] retinoic acid act as ligand for RARs, converting RARa from transcriptional repressor to activators [2,12,119-122]. Numerous RAR target genes after RA induction have been identified including genes within retinoid pathway, such as RARB,Crbp1/2 (Rbp1/2),Crabp1/2 and CYP26a1.And also, several members of HOX gene family, including HOXa1,HOXb1,HOXb4 and HOXd4,and other genes Tshz1 and Cdx1 [123] the function of which has been demonstrated in vivo in the normal roles of retinoids in patterning vertebrate embryogenesis, early neurogenesis, cell growth and differentiation (Figure 6).
Figure 6:Retinoid receptor-dependent gene regulation [116] & (b): Gene regulation by retinoic acid signalling [2].
Molecular Model of the Gene Regulation of Retinoic Acid Action in APL
Acute promyelocytic leukemia (APL) is a clonal expansion of promyelocytic precursors . Retinoic acid(RA) (initial 13-cis RA, later ATRA and tamibarotene) plus chemotherapy is currently the standard of care [124-131]. APL has a very good prognosis, with long-term survival rates up to near 70%-90% [132]. Molecular analysis has uncovered the facts that approximately 98% of APL, RARa translocates and fuses with the PML gene on chromosome 15 [133-136]. The resulting RAR chimeric genes encode pml/RARa fusion protein, which is specifically expressed in the promyelocytic lineage [20]. In addition to oncogenic receptor derivative pml/RARa [108,137-139] the translocation involves oncogenic TBL1XR1- RARB [140] and NUP98/RARG [141] and oncogenic PML-RARG [142] which share high homolog (90%) of three RAR family that were also detected in APL rare cases.
Most studies have shown in APL that oncogenic pml/ RARa act as constitutive transcriptional repressor that blocks neutrophil differentiation at the promyelocyte stage. Without its ligand, retinoic acid (RA), PML-RARA functions as a constitutive transcriptional repressor of RARE-containing target genes, abnormally associating NcoR/HDACs complex and blocking hematopoietic differentiation. In the presence of pharmacological concentration of RA (about 350ng/ml), RA induce the corepressors NcoR/ HDACs dissociation from PML-RARA, thereby activates transcription and stimulate differentiation [11,12,108,139]. In vitro by using a dominant negative RAR construct transfected with interleukin 3(IL-3)-dependent multipotent hematopoietic cell line (FDCP mix A4) and normal mouse bone marrow cells, GM-CSF induced neutrophil differentiation was blocked at the promyelocyte stage. The blocked promyelocytes could be induced to terminally differentiate into neutrophils with supraphysiological concentration of ATRA [143]. Similarly, overexpression of normal RARa transduced cells displayed promyelocyte like morphology in semisolid culture,and immature RARa transduced cells differentiate into mature granulocytes under high dose of RA(10-6M) [144]. Moreover, mutation of the N-CoR binding site abolishes the ability of PML-RARa to block differentiation [145,146]. Therefore, ectopic expression of RAR fusion protein in hematopoietic precursor cells blocks their ability to undergo terminal differentiation via recruiting nuclear corepressor N-CoR/histone deactylase complex and histone methyltransferase SUV39H1 [147]. In vivo, transgenic mice expressing PML-RARA fusion can disrupt normal hematopoiesis, give sufficient time, develop acute leukemia with a differentiation block at the promyelocytic stage that closely mimics human APL (APL-like syndrome, even in its response to RA in many studies. These results are conclusive in vivo evidence that PML/ RARa is indeed oncogenic, and oncogenic pml/RARa is etiology of APL pathogenesis [148-150]. This also represent a steroid receptor in tumorigenesis (Figure 7).
Figure 7: pml/RARa fusion in differentiation block at promyelocytic stage in transgenic mice [149].
Moreover, in Rousselot’s group experiments, HL-60 cells transfected with 15-30ug of PML-RARa fusion in culture show no features of granulocytic differentiation after 7 days of incubation with 10-7,10-6 uM RA (5.5-9.5% of differentiated cells by the NBT test). At 5ug of PML-RARa plasmid concentration, the blockage of RA-dependent myeloid differentiation could be overcomes with high doses(10-6M) of RA (99% of differentiated cells by NBT test) (Figure 8) [151]. The results clearly indicate that PMLRARa mediated transcriptional repression, as well as PML-RARa oncoprotein blocks RA-mediate promyelocyte differentiation. (Figure 8).
Figure 8: Expression of pml-RARa in HL-60 cells blocks ATRA-induced promyelocytic differentiation a(in the presence of 10-7 M RA, top), and transcriptional repressive properties of pml-RARa in human myeloid cells as βRARE-luc assay(bottom) [151].
By using Xenopus oocyte system to uniquely the comparison of the transcriptional properties of RAR and PML-RAR is due to the lack of endogenous nuclear receptors and the opportunity to evaluate the role of chromatin in transcriptional regulation. The results shown in (Figure 9) demonstrated that, indeed, PML-RARA is a stronger transcriptional repressor that is able to impose its silencing effect on chromatin state even in the absence of RXR. Only pharmacological concentration of RA,pml/RARA become transcriptional activator function [139]. (Figure 9).
Figure 9: Shows pml/RARa as a constitutive transcriptional repressor in xenopus oocyte system, as measured by RARE3 CAT and GAL4 assay [139].
In vitro experiments, ATRA induce pml-RARA itself cleavage into a 85-97kd delta PML-RARA product (a truncated pml/RARA form) in RA sensitive NB4 [152-156] (Figure 10). Delta PML-RARa is not formed in ATRA differentiation resistant NB4 subclones [152,155] which indicate the loss of PML/RARa may be directly linked to ATRA-induced differentiation [152,155].This induction of of PML-RARa cleavage and degradation by RA(ATRA,9-cis RA,Am80) involve the proteasome-dependent [152-154] and caspase mediated pathway [155] or independent of proteasome and caspase cleavage[156] and possibly ubiquitin-activating enzyme EI-like(UBEIL) induction in NB4 cells. This is reason that proteasome inhibitor MG-132 and caspase inhibitor ZVAD do not block ATRA-induced pml/RARa cleavage and differentiation whereas this delta pml-RARA is blocked by RARA itself antagonist Ro-41-5253 [156].The proteasome-dependent pml/RARA degradation, by using proteasome inhibitor lactacystin test, allows APL cells to differentiation by relieving the differentiation block [153]. These data suggest a set of multiple molecular mechanisms for restoration by RA induced myeloid differentiation in APL cells. (Figure 10).
Figure 10: Shows delta pml/RARa cleavage products independent of proteasome and caspase in the presence of ATRA(a,b), and pml/RARa act as transcriptional repressor even in the presence of ATRA(0.01uM,1uM) in RARE-tu-luc assay while delta pml/ RARa is less potent activator of RARE-tk-leu activation than wild-type RARa(c) in NB4 cells [155].
Next we further examine the pml/RARa three region functions,in vitro deletion of the RARa DNA binding domain decreased the ability of pml/RARa to inhibit vitD3 and TGFinduced the myeloid precursor U937and TF-1 cell differentiation [145]. This is also supported by functional analysis of DNA binding domain mutation in vitro. The RARa zinc finger is a sequencespecific DNA binding through which RARa contacts the RA target genes. Moreover, deletion of PML coiled-coil region also blocked the differentiation capacity of TF-1 cells [145]. The coiled-coil region directs the formation of pml/RARa homodimers tightly interact with the N-CoR/HDACs complex, so that transcriptional derepression cannot occur at RARA target gene promoter even if the presence of ATRA [RA resistant, 12,157]. In vitro, using established subclones of NB4 resistant to both ATRA and 9-cis RA, they were significantly less able to stimulate transcription of a RARE driven CAT-reporter gene induction by ATRA and showed altered DNA binding activaty on a RARE [158]. In the resistant cases, mut PML stabilizes PML-RARa [159]. PML-RARA with ligand-binding domain (LBD) mutation, ligand RA binding with LBD is impaired. These results have clearly shown that PML protein dimerization and RARa DNA binding domain are indispensible for the myeloid precursors differentiation which was blocked by PML/RARA and eventually leukemic transformation.
In accordance,the pml/RARa/RXR target genes is found to block differentiation by consitutively silencing a set of RA-responsive genes in the control of hematopoietic precursor cells. Five major transcription factors, Ap-1 [160] C/EBPepsilon [161,162] Pu.1/ DAPK2 [163] PTEN [164] and p21WAF/CCKN1A [165] directly regulate genes important in myeloid differentiation. PML/RARA fusion is oncogenic transcriptional repressor of five genes. Inhibited expression or functions of these five transcription factors lead to a block in myeloid differentiation, which is a hallmark of APL.
In vitro cotransfection of pml/RARA with plasmid expressing AP-1 of c-Jun and c-fos proteins in MCF-7 cells, by using CAT assay, PML-RARa is a repressor of AP-1 transcriptional activity in the absence of RA while RA treatment converted the chimera into a strong activator [160]. Since high AP-1 activity is associated with differentiation of leukemic cells in several context [160] the stimulatory effects in the presence of RA could be relevance to its reversal by provoking differentiation. Another, in pml/RARacontaining cell lines, a close link exists between induction of differentiation and induction of C/EBP epsilon expression [161]. C/ EBPepsilon knockout mice had a block in myeloid differentiation [162]. In absence of retinoic acid (RA), induction of pml/RARa expression in U937PR9 cells stably transfected with zinc-inducible pml/RARa suppressed the expression of C/EBPepsilon. In contrast to its repression, in the presence of a pharmacologic concentration of RA, pml/RARa significantly increased the level of C/EBPepsilon expression in a time and dose-dependent manner [161]. The findings implicate that C/EBPepsilon is critical downstream target gene in RA-dependent granulocytic differentiation in the treatment of APL [163-165].
Phosphotase and Tensin homolog (PTEN) is a protein and lipid phosphatase, which plays a pivotal dual role in tumor suppression and self-renewal of hematopoietic stem cells as its promoting exhaustion of normal hematopoietic stem cells (HSCs) and generation of leukemia-initiating cells (LICs) [166,167]. PTEN expression is downregulated in APL, while ATRA treatment increases PTEN leves by inducing PU.1 transcriptional activity via pml/RARa degradation, allowing the binding of PU.1 in PTEN promoter, in turn promotes PTEN nuclear re-location and decreases expression of the PTEN target Aurora A kinases. Therefore, PTEN is one of the primary target gene of oncogenic pml/RARa in APL
Importantly, restoring DAPK2 expression in PU.1 knockdown APL cells partially rescued neutrophil differentiation [168]. In addition, DAPK2 interacts with other cyclin- dependent kinase inhibitors such as p15INK4b and p21WAF1/CIP, which is needed for the cell-cycle arrest in terminal differentiation of neutrophils. Moreover, DAPK2 can bind and activate the key autophagy gene beclin-1 [169]. DAPK phosphorylates beclin 1 on Thr 119 located at a crucial position within its BH3 domain and thus promotes the dissociation of beclin 1 from BCL-XL inhibitor and induction of autophagy [169]. Here, beclin 1 was initially identified as a BCL-2- binding protein, which is part of a class III PI3K (phosphatidylinositol -3-kinase) multiprotein complex that participate in autophagosome nucleation. Death- associated protein kinase (DAPK1) is a calcium/ calmodulin (CaM) serine/threonine kinase for mediator of cell death [170]. PU.1, an ETS transcription factor known to regulate myeloid differentiation. Silencing of PU.1 in the adult hematopoietic tissue produces dysfunctional stem cells and impaires granulopoiesis by inducing a maturation block. Overexpression of PU.1 overcomes the differentiation block in SCa 1+/Lin- HSC with transduction of PML/ RARa fusion, as measured by the Gr-1 and Mac-1 expression [171]. Thus, pml/RARa represses PAPK2/PU.1 - mediated transcription of myeloid genes in APL,linking a novel autophagy mechanism of pml/ RARA degradation [172].
Figure 11:Molecular model of the gene regulation of retinoic acid (RA) action (George Zhu, January 1991, revised in 2012, further revised in 2018 and in this paper). Schematic alignment of the receptor protein. The two highly conserved regions, identified as the putative DNA-binding (C) and hormone- binding (E), a hinge region (D) and the non-conserved variable NH4-terminus (A/B) as described above. CAT:CAAT box, CCAAT-enhancer binding proteins(or C/EBPs); GC:GC box; TATA:TATA box. Note: In APLcells, pml/RARa fusion point is located in the first 60 amino acids from the N-terminus(A/B) of RARa [12,182].
The elucidation of the molecular basis of retinoic acid and retinoid pharmacology in APL has been illustrated in several publications [157,173-176] the detail molecular model of gene regulation had also been proposed by Zhu in 1990s [12,177,178]. As an approach to APL treatment, one possible the action of retinoic acid, A consensus sequence (TCAGGTCA motif) has been postulated for thyroid hormone (TRE) and retinoic acid responsive element(RARE)-containing in the promoter region of target genes [112]. High dose of RA-RARE-PML/RARa complexes in intracellular localization appears to relieve repressors from DNA-bound receptor [11,12,117,145,158,179] including the dissociation of corepressor complexes N-CoR, SMRT and HDACs from PML- RARa or partially PML-RARa/RXR [11,12,146,157,179]. Also release PML/RARa -mediated transcription repression [175]. This transcriptional derepression occurs at RARa target gene promoter [12,157,180]. Consquentially, PML-RARa chimera converted receptor from a repressor to a RA-dependent activator of transcription [156,157,160]. Co-activator complexes containing histone acetyltransferase (e.g. p300/CBP) are recruited. The resulting pml-RARA oncoprotein proteolytic degradation occurs through the autophagy- lysosome pathway [172] and the ubiquitin SUMO-proteasome system (UPS) [152-155] as well as caspase 3 [155] or lysosomal protease (cathepsin D) enzyme or/and EI-like ubiquitin-activating enzyme (UBEIL) induction [181]. An effect is to relieve the blockade of pml/RARa-mediated RA dependent promyelocytic differentiation and retinoic acid (9-cid RA, ATRA, Am80) in APL therapy Zhu G, March 1990- January 1991, revised in 2012). Here, RA can overcome the transcriptional repressor activity of pml/RARa [11,12,156,179,182]. The oncogenic pml/ RARa uncover a pathogenic role in leukemogenesis of APL through blocking promyelocytic differentiation. This oncogenic receptor derivative pml/RARa chimera is locked in their “off” regular mode thereby constitutively repressing transcription of target genes or key enzymes (such as AP-1, PTEN, DAPK2, UP.1, p21WAF/CCKN1A) [160-165] that are critical for differentiation of hematopoietic cells. This is first described in eukaryotes (Figure 11).
Read More About this Article: https://biomedgrid.com/fulltext/volume3/vitamin-a-and-its-derivatives-retinoic-acid-and-retinoid-pharmacology.000656.php
For more about: Journals on Biomedical Science :Biomed Grid
#biomedgrid#medical and medicinal journal#Journals on Biomedical Imaging#Journals on Medical drug and theraputics#American medical journal#Journals on Biomedical Science
0 notes
Text
The gene code of growing limbs
21.09.18 - Scientists from EPFL and the University of Geneva have discovered a “code” of architect genes that are expressed in specific combinations during the development of hands and fingers. The study decrypts developmental gene expression at the level of the single cell in developing limbs and expands our understanding of the genetics behind growing limbs. Image: Growing hand with HoxD combinatorial code. The image below is an overlay of the large-scale hand sculpture with Hox genes combinatorial code distribution along a diffusion map, illustrating genetic diversity in the growing limb. Each dot being one cell, the colors represent the single-cell combinatorial Hoxd code that match with different degree of differentiation, a recent concept also described as pseudo-temporal ordering of single-cells. The graphic highlights the switch between cellular temporal states involved in a regulatory network. The cells with only one gene are in a more premature state while the one expressing a higher set of these genes being at the end of their maturation. Credit: Sculpture and “del desierto” from Chilean sculptor Mario Irrarazabal in Atacama Desert. Graphical representation of pseudotime by P. Fabre and Q. Lo Giudice, University of Geneva. When a fetus develops, everything must be timed to perfection: cell division and differentiation, gene expression, cell-to-cell signaling, and morphogenesis must be carefully coordinated to occur in the correct sequence and for the proper amount of time. Failures in timing can result in congenital deformities, disabilities, and even death. The big question that developmental biologists have been asking for a long time is this: what sets the pace and the order of developmental events? Looking at the development of the paw and digits in mice, scientists from the lab of Denis Duboule at EPFL, with Pierre Fabre at the University of Geneva, have now discovered a “code” of architect genes that play a central role in the developmental cascade. The genes belong to the Hoxd group, developmental genes that are active in various combinations inside so-called “progenitor” cells – cells that are just a step more specialized than stem cells. By pushing a cutting-edge single-cell RNA sequencing technique to its maximal resolution, the scientists were able to study the expression patterns of thousands of genes inside single progenitor cells. The analysis showed that each cell contains 343 genes that are associated with specific cellular states, many of which are involved in the packaging and ordering of DNA in the cell, as well as patterning how the paw’s fingers will develop. The scientists identified a very restricted set of six main combinations of five Hoxd genes (Hoxd9, 10, 11, 12, and 13) in the development of digits in mice. Each combination includes one, two, or four of the genes, with the simplest (one gene) at the beginning of development and the more complex (four genes) combination occurring at the later stages of maturation. The pattern by which Hox genes are expressed when they “build” the entire body – in both mice and humans – has been known and studied for over thirty years. But most of that information comes from looking at entire tissues of developing organs. In contrast, this study is the first to look at it in single cells, offering a higher resolution and clarity in the way Hox genes orchestrate the rhythm of development. A graph showing the five combinations of Hoxd genes in precursor cells during development of the paw and digits of mice. Each dot represents a single cell; each color represents one of the Hoxd combinations, which match different degrees of differentiation (earliest stages on the left). The graphic highlights the switch between cellular temporal states involved in a regulatory network. The cells with only one gene are in a more premature state while the one expressing a higher set of these genes being at the end of their maturation. Credit: Pierre J. Fabre and Quentin Lo Giudice, University of Geneva. “The study shows how architect genes act in concert, following a gradual progression in every developing cell to generate our fully mature arms and hands at the right time and the right place,” says Pierre Fabre. “The Hoxd gene combinations provide a machinery that generate a spectrum of functionally different cells within genetically-defined classes of limb patterning motifs. This will pave the way for future genetic work to understand how cells get to synchronize the combined activation of multiple genes.” This study is a collaboration between the faculties of Science and Medicine of the University of Geneva with EPFL’s ISREC Foundation and BBCF. Nik Papageorgiou http://actu.epfl.ch/news/the-gene-code-of-growing-limbs (Source of the original content)
0 notes
Text
New Post has been published on Biotech Advisers
New Post has been published on http://www.bioadvisers.com/differentiation-of-human-pluripotent-stem-cells-into-colonic-organoids-via-transient-activation-of-bmp-signaling/
Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling
Topics overview: Highlight ITGA7 can act as a glioblastoma biomarker and candidate therapeutic target, Transient Activation of BMP Signaling, The function of Intestinal Enteroendocrine Lineage Cells, How stem cells are triggered to enter this proliferative TA state, The role of immune cells in lung regeneration.
1. Integrin α7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma
Functionally relevant markers of glioblastoma stem-like cells (GSCs) have potential for therapeutic targeting to treat this aggressive disease. Here Tobias L. Haas at Institute of General Pathology, Università Cattolica del Sacro Cuore in Rome, Italy and his colleagues used generation and screening of thousands of monoclonal antibodies to search for receptors and signaling pathways preferentially enriched in GSCs. They identified integrin α7 (ITGA7) as a major laminin receptor in GSCs and in primary high-grade glioma specimens. Analyses of mRNA profiles in comprehensive datasets revealed that high ITGA7 expression negatively correlated with survival of patients with both low- and high-grade glioma. In vitro and in vivo analyses showed that ITGA7 plays a key functional role in growth and invasiveness of GSCs. They also found that targeting of ITGA7 by RNAi or blocking mAbs impaired laminin-induced signaling, and it led to a significant delay in tumor engraftment plus a strong reduction in tumor size and invasion. Their data, therefore, highlight ITGA7 as a glioblastoma biomarker and candidate therapeutic target.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30137-6
2. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling
Gastric and small intestinal organoids differentiated from human pluripotent stem cells (hPSCs) have revolutionized the study of gastrointestinal development and disease. Distal gut tissues such as cecum and colon, however, have proved considerably more challenging to derive in vitro. Here Jorge O. Múnera at Division of Developmental Biology, Cincinnati Children’s Hospital Research Foundation in Cincinnati, USA and his colleagues report the differentiation of human colonic organoids (HCOs) from hPSCs. They found that BMP signaling is required to establish a posterior SATB2+ domain in developing and postnatal intestinal epithelium. Brief activation of BMP signaling is sufficient to activate a posterior HOX code and direct hPSC-derived gut tube cultures into HCOs. In vitro, HCOs express colonic markers and contained colon-specific cell populations. Following transplantation into mice, HCOs undergo morphogenesis and maturation to form tissue that exhibits molecular, cellular, and morphologic properties of human colon. Together these data show BMP-dependent patterning of human hindgut into HCOs, which will be valuable for studying diseases including colitis and colon cancer.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30226-6
3. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity
Several cell populations have been reported to possess intestinal stem cell (ISC) activity during homeostasis and injury-induced regeneration. Here, Kelley S. Yan at Stanford University School of Medicine in Stanford, USA and his colleagues explored inter-relationships between putative mouse ISC populations by comparative RNA-sequencing (RNA-seq). The transcriptomes of multiple cycling ISC populations closely resembled Lgr5+ ISCs, the most well-defined ISC pool, but Bmi1-GFP+ cells were distinct and enriched for enteroendocrine (EE) markers, including Prox1. Prox1-GFP+ cells exhibited sustained clonogenic growth in vitro, and lineage-tracing of Prox1+ cells revealed long-lived clones during homeostasis and after radiation-induced injury in vivo. Single-cell mRNA-seq revealed two subsets of Prox1-GFP+ cells, one of which resembled mature EE cells while the other displayed low-level EE gene expression but co-expressed tuft cell markers, Lgr5 and Ascl2, reminiscent of label-retaining secretory progenitors. Their data suggest that the EE lineage, including mature EE cells, comprises a reservoir of homeostatic and injury-inducible ISCs, extending our understanding of cellular plasticity and stemness.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30240-0
4. An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice
Tissue homeostasis requires the production of newly differentiated cells from resident adult stem cells. Central to this process is the expansion of undifferentiated intermediates known as transit-amplifying (TA) cells, but how stem cells are triggered to enter this proliferative TA state remains an important open question. Using the continuously growing mouse incisor as a model of stem cell-based tissue renewal, Jimmy Kuang-Hsien Hu at University of California in San Francisco, USA and his colleagues found that the transcriptional cofactors YAP and TAZ are required both to maintain TA cell proliferation and to inhibit differentiation. Specifically, they identified a pathway involving activation of integrin α3 in TA cells that signals through an LATS-independent FAK/CDC42/PP1A cascade to control YAP-S397 phosphorylation and nuclear localization. This leads to Rheb expression and potentiates mTOR signaling to drive the proliferation of TA cells. These findings thus reveal a YAP/TAZ signaling mechanism that coordinates stem cell expansion and differentiation during organ renewal, the authors suggest.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30094-2
5. Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration following Pneumonectomy
To investigate the role of immune cells in lung regeneration, Andrew J. Lechner at University of California in San Francisco, USA and his colleagues used a unilateral pneumonectomy model that promotes the formation of new alveoli in the remaining lobes. Immunofluorescence and single-cell RNA sequencing found CD115+ and CCR2+ monocytes and M2-like macrophages accumulating in the lung during the peak of type 2 alveolar epithelial stem cell (AEC2) proliferation. Genetic loss of function in mice and adoptive transfer studies revealed that bone marrow-derived macrophages (BMDMs) traffic to the lung through a CCL2-CCR2 chemokine axis and are required for optimal lung regeneration, along with Il4ra-expressing leukocytes. Their data suggest that these cells modulate AEC2 proliferation and differentiation. Finally, they provide evidence that group 2 innate lymphoid cells are a source of IL-13, which promotes lung regeneration. Together, their data highlight the potential for immunomodulatory therapies to stimulate alveologenesis in adults.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30095-4
#BMP Signaling#Enteroendocrine Lineage Cells#FAK-YAP-mTOR Signaling Axis#integrin α7#Lung Regeneration
0 notes
Text
New Post has been published on Biotech Advisers
New Post has been published on http://www.bioadvisers.com/differentiation-of-human-pluripotent-stem-cells-into-colonic-organoids-via-transient-activation-of-bmp-signaling/
Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling
Topics overview: Highlight ITGA7 can act as a glioblastoma biomarker and candidate therapeutic target, Transient Activation of BMP Signaling, The function of Intestinal Enteroendocrine Lineage Cells, How stem cells are triggered to enter this proliferative TA state, The role of immune cells in lung regeneration.
1. Integrin α7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma
Functionally relevant markers of glioblastoma stem-like cells (GSCs) have potential for therapeutic targeting to treat this aggressive disease. Here Tobias L. Haas at Institute of General Pathology, Università Cattolica del Sacro Cuore in Rome, Italy and his colleagues used generation and screening of thousands of monoclonal antibodies to search for receptors and signaling pathways preferentially enriched in GSCs. They identified integrin α7 (ITGA7) as a major laminin receptor in GSCs and in primary high-grade glioma specimens. Analyses of mRNA profiles in comprehensive datasets revealed that high ITGA7 expression negatively correlated with survival of patients with both low- and high-grade glioma. In vitro and in vivo analyses showed that ITGA7 plays a key functional role in growth and invasiveness of GSCs. They also found that targeting of ITGA7 by RNAi or blocking mAbs impaired laminin-induced signaling, and it led to a significant delay in tumor engraftment plus a strong reduction in tumor size and invasion. Their data, therefore, highlight ITGA7 as a glioblastoma biomarker and candidate therapeutic target.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30137-6
2. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling
Gastric and small intestinal organoids differentiated from human pluripotent stem cells (hPSCs) have revolutionized the study of gastrointestinal development and disease. Distal gut tissues such as cecum and colon, however, have proved considerably more challenging to derive in vitro. Here Jorge O. Múnera at Division of Developmental Biology, Cincinnati Children’s Hospital Research Foundation in Cincinnati, USA and his colleagues report the differentiation of human colonic organoids (HCOs) from hPSCs. They found that BMP signaling is required to establish a posterior SATB2+ domain in developing and postnatal intestinal epithelium. Brief activation of BMP signaling is sufficient to activate a posterior HOX code and direct hPSC-derived gut tube cultures into HCOs. In vitro, HCOs express colonic markers and contained colon-specific cell populations. Following transplantation into mice, HCOs undergo morphogenesis and maturation to form tissue that exhibits molecular, cellular, and morphologic properties of human colon. Together these data show BMP-dependent patterning of human hindgut into HCOs, which will be valuable for studying diseases including colitis and colon cancer.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30226-6
3. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity
Several cell populations have been reported to possess intestinal stem cell (ISC) activity during homeostasis and injury-induced regeneration. Here, Kelley S. Yan at Stanford University School of Medicine in Stanford, USA and his colleagues explored inter-relationships between putative mouse ISC populations by comparative RNA-sequencing (RNA-seq). The transcriptomes of multiple cycling ISC populations closely resembled Lgr5+ ISCs, the most well-defined ISC pool, but Bmi1-GFP+ cells were distinct and enriched for enteroendocrine (EE) markers, including Prox1. Prox1-GFP+ cells exhibited sustained clonogenic growth in vitro, and lineage-tracing of Prox1+ cells revealed long-lived clones during homeostasis and after radiation-induced injury in vivo. Single-cell mRNA-seq revealed two subsets of Prox1-GFP+ cells, one of which resembled mature EE cells while the other displayed low-level EE gene expression but co-expressed tuft cell markers, Lgr5 and Ascl2, reminiscent of label-retaining secretory progenitors. Their data suggest that the EE lineage, including mature EE cells, comprises a reservoir of homeostatic and injury-inducible ISCs, extending our understanding of cellular plasticity and stemness.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30240-0
4. An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice
Tissue homeostasis requires the production of newly differentiated cells from resident adult stem cells. Central to this process is the expansion of undifferentiated intermediates known as transit-amplifying (TA) cells, but how stem cells are triggered to enter this proliferative TA state remains an important open question. Using the continuously growing mouse incisor as a model of stem cell-based tissue renewal, Jimmy Kuang-Hsien Hu at University of California in San Francisco, USA and his colleagues found that the transcriptional cofactors YAP and TAZ are required both to maintain TA cell proliferation and to inhibit differentiation. Specifically, they identified a pathway involving activation of integrin α3 in TA cells that signals through an LATS-independent FAK/CDC42/PP1A cascade to control YAP-S397 phosphorylation and nuclear localization. This leads to Rheb expression and potentiates mTOR signaling to drive the proliferation of TA cells. These findings thus reveal a YAP/TAZ signaling mechanism that coordinates stem cell expansion and differentiation during organ renewal, the authors suggest.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30094-2
5. Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration following Pneumonectomy
To investigate the role of immune cells in lung regeneration, Andrew J. Lechner at University of California in San Francisco, USA and his colleagues used a unilateral pneumonectomy model that promotes the formation of new alveoli in the remaining lobes. Immunofluorescence and single-cell RNA sequencing found CD115+ and CCR2+ monocytes and M2-like macrophages accumulating in the lung during the peak of type 2 alveolar epithelial stem cell (AEC2) proliferation. Genetic loss of function in mice and adoptive transfer studies revealed that bone marrow-derived macrophages (BMDMs) traffic to the lung through a CCL2-CCR2 chemokine axis and are required for optimal lung regeneration, along with Il4ra-expressing leukocytes. Their data suggest that these cells modulate AEC2 proliferation and differentiation. Finally, they provide evidence that group 2 innate lymphoid cells are a source of IL-13, which promotes lung regeneration. Together, their data highlight the potential for immunomodulatory therapies to stimulate alveologenesis in adults.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30095-4
#BMP Signaling#Enteroendocrine Lineage Cells#FAK-YAP-mTOR Signaling Axis#integrin α7#Lung Regeneration
0 notes
Text
New Post has been published on Biotech Advisers
Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling
Topics overview: Highlight ITGA7 can act as a glioblastoma biomarker and candidate therapeutic target, Transient Activation of BMP Signaling, The function of Intestinal Enteroendocrine Lineage Cells, How stem cells are triggered to enter this proliferative TA state, The role of immune cells in lung regeneration.
1. Integrin α7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma
Functionally relevant markers of glioblastoma stem-like cells (GSCs) have potential for therapeutic targeting to treat this aggressive disease. Here Tobias L. Haas at Institute of General Pathology, Università Cattolica del Sacro Cuore in Rome, Italy and his colleagues used generation and screening of thousands of monoclonal antibodies to search for receptors and signaling pathways preferentially enriched in GSCs. They identified integrin α7 (ITGA7) as a major laminin receptor in GSCs and in primary high-grade glioma specimens. Analyses of mRNA profiles in comprehensive datasets revealed that high ITGA7 expression negatively correlated with survival of patients with both low- and high-grade glioma. In vitro and in vivo analyses showed that ITGA7 plays a key functional role in growth and invasiveness of GSCs. They also found that targeting of ITGA7 by RNAi or blocking mAbs impaired laminin-induced signaling, and it led to a significant delay in tumor engraftment plus a strong reduction in tumor size and invasion. Their data, therefore, highlight ITGA7 as a glioblastoma biomarker and candidate therapeutic target.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30137-6
2. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling
Gastric and small intestinal organoids differentiated from human pluripotent stem cells (hPSCs) have revolutionized the study of gastrointestinal development and disease. Distal gut tissues such as cecum and colon, however, have proved considerably more challenging to derive in vitro. Here Jorge O. Múnera at Division of Developmental Biology, Cincinnati Children’s Hospital Research Foundation in Cincinnati, USA and his colleagues report the differentiation of human colonic organoids (HCOs) from hPSCs. They found that BMP signaling is required to establish a posterior SATB2+ domain in developing and postnatal intestinal epithelium. Brief activation of BMP signaling is sufficient to activate a posterior HOX code and direct hPSC-derived gut tube cultures into HCOs. In vitro, HCOs express colonic markers and contained colon-specific cell populations. Following transplantation into mice, HCOs undergo morphogenesis and maturation to form tissue that exhibits molecular, cellular, and morphologic properties of human colon. Together these data show BMP-dependent patterning of human hindgut into HCOs, which will be valuable for studying diseases including colitis and colon cancer.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30226-6
3. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity
Several cell populations have been reported to possess intestinal stem cell (ISC) activity during homeostasis and injury-induced regeneration. Here, Kelley S. Yan at Stanford University School of Medicine in Stanford, USA and his colleagues explored inter-relationships between putative mouse ISC populations by comparative RNA-sequencing (RNA-seq). The transcriptomes of multiple cycling ISC populations closely resembled Lgr5+ ISCs, the most well-defined ISC pool, but Bmi1-GFP+ cells were distinct and enriched for enteroendocrine (EE) markers, including Prox1. Prox1-GFP+ cells exhibited sustained clonogenic growth in vitro, and lineage-tracing of Prox1+ cells revealed long-lived clones during homeostasis and after radiation-induced injury in vivo. Single-cell mRNA-seq revealed two subsets of Prox1-GFP+ cells, one of which resembled mature EE cells while the other displayed low-level EE gene expression but co-expressed tuft cell markers, Lgr5 and Ascl2, reminiscent of label-retaining secretory progenitors. Their data suggest that the EE lineage, including mature EE cells, comprises a reservoir of homeostatic and injury-inducible ISCs, extending our understanding of cellular plasticity and stemness.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30240-0
4. An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice
Tissue homeostasis requires the production of newly differentiated cells from resident adult stem cells. Central to this process is the expansion of undifferentiated intermediates known as transit-amplifying (TA) cells, but how stem cells are triggered to enter this proliferative TA state remains an important open question. Using the continuously growing mouse incisor as a model of stem cell-based tissue renewal, Jimmy Kuang-Hsien Hu at University of California in San Francisco, USA and his colleagues found that the transcriptional cofactors YAP and TAZ are required both to maintain TA cell proliferation and to inhibit differentiation. Specifically, they identified a pathway involving activation of integrin α3 in TA cells that signals through an LATS-independent FAK/CDC42/PP1A cascade to control YAP-S397 phosphorylation and nuclear localization. This leads to Rheb expression and potentiates mTOR signaling to drive the proliferation of TA cells. These findings thus reveal a YAP/TAZ signaling mechanism that coordinates stem cell expansion and differentiation during organ renewal, the authors suggest.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30094-2
5. Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration following Pneumonectomy
To investigate the role of immune cells in lung regeneration, Andrew J. Lechner at University of California in San Francisco, USA and his colleagues used a unilateral pneumonectomy model that promotes the formation of new alveoli in the remaining lobes. Immunofluorescence and single-cell RNA sequencing found CD115+ and CCR2+ monocytes and M2-like macrophages accumulating in the lung during the peak of type 2 alveolar epithelial stem cell (AEC2) proliferation. Genetic loss of function in mice and adoptive transfer studies revealed that bone marrow-derived macrophages (BMDMs) traffic to the lung through a CCL2-CCR2 chemokine axis and are required for optimal lung regeneration, along with Il4ra-expressing leukocytes. Their data suggest that these cells modulate AEC2 proliferation and differentiation. Finally, they provide evidence that group 2 innate lymphoid cells are a source of IL-13, which promotes lung regeneration. Together, their data highlight the potential for immunomodulatory therapies to stimulate alveologenesis in adults.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30095-4
#BMP Signaling#Enteroendocrine Lineage Cells#FAK-YAP-mTOR Signaling Axis#integrin α7#Lung Regeneration
0 notes
Text
New Post has been published on Biotech Advisers
New Post has been published on http://www.bioadvisers.com/differentiation-of-human-pluripotent-stem-cells-into-colonic-organoids-via-transient-activation-of-bmp-signaling/
Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling
Topics overview: Highlight ITGA7 can act as a glioblastoma biomarker and candidate therapeutic target, Transient Activation of BMP Signaling, The function of Intestinal Enteroendocrine Lineage Cells, How stem cells are triggered to enter this proliferative TA state, The role of immune cells in lung regeneration.
1. Integrin α7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma
Functionally relevant markers of glioblastoma stem-like cells (GSCs) have potential for therapeutic targeting to treat this aggressive disease. Here Tobias L. Haas at Institute of General Pathology, Università Cattolica del Sacro Cuore in Rome, Italy and his colleagues used generation and screening of thousands of monoclonal antibodies to search for receptors and signaling pathways preferentially enriched in GSCs. They identified integrin α7 (ITGA7) as a major laminin receptor in GSCs and in primary high-grade glioma specimens. Analyses of mRNA profiles in comprehensive datasets revealed that high ITGA7 expression negatively correlated with survival of patients with both low- and high-grade glioma. In vitro and in vivo analyses showed that ITGA7 plays a key functional role in growth and invasiveness of GSCs. They also found that targeting of ITGA7 by RNAi or blocking mAbs impaired laminin-induced signaling, and it led to a significant delay in tumor engraftment plus a strong reduction in tumor size and invasion. Their data, therefore, highlight ITGA7 as a glioblastoma biomarker and candidate therapeutic target.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30137-6
2. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling
Gastric and small intestinal organoids differentiated from human pluripotent stem cells (hPSCs) have revolutionized the study of gastrointestinal development and disease. Distal gut tissues such as cecum and colon, however, have proved considerably more challenging to derive in vitro. Here Jorge O. Múnera at Division of Developmental Biology, Cincinnati Children’s Hospital Research Foundation in Cincinnati, USA and his colleagues report the differentiation of human colonic organoids (HCOs) from hPSCs. They found that BMP signaling is required to establish a posterior SATB2+ domain in developing and postnatal intestinal epithelium. Brief activation of BMP signaling is sufficient to activate a posterior HOX code and direct hPSC-derived gut tube cultures into HCOs. In vitro, HCOs express colonic markers and contained colon-specific cell populations. Following transplantation into mice, HCOs undergo morphogenesis and maturation to form tissue that exhibits molecular, cellular, and morphologic properties of human colon. Together these data show BMP-dependent patterning of human hindgut into HCOs, which will be valuable for studying diseases including colitis and colon cancer.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30226-6
3. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity
Several cell populations have been reported to possess intestinal stem cell (ISC) activity during homeostasis and injury-induced regeneration. Here, Kelley S. Yan at Stanford University School of Medicine in Stanford, USA and his colleagues explored inter-relationships between putative mouse ISC populations by comparative RNA-sequencing (RNA-seq). The transcriptomes of multiple cycling ISC populations closely resembled Lgr5+ ISCs, the most well-defined ISC pool, but Bmi1-GFP+ cells were distinct and enriched for enteroendocrine (EE) markers, including Prox1. Prox1-GFP+ cells exhibited sustained clonogenic growth in vitro, and lineage-tracing of Prox1+ cells revealed long-lived clones during homeostasis and after radiation-induced injury in vivo. Single-cell mRNA-seq revealed two subsets of Prox1-GFP+ cells, one of which resembled mature EE cells while the other displayed low-level EE gene expression but co-expressed tuft cell markers, Lgr5 and Ascl2, reminiscent of label-retaining secretory progenitors. Their data suggest that the EE lineage, including mature EE cells, comprises a reservoir of homeostatic and injury-inducible ISCs, extending our understanding of cellular plasticity and stemness.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30240-0
4. An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice
Tissue homeostasis requires the production of newly differentiated cells from resident adult stem cells. Central to this process is the expansion of undifferentiated intermediates known as transit-amplifying (TA) cells, but how stem cells are triggered to enter this proliferative TA state remains an important open question. Using the continuously growing mouse incisor as a model of stem cell-based tissue renewal, Jimmy Kuang-Hsien Hu at University of California in San Francisco, USA and his colleagues found that the transcriptional cofactors YAP and TAZ are required both to maintain TA cell proliferation and to inhibit differentiation. Specifically, they identified a pathway involving activation of integrin α3 in TA cells that signals through an LATS-independent FAK/CDC42/PP1A cascade to control YAP-S397 phosphorylation and nuclear localization. This leads to Rheb expression and potentiates mTOR signaling to drive the proliferation of TA cells. These findings thus reveal a YAP/TAZ signaling mechanism that coordinates stem cell expansion and differentiation during organ renewal, the authors suggest.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30094-2
5. Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration following Pneumonectomy
To investigate the role of immune cells in lung regeneration, Andrew J. Lechner at University of California in San Francisco, USA and his colleagues used a unilateral pneumonectomy model that promotes the formation of new alveoli in the remaining lobes. Immunofluorescence and single-cell RNA sequencing found CD115+ and CCR2+ monocytes and M2-like macrophages accumulating in the lung during the peak of type 2 alveolar epithelial stem cell (AEC2) proliferation. Genetic loss of function in mice and adoptive transfer studies revealed that bone marrow-derived macrophages (BMDMs) traffic to the lung through a CCL2-CCR2 chemokine axis and are required for optimal lung regeneration, along with Il4ra-expressing leukocytes. Their data suggest that these cells modulate AEC2 proliferation and differentiation. Finally, they provide evidence that group 2 innate lymphoid cells are a source of IL-13, which promotes lung regeneration. Together, their data highlight the potential for immunomodulatory therapies to stimulate alveologenesis in adults.
Read more, please click http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30095-4
#BMP Signaling#Enteroendocrine Lineage Cells#FAK-YAP-mTOR Signaling Axis#integrin α7#Lung Regeneration
0 notes