#petrographic microscope
Text
woag, look at that feldspar's chemical zoning... now in MULTIPLE colors!
#fuckign mc's GASP and “oooooooh my gosh... nofair!” at the end kills me#she then took over the microscope to get her own video#tuna talks geology#thin section#microscope#petrographic microscope#mineralogy
173 notes
·
View notes
Text




sample vs. thin section part two!
#science with sherrie#sherrie’s shit#geology#finished my petrology course w/ an A/93% 😎#very happy with myself#loved the class too :’)#petrology#igneous rocks#thin section#both thin sections taken at 2x/XPL#via leica petrographic microscope
55 notes
·
View notes
Text
Reflective polarizing microscope
Reflective polarizing microscope bench-top unit integrated with dual polarizing filters. Halogen lamp provide bright illumination. Optimized stress and strain free objectives are used with polarized light providing bright crisp images. Coaxial coarse and fine adjustment focus knob with tension adjustment aids in mobility of the objective lenses. Optional digital camera can be incorporated for image analysis.
0 notes
Text
Mineral Swag Round 1: The Poll Maker's Favorites


More information under the cut (this is a long one, I love these minerals!)
Garnet and Peridot. These are literally my two favorite minerals and here I am pitting them against each other in round one. But it must be done. Their vibes are too similar for them to be paired with any other mineral.
Garnet is #1 on my list. She can be red or orange or green. The most well-known of the garnet species is probably pyrope garnet. She’s the one with that classic deep red color associated with garnet! (For more information about mineral species and what they are and why they form, see when I talk about olivine further down).

The picture above the poll is an almandine garnet and it’s probably my favorite garnet sample of ALL TIME. Those garnet crystals formed like that naturally. Someone did NOT go in and cut that up. They removed some of the schist (the rock matrix that the garnet crystals are stuck in) but garnet just looks like that naturally, babey! Garnet naturally forms as a dodecahedron.
I also love that garnet forms during the metamorphosis of rocks! Shale is a dull, mostly boring-to-look-at rock made of mud and clay. When it experiences high enough pressures and temperatures, it is metamorphosed into schist, a delightfully shinny slippery rock that sometimes has big old garnets in it, as shown above! The rock was smooth and boring, then the minerals recrystallized until it became a weirdly chunky rock!
Another fun fact about garnet has to do with petrographic microscopes and optical mineralogy. Geologists can look at rocks on an outcrop (think big rocks and cliff faces on the side of the road) or in their hand. But to see the minute details of specific minerals, we use thin sections of rock. Thin sections are exactly what they sound like! Super super super thin (30 microns, or 0.003 centimeters thick) slices of rock. Geology students can make them! But if you want good quality ones, you usually send them to a company.
Anyway, when rocks are sliced that thin you can shine light through the minerals (using a petrographic microscope). Minerals have properties you can see and test when you’re holding a sample, but they also have optical properties when you shine plane polarized light (PPL) or cross polarized light (XPL) through it that can be used to identify it. One of garnet’s optical properties is that it looks clear in PPL, but completely dark in XPL and I think that’s a super neat trick.


The same sample in PPL (left) and XPL (right). Pictures from here, annotated by me.
Maybe if people are super geeked about petrology and mineralogy, I’ll talk about it more in another post, but this post is already long and I do not remember THAT much about mineralogy to write a concise summary about any of it!
Now for my other favorite mineral, olivine, aka peridot. Peridot and olivine are the same mineral, but peridot is gem-quality olivine.
Olivine is the real forbidden rock candy. Most of the time it forms these little crunchy looking crystals instead of big impressive crystals like garnet. But it’s such a brilliant green color, and the texture is really very fun.

As for its geologic significance, most of the mantle is olivine! The gooey plastic rock that the tectonic plates float on is mostly this cool green rock. Also, olivine is a great example to use when learning about mineral species. Taken from the preliminary polls:
Olivine has two prominent "end members" or mineral species. The chemical formula for olivine is
(SOMETHING)2Si2O6
That (SOMETHING) is almost always magnesium (Mg) or iron (Fe) or some combination of the two. If an olivine sample has ALL magnesium in that spot, it is known as forsterite (one of the olivine "end members"). If it has ALL iron in that spot, it is known as fayalite (the other end member). And, of course, because nature is not perfect, there can be an olivine with any combination of mangesium and iron (between these two end members).
At their core, fayalite and forsterite are both olivine. They are both silicate minerals (see the part of their formula with Si) and regardless of whether they have magnesium or iron, their structure is the same. They have silicate tetrahedrons (the 3D shape that the molecule Si2O6 makes - think a d4 in D&D) bonded with some iron or magnesium atoms.
In this case, whether you vote garnet or olivine, you have chosen a wonderfully amazing mineral.
52 notes
·
View notes
Text






Feldspar, Garnet, Quartz, Olivine, Biotite, and Calcite under a petrographic microscope
19 notes
·
View notes
Text
VARIOUS GDC TENDER NOTICE JANUARY 2024
GEOTHERMAL DEVELOPMENT COMPANY LIMITED TENDER JANUARY 2024
TENDER NOTICE
The Geothermal Development Company Limited (GDC) invites sealed tenders from eligible candidates for
the following:
TENDER NO.
TENDER DESCRIPTION
TENDER SECURITY
MANDATORY SITE VISIT
CLOSING DATE
GDC/GRA/OT/032/ 2023-2024
Tender for Supply and Delivery of Petrographic Microscope and Desktop Auto Petro Thin
Kshs.…

View On WordPress
0 notes
Text
Trying to figure out how to convert a normal microscope (which I do not have) to a polarising petrographic microscope (which I cannot afford) for the shits and giggles.
I can't quite believe how expensive polarising microscopes are. The price jump is insane. A passable bog standard light microscope is about 100 bucks, 150 if you include the camera attachment. Petrographic microscopes, even the so called "budget" ones, are £600+ (going up to ridiculously expensive several thousand pound microscopes which venture, almost, into tabletop SEM price ranges).
I barely (if ever) use Bertrand lenses, so really the only special features I'd need are a) a rotating stage and b) polarising light filters before and after the stage, a.k.a. a polariser and an analyser. For the former - screw it, I can literally rotate my sample by hand if I must; I will live. For the latter - surely, for something that costs about 10 bucks on amazon (polarising filters), someone, somewhere, has engineered a better solution than taking a microscope apart to glue gun in penknifed filter cut outs, but apparently not!
A normal microscope is alas a bit useless for geological purposes so if I do take the jump and buy one (being otherwise very extremely broke on my grad student budget) and I cannot engineer a working solution, I will be a bit extremely miffed.
But also, I would love to take home my thin sections after this PhD and not just stick them on a shelf! Hence, I need a polarising microscope.
#conundrums conundrums#have to see what role if any a 3d printer might play into this#the polariser is easy enough to add to any microscope#it's the analyser which creates issues#if I add a fixed analyser and a removable polariser it would probably make my life much easier#(probably)#and the less I need to unscrew to place said analyser the better#comparatively speaking the rotating stage is a non issue#that seems a part one can buy#if not print bits for#. so much work. if I could buy I would but it's SO EXPENSIVE#and it's not that all microscopes are that expensive so it would sting me greatly to set that amount aside for a mic#also. i know how often these things have problems#the number of mics with blown bulbs in the lab I... I need something long term and easy to fix
1 note
·
View note
Text
#mortar testing services#mortar testing#heritage mortar#ceramic tiles#mortar analysis#heritage services
0 notes
Text
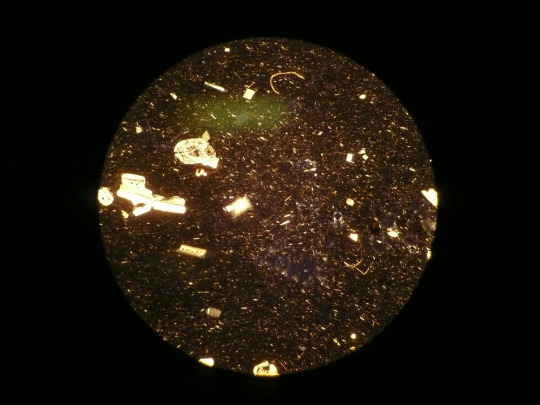
This is possibly the worst photograph taken through a petrographic microscope, somehow the canada balsam is more in focus than the minerals. I thought I'd start here to keep expectations in line with my ability. There is some feldspar here and what I'm speculating was mica at some juncture
1 note
·
View note
Text
another video of the Circles TM
84 notes
·
View notes
Text
Halogen lamp bulb

Because of the new components, these advanced lamps were originally referred to using the term: quartz-iodide. Replacement of the lower-melting glass by quartz was necessary because the halogen regenerative cycle of the lamp (discussed in detail below) requires the envelope to be maintained at a high temperature (in excess of those tolerated by ordinary glass) to prevent tungsten halogen compounds from solidifying on the inside surface. In addition, minute amounts of iodine vapor were sealed inside the envelope. Tungsten-halogen lamps were first developed in the early 1960s by replacing the traditional glass bulb with a higher performance quartz envelope that was no longer spherical, but tubular in shape. Likewise, the loss of tungsten from the filament reduces the diameter, leaving it so thin that it ultimately fails. Over time, the lamp output diminishes as the residue of deposited tungsten on the inner envelope walls grows thicker and absorbs increasing amounts of the shorter visible wavelengths. The major concern with tungsten lamps is that, during normal operation, the filament continuously vaporizes to produce gaseous tungsten that slowly reduces the filament diameter and eventually solidifies on the inside of the glass envelope as a blackened, sooty deposit. In contrast, tungsten has a melting point of approximately 3380° C and can be heated to almost this temperature within a glass envelope to generate light having a higher color temperature and life span than any of the previous materials used for lamp filaments. Carbon lamps suffer from rapid vaporization of the filament at temperatures above 2500° C and thus, must be operated at lower voltages to produce light having a relatively low color temperature (yellowish). These advanced filaments, which could be looped, coiled, and operated at very high temperatures, were found to be far more versatile than their carbon and osmium-based predecessors. The first commercial incandescent lamps equipped with tungsten filaments were introduced in the early 1900s. In long-term experiments (typically, those requiring hundreds to thousands of image captures), this lamp is particularly stable and is subject to only minor levels of temporal and spatial output fluctuation under normal operating conditions. For imaging living cells with contrast-enhancing techniques (principally differential interference contrast ( DIC) and phase contrast) in transmitted light compound microscopes, the most common light source currently in use is the 12-volt, 100-watt tungsten-halogen lamp. Stereomicroscopes also take advantage of this ubiquitous light source in both entry-level and advanced models. Polarized light microscopes used for particle identification, fiber analysis, and birefringence measurements, as well as routine petrographic geological applications, typically use high power tungsten-halogen lamps to provide the necessary light intensity through crossed polarizers. They are excellent for brightfield examination, photomicrography, and digital imaging of stained cells and tissue sections, as well as numerous reflected light applications for industrial manufacturing and development. Several varieties of tungsten-halogen lamps are now the default incandescent illumination source (and are provided by the manufacturer) for most of the teaching and research-level microscopes marketed around the world. Due to their relatively weak emission in the ultraviolet portion of the spectrum, tungsten-halogen lamps are not as useful as arc lamps and lasers for examining specimens that must be illuminated with wavelengths below 400 nanometers. Tungsten-halogen lamps, the most advanced design in this class, generate a continuous distribution of light across the visible spectrum, although most of the energy emitted by these lamps is dissipated as heat in the infrared wavelengths (see Figure 1). These features are primarily responsible for the widespread popularity of incandescent light sources in all forms of optical microscopy. Tungsten lamps are relatively inexpensive (compared to many other light sources), easy to replace, and provide adequate illumination when coupled to a ground glass diffusion filter. Older lamps equipped with tungsten wire filaments and filled with inert argon gas are frequently used in student microscopes for brightfield and phase contrast imaging, and these sources may be sufficiently bright enough for some applications requiring polarized light. Incandescent light sources, including older versions with tungsten and carbon filaments, as well as the newer, more advanced tungsten-halogen lamps, have been successfully employed as a highly reliable light source in optical microscopy for many decades and continue to be the one of the illumination mechanisms of choice for a variety of imaging modalities.

0 notes
Text
Argonite - What Is It?
Argonite is a solid material that can reduce oxygen levels and suffocate fires. It is used in computer and data centers and other enclosed rooms that contain sensitive materials. The material's low oxygen content makes it unsustainable for humans, but it is safe for a short period of time. Some systems also incorporate a delay to allow people to safely leave the room. For this reason, argonite is also often used in emergency response plans.
In crystalline form, aragonite is a stable form of calcium carbonate under high pressures. It is harder than calcite and has a higher specific gravity. It is found exclusively in low-temperature deposits and is often present in pearls. Although aragonite and calcite share the same chemical formula, their crystal structures are very different. In fact, aragonite is polymorphic with vaterite, which suggests that aragonite can invert under normal conditions.
These stones range in size, characteristics, and color. Argonite is an A grade mineral. Its beauty and detail make it an ideal choice for a home or business display. This mineral can be used as a centerpiece, or as an attractive accessory for a mantel. If you're looking for a conversation piece or a gift for someone special, argonite is the right choice. The beauty of this stone is its ability to captivate others.

To determine the exact properties of argonite, it is necessary to look at it under a petrographic microscope. A thin section of the mineral is placed on the microscope stage and is placed above a pair of linear polarisers. The first polariser is placed above the second. The second polariser is placed between the objective lens and eyepiece. The microscope condenser is then brought closer to the specimen. Then the light intensity is increased by opening the diaphragm and turning up the bulb. The polarisation is performed with a high power objective lens.
Argonite is also a great solution for data centers. It has low toxicity, which makes it a great choice for a number of applications. It is also non-conductive, which means that it won't damage sensitive electronic equipment. Moreover, it doesn't damage the environment and doesn't harm the oxygen layer. Moreover, it can be used in hospitals, data centers, and other environments. In fact, it is widely used as an alternative to chemical fire suppression.
0 notes
Text

LCD digital polarizing microscope
LCD digital polarizing microscope is a bench-top unit integrated with Siedentopf binocular head for fine focusing. HD LCD screen for viewing the images. Built-in provision to capture quick and short video. Coaxial coarse and fine adjustment focus knob with tension adjustment aids in mobility of the objective lenses. Polarizing unit contains 360 degree rotatable polarizer and analyzer.
#petrographic microscope price#leica polarizing microscope#nikon petrographic microscope#zeiss petrographic microscope#polarizer for microscope
0 notes
Photo

its been scrutinized.
this is clinopyroxene
6 notes
·
View notes
Text


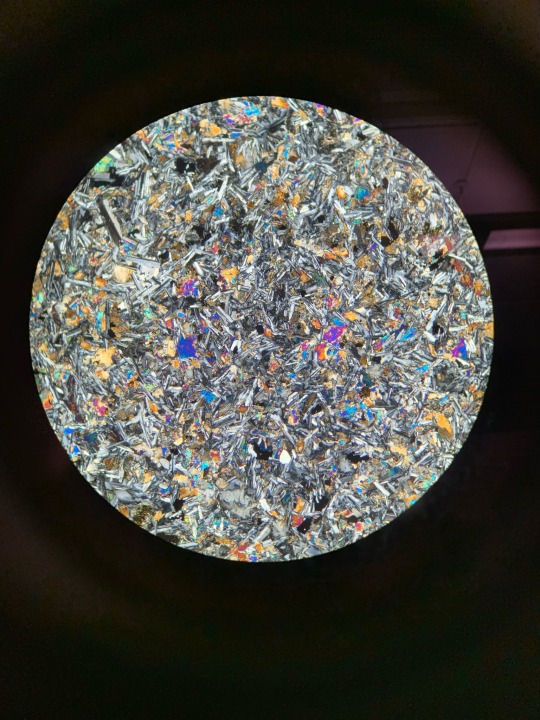
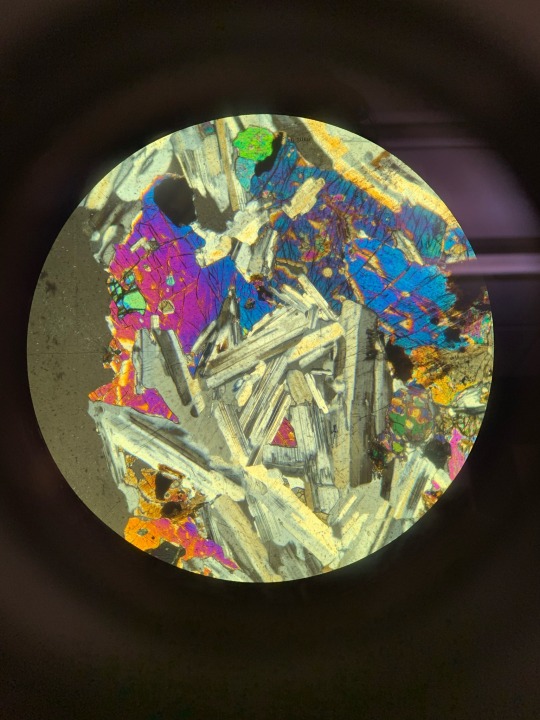
Here’s some igneous rock samples through the petrographic microscope that im supposed to be identifying ^_^
11 notes
·
View notes
Photo

Just a typical Thursday night with my microscope, Sméagol XD (at Central Michigan University)
https://www.instagram.com/p/BpX9rzoFJWJ/?utm_source=ig_tumblr_share&igshid=osnhl8k7jpsz
#geology undergrad#geology studyblr#geology#science#sciblr#studyblr#petrographic microscope#microscopy#microscope
2 notes
·
View notes