#iso17025:2017
Text
ISO/IEC 17025:2017 Fundamental Training Course

This course helps to gain the confidence to be able to effectively understand the purpose, intent, and apply the requirements of ISO/IEC 17025:2017 offers Learners a thorough understanding of the laboratory accreditation process.
#iso17025
0 notes
Text
ISO 17025 Certification Procedure in Indonesia
Introduction
ISO 17025 certification demonstrates that it has a workable quality management system in place as well as the capacity and expertise to deliver test and calibration findings.ISO 17025 Certification in indonesia The laboratory's quality management system and technical proficiency are carefully assessed by a third party to receive ISO 17025 certification.In order to keep certification, audits are performed frequently.Only an certified authority with authorization may grant ISO certification.When a laboratory certified, it indicates that it has complied with the management and technical requirements of and has been found to be technically capable of producing calibration and testing finding a laboratory can demonstrate that it has a functional quality management system and that it is qualified to deliver test and calibration findings.The findings of tests conducted by certified labs are accepted by many governmental and regulatory entities.The tests are conducted in accordance with international standards.According to the agreement between certification bodies, the calibration results of certified members would be accepted as if they had performed the calibration themselves, and their test results would fulfil the same minimal quality requirement regardless of the certification body.
For use by laboratories in creating their management system for quality, administrative, and technical operations, see ISO 17025:2005. It may also be used by laboratory clients, regulatory bodies, and auditors to verify or acknowledge the proficiency of laboratories.The purpose of ISO is not to serve as the foundation for laboratory certification.laboratories must adhere to ISO regardless of the number of employees or the scope of the testing and calibration activities.The requirements of those clauses do not apply when a laboratory does not carry out one or more of the tasks defined by ISO 17025,such as sampling and the design/development of new procedures.
What is the purpose of ISO 17025?
By demonstrating their ability to operate competently and produce reliable findings, laboratories can use ISO17025 to boost trust in their work on a national and international level.ISO 17025 Certification in India By increasing the acceptance of results across nations,it also promotes collaboration between laboratories and other organisations.Without the need for additional testing, test reports and certifications can be accepted from one nation to the next, which enhances international trade.Any organisation that does testing, sampling, or calibration and seeks dependable data should find ISO17025 valuable.All types of laboratories are included in this, regardless of whether they are run by the government, business, or any other group.The standard is also helpful to government agencies,inspection bodies, product certification organisations, and other conformity assessment organisations.
How long does it take to get ISO 17025 Certification?
A research institute typically needs six months to a year to get ready for the accreditation assessment.From the day that any eligible non conformances are closed until the certificate is issued, the evaluation itself takes about 8 weeks to complete.The group will look over the goals and parameters of your assessment. They will go over the methods for finding and reporting nonconformances or deficiencies and they will check timeframes, schedules, and resources with you.ISO 17025 in Indonesia, creation of a thorough Gap Analysis report, and micro-level survey for each and every calibration parameter of the laboratory preparation of relevant documentation necessary by ISO 17025:2017 in Indonesia based on a thorough analysis of all laboratory activities, aids in the efficient implementation of the laboratory system by recurrent visits until Indonesia receives ISO certification.
What are the requirements of ISO 17025 in indonesia?
Organisations must be aware of the numerous ISO 17025 regulations in order to create the necessary compliance protocols that apply to all departments.First off, the 2017 edition differs significantly from the 2005 version in a number of important ways.ISO 17025 Certification in Nepal Some of these are the General Requirements and Structural Requirements Clauses 6 to 8 outlining the Resource, Process, and Management System Requirements were added to the Management Requirements and Technical Requirements Clauses that were previously included in the 2005 version.With this, it's important to note that the 2017 version now outlines the fundamental standards needed to direct laboratories in their activities, particularly with regard to assuring their expertise and objectivity.Objectives for Management Systems emphasises the two choices that companies can choose from to comply with the standard, their differences,and the different tasks associated in this part.Requirements the fundamental organisational components,operations,and adherence to a management system of a laboratory.
Benefits of ISO 17025 in indonesia
It increases the dependability of the laboratory's evaluation findings and reports. It ensures adequate documentation and offers an effective and efficient management system for all procedures.By adhering to the standards of ISO 17025 standard, a successful work management system can be ensured while saving significant time and money.The gap, which is well-preserved, will ensure that their equipment is tested and calibrated in a proper and legitimate manner.In order to establish a strategic target for a firm, it helps the labs to specify policies, operations, and goals. The requirement develops a proactive, risk-based company culture.Technology testing and calibration processes must be reviewed and audited using the most latest documentation, and ISO 17025 ensures.
Certvalue is a global leader in consulting, training and certification as a one solution for ISO,17025 and many more high quality services with complete focus on Customer satisfaction.Certvalue is the top ISO Consultants in Indonesia for providing ISO Certifications.
0 notes
Text
Why is accreditation with ISO 17025 important?
A third party extensively assesses the laboratory's quality management system and technical proficiency in order to grant ISO 17025 Consultant cost in Bahrain accreditation. To keep accreditation, audits are carried out frequently. Only a recognized accreditation authority may grant ISO 17025 accreditation. In order to produce calibration and test results, a laboratory must meet both the management requirements and the technical requirements of ISO17025 in order to be accredited.
What are the benefits of ISO/IEC 17025 accreditation for testing laboratories?
Due to differences in personnel expertise, equipment used, and Standard Operating Procedures (SOPs) followed when performing the test, several analytical testing laboratories frequently generate different test findings for a particular sample. These differences result from the use of inaccurate and poorly maintained instruments, ambient conditions in the laboratory, and the skill level of the laboratory personnel conducting the test. The fabrication of potency reports to raise retail value is another factor contributing to this discrepancy. This is frequently accomplished by fiddling with test findings and sampling techniques. Well, a plethora of variables may be at play in the consistency and accuracy of test results and the conclusions drawn from them.
So, are there any rules a testing laboratory must abide by?
One such law that outlines specifications for testing facilities is ISO/IEC 17025. Accreditation to ISO/IEC 17025 is required for testing facilities in order to prove their technical proficiency and guarantee the reliability of test results. The importance of ISO/IEC 17025 accreditation for your laboratory will be highlighted in this article.
First of all, one of the most important accreditations for all kinds of laboratories, institutions, and research facilities that conduct tests and calibration is ISO/IEC 17025. A testing or calibration laboratory may cite ISO/IEC 17025 as the standard that best meets their management and technical needs. Additionally, accreditation by regulatory agencies attests to the reliability and accuracy of the results, which are produced in accordance with best practices.
Exactly why was ISO/IEC 17025 updated?
It is essential to stay abreast of revisions and modifications to the standard and to set up your lab for compliance.
Due to an increase in errors and inaccurate data, ISO/IEC 17025 was updated. To stay current with the industry and as nearly as possible adhere to the ISO 9001:2015 standard, ISO/IEC 17025 has already undergone two updates, the first in 2005 and the second in 2017.
The most recent adjustments to the laboratory environment and work procedures were made to ISO/IEC 17025. The most recent revisions to ISO/IEC 17025 add a new chapter on risk-based thinking, control over nonconforming testing and calibration operations, new terminology, incorporating computer systems, and tightening data control, among other things.
scope to incorporate sampling, calibration, and testing. New standards like ISO 9001 (quality management), ISO 15189 (quality of medical laboratories), and ISO/IEC 17021-1 now align with the process approach (which states the requirements for auditing and certification bodies).
Is obtaining ISO/IEC 17025 accreditation for your laboratory a difficult task?
The ISO 17025 standard is extensive and complex, thus it necessitates a full comprehension of and training in its application. Conducting routine internal audits to get a lab ready for third-party evaluations proves to be a huge difficulty because the goal is to standardize the operations of all testing laboratories and to assure the authenticity of the data collected. Finding a trustworthy and integrated software solution that aids in meeting the regulatory criteria is also difficult as well.
It is crucial to implement a Laboratory Information Management System (LIMS) that enables your laboratory to adhere to ISO 17025 Certification in Oman standards as well as other national, regional, and municipal laws.
0 notes
Text
وزارة الزراعة: تجديد الاعتماد الدولي لمعامل مكافحة «العفن البني» بالبطاطس
وزارة الزراعة: تجديد الاعتماد الدولي لمعامل مكافحة «العفن البني» بالبطاطس
أعلنت الدكتورة نجلاء بلابل مدير مشروع حصر ومكافحة مرض العفن البني في البطاطس عن تجديد اعتماد معامل المشروع للايزو 17025: 2017 من المجلس الوطنى للإعتماد “إيجاك “.
جاء ذلك تنفيذا لتوجيهات السيد القصير وزير الزراعة واستصلاح الأراضي بالعمل على التطوير المستمر للمعامل التابعة للوزارة.
وقالت إن المشروع قد حصل على شهادة الاعتماد الدولى فى المتطلبات العامة لكفاءة مختبرات الفحص والمعايرة ISO17025:2017،…

View On WordPress
0 notes
Photo

ISO 17025 Training on New Standard and Certification for Systems Implementation in UAE. Contact Ibex Systems for ISO 17025:2017 Training. For more info - https://www.ibexsystems.net/isoiec-17025-laboratory-accreditation/
#iso17025training#iso17025awarenesstraining#isotraining#labaccreditationtraining#labaccreditation#trainingforisoaccreditation#laboratoryaccreditation#onlineiso17025training#iso17025:2017#onlineiso17025#iso17025:2017training#onlinetrainingforiso17025#iso17025#iso17025certification#iso17025consulting#consulting services
0 notes
Text
ISO 17025 consultancy, ISO 17025 accreditation

ISO / IEC 17025:2017 Accreditation Consultancy and Documentation
Accreditationconsultancy.com offers complete solution to ISO 17020:2012 Accreditation for various types of inspection agencies. ISO 17020 Accreditation gives recognition to the competence of inspection agency for carrying out examination of materials, products, installations, plants, processes, work procedures or services, etc. ISO 17020 Accreditation allows an inspection agency to demonstrate integrity, reliability, and technical competence as well as compliance with internationally recognized good practices. ISO 17020:2012 is internationally recognized standard that provides requirements for the competence of operation of various types of inspection agencies. This International Standard has been drawn up with the objective of promoting confidence in the agencies performing inspection. ISO 17020 covers all the activities of inspection agencies and used for the determination of their conformity with requirements.
ISO/IEC 17025:2017 Accreditation Consultancy
We are the pioneer in providing ISO/IEC 17025 Accreditation consultancy. We offer consultancy for ISO17025: 2017 Accreditation for system implementation, documentation, internal auditing, auditors training, etc. We have rich experience of providing ISO 17025 Accreditation consultancy to all types of calibration and testing laboratories and organizations by helping them in system implementation, preparation of ISO17025: 2017 documents, including ISO17025: 2017 Manual, as well as conducting training programs on system awareness, method validation, uncertainty of measurement and auditor training for ISO/IEC 17025:2017. Our ISO17025: 2017 Accreditation consultancy helps laboratories in confirming and recognizing the competence of laboratories from MRA partnersas per ISO/IEC 17025:2017. We have a team of ISO17025: 2017 accreditation consultants, having approximately 25 years of experience in ISO 17025 accreditation. So far, we haveprovided consultancy to more than 100 national and international laboratories in the areas of food, calibration, material, civil, metal, chemical, environmental testing, etc. to achieve ISO/IEC 17025 accreditation in a quick and effective manner. We help our clients to achieve accreditation by less repetitive work, which reduces time and cost of overall project.
ISO 17025:2017 Accreditation Documents - ISO 17025Manual, Procedures, Audit Checklist
Well-written and organized ISO/IEC 17025:2017 documents are essential forany professional laboratory that wants to achieve accreditation. ISO17025: 2017 accreditation documents must contain ISO 17025:2017 manual, ISO17025 procedures, formats, records, SOPs, formats for technical areas specific to laboratory activities, audit checklist, etc. Accreditationconsultancy.com offers ISO 17025: 2017 documents that cover all such requirements of upgraded standard and can be used for ISO/IEC 17025:2017 re-accreditation also. Our ISO 17025: 2017 manual, ISO 17025 2017 audit checklist and ISO 17025 2017 procedures are provided in editable format. They serve as the primary source of documentation by which the auditors and consultants carry out the process of assessment.
What do ISO 17025:2017 accreditation documentsinclude?
The ISO 17025:2017 documents for testing and calibration labs include the following:
1. ISO 17025:2017 Manual
2. ISO 17025 2017 Procedures
3. Exhibits, including exhibit for calibration periodicity of instruments.
4. Standard Operating Procedures (02 for good work practices.
5. Blank and Filled forms to establish the system for the laboratory.
6. Sample risk template
7. ISO 17025 2017 Audit checklist
Features
• Written in plain English.
• Editable softcopy of documents is provided
• Easy to learn, user-friendly and comply with all accreditation requirements.
• Developed by a team of experienced ISO 17025:2017 consultants.
Benefits of ISO 17025:2017 accreditation documents
• Helps in fine-tuning the processes and establishinga good ISO/IEC 17025 system .
• Saves much time and cost in document preparation.
• Easily modifiable templates according to your requirements
For more information, Inquire us at [email protected]
Website - http://www.accreditationconsultancy.com/
Visit Link - http://www.accreditationconsultancy.com/iso-17025-accreditation-consultancy.htm
ISO 17024 consultancy, ISO 15189 consultancy, ISO 17021 consultancy
1 note
·
View note
Link
ISO/IEC 17025:2017 is a standard developed by the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) that specifies general requirements for the competence of testing and calibration laboratories. ISO/IEC 17025:2017 is designed to ensure that laboratory results are accurate, credible, reproducible, traceable, and verifiable.
0 notes
Text
Phòng kiểm nghiệm được công nhận phù hợp ISO 17025
ISO / IEC 17025 là tiêu chuẩn quốc tế quy định các yêu cầu đảm bảo năng lực của các phòng thử nghiệm và hiệu chuẩn (gọi tắt là phòng thử nghiệm). Tiêu chuẩn này đã được phát triển để thúc đẩy độ tin cậy trong hoạt động của các phòng thí nghiệm. Tiêu chuẩn này bao gồm các yêu cầu đối với các phòng thử nghiệm, cho phép họ chứng minh rằng họ đang hoạt động thành thạo và có khả năng cung cấp các kết quả có giá trị khi sử dụng. Nói chung, các phòng thí nghiệm tuân thủ tiêu chuẩn này cũng sẽ hoạt động theo các nguyên tắc của ISO 9001.
Tiêu chuẩn này yêu cầu các phòng thí nghiệm lập kế hoạch và thực hiện các hành động để giải quyết các rủi ro và cơ hội. Giải quyết cả rủi ro và cơ hội là cơ sở để nâng cao hiệu lực của hệ thống quản lý, đạt được kết quả tốt hơn và ngăn ngừa các tác động tiêu cực. Phòng thí nghiệm chịu trách nhiệm quyết định những rủi ro và cơ hội nào cần được giải quyết.
Để đạt được chứng nhận ISO 17025, hệ thống quản lý chất lượng và năng lực kỹ thuật của phòng thí nghiệm được bên thứ ba đánh giá. Các cuộc đánh giá được thực hiện thường xuyên để duy trì sự công nhận. Công nhận ISO 17025 chỉ có thể được cấp bởi cơ quan công nhận có thẩm quyền. Công nhận rằng phòng thí nghiệm đã đáp ứng các Yêu cầu Quản lý và Yêu cầu Kỹ thuật của ISO17025 và được coi là có đủ năng lực về mặt kỹ thuật để đưa ra các kết quả hiệu chuẩn và thử nghiệm.
Công nhận ISO 17025 chứng minh một phòng thí nghiệm có hệ thống quản lý chất lượng được chấp nhận và có khả năng và năng lực cung cấp các kết quả thử nghiệm và hiệu chuẩn. Các phòng thí nghiệm được công nhận thực hiện các thử nghiệm theo tiêu chuẩn quốc tế (ISO 17025) và kết quả được các tổ chức chính phủ và cơ quan quản lý khác nhau chấp nhận được. Sự sắp xếp giữa các cơ quan công nhận là kết quả thử nghiệm của các thành viên được công nhận sẽ đáp ứng cùng một tiêu chuẩn chất lượng tối thiểu bất kể tổ chức công nhận nào và kết quả hiệu chuẩn sẽ được công nhận như thể họ đã tự thực hiện hiệu chuẩn.
Tiêu chuẩn iso 17025 áp dụng cho tất cả các tổ chức thực hiện thử nghiệm hoặc hiệu chuẩn bất kể số lượng nhân viên hoặc phạm vi hoạt động thử nghiệm hoặc hiệu chuẩn:
- Phòng thí nghiệm hóa chất / phòng xét nghiệm mẫu thực phẩm, mỹ phẩm, hóa dầu, vật tư nông nghiệp, phân bón, thức ăn chăn nuôi, thuốc bảo vệ thực vật…;
- Phòng thí nghiệm / xét nghiệm sinh học (vi sinh) đối với các mẫu thực phẩm, mỹ phẩm, vật tư nông nghiệp, phân bón, thức ăn chăn nuôi, thuốc bảo vệ thực vật…;
- Phòng kiểm nghiệm / kiểm nghiệm dược phẩm;
- Thí nghiệm / phòng thí nghiệm cơ giới đối với các mẫu sắt thép, cơ khí, vật liệu xây dựng, kết cấu xây dựng;
- Phòng thử nghiệm / phòng thí nghiệm nguyên mẫu thiết bị điện, điện tử;
- Các phòng thí nghiệm kiểm tra không phá hủy kết cấu công trình, cầu đường, giao thông…;
- Phòng hiệu chuẩn, phòng đo lường phục vụ công tác đo lường, hiệu chuẩn phương tiện đo, thiết bị đo lường.
Yêu cầu chung khi áp dụng chứng nhận ISO / IEC 17025: 2017
Phòng thí nghiệm phải có sẵn nhân sự, phương tiện, thiết bị, hệ thống và dịch vụ hỗ trợ cần thiết để quản lý và thực hiện các hoạt động của phòng thí nghiệm;
Tất cả nhân viên phòng thí nghiệm, cả bên trong hoặc bên ngoài, những người có thể ảnh hưởng đến hoạt động của phòng thí nghiệm phải có năng lực, hành động khách quan và thực hiện nhiệm vụ của họ phù hợp với hệ thống quản lý của phòng thí nghiệm. thí nghiệm;
Phòng thí nghiệm phải ghi lại các yêu cầu về năng lực đối với từng vị trí chức năng có ảnh hưởng đến kết quả của các hoạt động trong phòng thí nghiệm, bao gồm các yêu cầu về giáo dục, trình độ, đào tạo, kiến thức và kinh nghiệm. kiến thức kỹ thuật, kỹ năng và kinh nghiệm;
Phòng thí nghiệm phải đảm bảo rằng nhân viên có đủ năng lực để thực hiện các hoạt động của phòng thí nghiệm mà họ chịu trách nhiệm và đánh giá mức độ nghiêm trọng của các sai lệch;
Lãnh đạo phòng thí nghiệm là trao đổi với nhân viên về nhiệm vụ, trách nhiệm và quyền hạn của họ.
0 notes
Quote
Calibration Awareness
What is Calibration?
In information technology and other fields, calibration is the process of arrangement of an instrument to provide a result for a sample within bearable bounds. To get rid of or minimizing components that cause inaccurate measurements is a fundamental aspect of instrumentation design.
Why Calibration is important?
In today’s world, technology, and accuracy in tools and machinery are very important. Calibrating your equipment from time to time is a must to maintain a smooth flow in work. The main reason to calibrate instruments and tools is to ensure accurate measurements. The accuracy of the instruments decreases over time due to wear and tear.
For the accurate measurements to avoid system failure or faulty results for the smooth functioning of the instrument, after a repair or installation It assures the quality of the final product in the production line.
You must be very careful while calibrating the device\instrument without damaging or voiding the warranty, there are always instructions given by the manufacturer on how to calibrate properly the device.
Calibration Process
Master Instrument List:
Serial Number
Number of Instrument
Location
Accuracy Required
Range of Measurement
Calibration Done Date
Due Date for Next Calibration
Calibration Certificate Number and Date
Which instruments require calibration?
All measuring devices can be calibrated. It will help you to make sure they are working correctly and are providing the accuracy necessary for the application. How the calibration is done differently according to the device.
The Benefits and Purpose of Calibration:
The purpose of calibration is to help convince precise measurements. The benefits of calibration include improving safety as well as saving money by avoiding the costs of false acceptance and rejection of products, increasing production efficiency, and extending the life of the equipment.
Generally, profitability can be increased by either increasing revenues or cutting costs; calibration helps do both.
The requirement of the Calibration Management system:
Unique Identification
Recorded history and current calibration status
Calibration Procedures Require:
1. Approved Procedures for calibration
2. Schedule for Calibration
3. Process range Limits
Quality Consulting:
Our team consists of qualified engineers, who have years of experience working with implementing and maintaining standards like ISO 9001:2015, AS9100D:2015, ISO 13485:2016, ISO17025:2017, and Transport Canada standards. Contact us if you need help with implementing and maintaining these standards for your workplace.
ISO certifications are very crucial in the industry these days. Our services range from the highly regulated industry from Medical, Aerospace, Marine to others. A lot of companies want this implemented in a timely manner, and once obtained, it needs to be maintained.
It will give all support services, both for companies getting a new ISO and to maintain what they already have.
The most general ISO9001:2015 is quite crucial to a lot of other businesses. It can support those businesses as well. Once the quality standard is obtained, the caliber of the business overall increases, business becomes more reliable to clients and henceforth the importance of getting quality certified.
AS9100D:2017
ISO9001:2015
ISO13485
ISO17025
CAR561 Transport Canada Manufacturing
CAR571 Transport Canada Certification of Aeronautical Products
CAR 573 Transport Canada Maintenance and service (AMO)
0 notes
Photo

Le novità della revisione della norma Iso 17025 2017 Le novità introdotte dalla terza revisione della norma #ISO17025 2017 sono attribuibili ad una armonizzazione dei requisiti tecnici e delle norme Iso 9001, sistemi di gestione per la qualità, e Iso 17021-1 sui principi ed i requisiti per la competenza, la coerenza e l’imparzialità degli organismi che forniscono audit e certificazione di tutti i tipi di sistemi di gestione. E’ stata fondamentale il lavoro collaborativo tra ISO e IEC attraverso il Comitato sulla valutazione della conformità noto come CASCO... https://www.instagram.com/p/B98oMktpBsa/?igshid=njbagdsbmwzf
0 notes
Photo
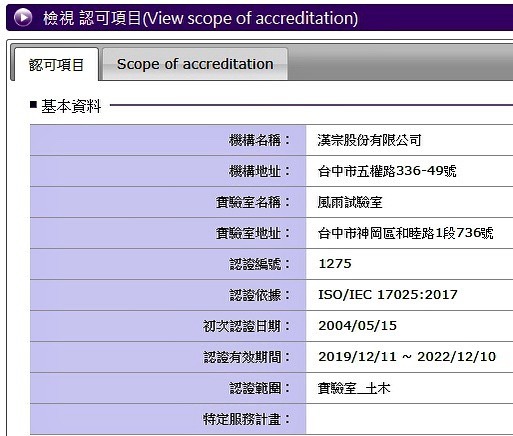
熱呼呼 實驗室認證版本更新到ISO17025:2017最新年版 #iso170252017 #TAF https://www.instagram.com/p/B592e3oldxs/?igshid=okoliy2gaap6
0 notes
Text
Implementation Of ISO 17025 Certification In New York
Introduction
The major ISO standard utilised by testing and calibration laboratories is ISO17025 General Requirements for the Competence of Testing and Calibration Laboratories.For the majority of laboratories to be considered technically competent, ISO 17025 certification is required in the majority of nations.The findings of tests or calibrations performed by a lab that is not certified are frequently rejected by suppliers and regulatory bodies.ISO 17025 Certification in new york In 1999, ISO released the first version of what was once known as ISO.While the ISO 17025 standard and the ISO 9000 standard have many similarities,the latter is more technical in nature, more directly applicable to businesses that generate testing and calibration results,and more explicit in its competency criteria.The ISO17025 quality system is used by laboratories to improve their capacity to consistently generate reliable findings.Additionally, it forms the foundation for certification by an organisation.Three releases occurred in 1999, 2005, and 2017.The emphasis on senior management's duties, specific demands for ongoing development of the management system itself, and customer communication were the three biggest changes between the 1999 and 2005 releases. Additionally, it was more in line with the 2000 edition.
This standard includes requirements for the management system, resources, processes, and general needs.The operation and efficacy of the laboratory's quality management system as well as the organisation of the laboratory itself are largely addressed by the general, structural, and management system requirements.The elements that impact the accuracy and dependability of the tests and calibrations the laboratory performs are included in the resource and process requirements.The general requirements for competence to conduct tests and calibrations, including sampling, are outlined in ISO 17025.It encompasses testing and calibrating quality systems utilising conventional techniques as well as novel or altered laboratory techniques.
How to Get an ISO 17025 Certification ?
Begin a search into 17025.Learn about the fundamentals of ISO 17025 and the many requirements.Learn about the extent of the certification and the several standards, including the general, structural, resource, and process criteria, to better prepare yourself at all organisational levels. Identify the areas where your laboratory management system needs to be strengthened in order to meet the management and technical standards of ISO 17025 certification.ISO 17025 consultant in new york By using a gap analysis checklist, you can determine what your lab now has, where it can improve, and how long it will take to get certified.Make a structure for addressing certification organisations by using the preceding step's checklist as a guide.Create a workflow and timetable for the procedures you'll follow during the certification application process and thereafter.Your lab now wants to know if everything is operating as it should after you enhanced your quality management system and carefully vetted every paperwork. The lab will need to conduct internal audits to detect any non-conformities based on the audit report and develop an action plan in order to achieve this.This will assist your lab in resolving any issues prior to the start of the certification examinations.
The final examination of labs will be performed by a certified organisation.They might talk to some of your company's or lab's employees, go through documents and records, see some calibrations or tests, and inspect the equipment.They look for statements, written policies, records, and processes in order to assess the technical proficiency of your lab.If any non-conformities are found, you will be expected to make the necessary adjustments. For calibration and testing laboratories of all sizes, ISO 17025 is a quality assurance certification that is widely recognised.When all standards are completed, the certification, which focuses on the organisation's or lab's laboratory management system, verifies the lab's technical proficiency in carrying out testing and calibration processes.
Benefits of an ISO 17025 certification in New York
Time and money savings by eliminating product retesting, certification saves a considerable amount of time and operating money.Additionally, it does away with the need for independent supplier checks.ISO 17025 Certification in chicago Decreased waste a long-term strategy for labs to show their technical proficiency and earn the trust of their clients is through certification.The testing and calibrations are standardised to avoid any duplication of the steps or material waste.Greater precision the standard guarantees precise outcomes and verifies test and calibration procedures.Probably require general scientific equipment whether your study includes proteins, blood, cell cultures, soil analysis, or anything else.Any and every lab equipment, from standard lab equipment to specialist instruments and instrumentation, can be financed via Excedr's equipment lease programme.Recognition on a global scale: The widely recognised certification certificate verifies your lab's performance for your clients and stakeholders, giving you the upper hand over rivals and enhancing your reputation on a worldwide scale.
Technical proficiency When a lab is accredited, the strictest requirements for measurement traceability and calibrations have been satisfied.It demonstrates the lab team's technical capacity to produce dependable and accurate results.It assesses staff practises, approaches, and lab tools to make sure you can get reliable findings in your lab.A testing or calibration lab's ability to produce high-quality findings while minimising measurement uncertainties and mistakes is essential.
Certvalue is a global leader in consulting, training and certification as a one solution for ISO,17025 and many more high quality services with complete focus on Customer satisfaction.Certvalue is the top ISO Consultants in New York For providing ISO Certifications.
0 notes
Text
What is ISO 17025?
A quality management system called ISO 17025 consultant in Bahrain serves as the primary standard for testing and calibration facilities. While ISO 17025 and ISO 9000 have many similarities, ISO 17025 assesses technical proficiency in lab testing and calibration services and is applicable to businesses that generate testing and calibration results.
Why is accreditation important?
A third party extensively assesses the laboratory's quality management system and technical proficiency in order to grant ISO 17025 accreditation. To keep accreditation, audits are carried out frequently. Only a recognized accreditation authority may grant ISO 17025 accreditation. In order to produce calibration and test results, a laboratory must meet both the management requirements and the technical requirements of ISO17025 in order to be accredited.
Why is calibration crucial?
your equipment in a lab with ISO 17025 accreditation?
A laboratory's ISO 17025 accreditation demonstrates that it has a functional quality management system and that it is capable of producing accurate test and calibration results. A variety of governmental and regulatory agencies accept the findings of tests conducted by accredited labs against international standards (ISO 17025). The agreement between accrediting bodies states that regardless of the accreditation body, accredited members' test findings must fulfill the same minimal quality level, and their calibration results must be accepted as if they had conducted the calibration themselves.
Why might your company benefit from ISO/IEC 17025:2017 accreditation?
The advantages include strategic, business-related, and internal improvements. Here, a few are highlighted:
they are boosting consumer assurance. A laboratory's ability to deliver consistently valid results, the competence of the workers doing the work, and the ability to trace all accredited measurement results back to the International System of Units (SI) or the appropriate references are all evidenced by accreditation to ISO/IEC 17025:2017. This is the main goal for your clients in order for the results to be recognized internationally.
fostering a proactive, rather than a reactionary, culture of quality and risk-based business. The organization's strategic orientation is built on well-defined activities, rules, and quality goals. Cost-effective operations and evidence-based decisions are driven by a culture of risk-based thinking.
The lab must devise strategies to manage hazards, spur advancements, and guarantee that significant quality risks associated with tests and calibrations are understood and controlled (carried out the same way every time).
ensuring the credibility of your laboratory. To make sure you're utilizing the most recent technology and documentation available, your test and calibration processes need to be evaluated and verified. It is confirmed that tests and calibrations are performed accurately by qualified laboratory personnel through assessment by a third-party accreditation agency.
creating a culture of pride and professionalism. A third-party assessment is difficult since auditors are watching you and scrutinizing everything you do, but after it's finished, the auditee will feel proud of themselves. Third-party certification makes people feel proud of themselves.
What are the steps to obtain ISO/IEC 17025 accreditation, practically speaking?
Having a copy of the standard and understanding ISO 17025:2017 are prerequisites for accreditation. Employ an ISO 17025 Services in Saudi Arabia accreditation organization and meet its demands. Plan your training as all employees must complete some exercise, especially those who will be in charge of management and technical tasks as well as those who will serve as internal auditors.
0 notes
Photo




Take a look at one of our recent #NBQP accredited four-day #ISO17025 Internal #Auditor #Training completed in Ahmedabad #India.
Registration of our #upcoming four-day ISO 17025:2017 Internal Auditor Training on 6th to 9th March 2019 is underway....Few Seats are Left....Reserve your seat Now
For any further query
Kindly get in touch with us at [email protected]
0 notes
Photo

ISO 17025 Training on New Standard and Certification for Systems Implementation in Middle East. Contact for ISO 17025:2017 Training. For more info - https://www.ibexsystems.net/isoiec-17025-laboratory-accreditation/
#ISO17025Training#ISO17025AwarenessTraining#iso training#LabAccreditationTraining#LabAccreditation#TrainingforISOAccreditation#LaboratoryAccreditation#OnlineISO17025Training#iso17025:2017#onlineiso17025#iso17025:2017training#OnlineTrainingforISO17025#ISO#ISO17025#ISO17025Certification#ISO17025Consulting#ConsultingServices
0 notes
Text
ISO 17025 Certification in Michigan
New Post has been published on https://www.expertcertifier.com/iso-17025-certification-in-michigan/
ISO 17025 Certification in Michigan
“Expert certifier is a catalyst for business and process excellence, your business and process excellence is guaranteed through ISO certification with Expert certifier in Michigan”
Up your business, talk to our Expert certifier masters who are available for you to coach and on how to get your business and process certified with ISO 9001, ISO 14001, ISO 45001,ISO 22000,ISO 27001,ISO 20000-1 and HACCP.
What is ISO 17025 Standard All About?
Fundamentally, ISO 17025 in Michigan is a quality system which is structured to make sure the standard and repeatability of a product or service business. The quality doesn’t define exactly how the processes should be administered, but rather an overarching set of guidelines and controlled outcomes that specific procedures should ensure. For instance, the 17025 standard requires that the calibration of reference sensors have adequate control and calibration traceability. How each of those areas is accomplished is up to the practitioner (such as choosing an appropriate calibration interval); but within the end, the alternatives for process/procedure must be documented, repeatable, defensible, and supply a top quality output. The standard system is proven by its outcomes.
Prior to any work on a 17025 compliant quality system, you’ll get to choose your scope of competency. This scope defines the list of test and calibration services that your organizations are going to be operating under the 17025 quality system. For dynamic sensor users, this might be an inventory including items like accelerometer calibration, force sensor calibration, and/or pressure sensor calibration. The subsequent links are included as samples of different sorts of scope for dynamic calibration.
To fulfill the needs of the 17025 standard there are 5 basic components that compose the structure to satisfy the detailed clauses of the quality. They are: scope, procedures, control (including conformance/feedback), uncertainties, and proficiencies. Overall, this is often again very almost like the structure of ISO9001 within the use of procedures, control and audits. In fact, as far as procedures go, much of an equivalent verbiage in place for an existing quality system is often utilized in fulfillment of a 17025 compliant system. The common parts to the 2 quality systems are often in areas like purchasing, document control, quality systems auditing/findings, and correct/preventive action.
The definition of calibration uncertainties is exclusive to ISO17025 and one core to the precise concept of calibration. In fact, the repeatability of calibration within a prescribed uncertainty is the first level of performance proficiency. The world of uncertainties is usually one among the foremost intensely discussed during the 17025 compliant system formulations, also as during the certification audits. It’s during this area that practitioners can show that they clearly understand the inner workings / cause and effect of the calibration system measurement choices.
The concept of proficiency is extended through round robin testing. During a round robin, an equivalent test sensor is calibrated by multiple organizations. Each organization provides calibration data and uncertainties in order that the results could also be compared. Through the discussion of both calibration values and uncertainties, any unacceptably large deviation are often viewed, isolated, and discussed for root cause analysis, thereby improving the measurement and calibration process.
Ensuring of these parts conform to 17025, a laboratory can then enlist the services of a certifying body. Upon satisfactory audit examination, the power receives certification which is subject to regular renewal audits.
The ISO/IEC 17025 standard is formed from five elements:
Scope
Normative References
Terms and Definitions
Management Requirements
Technical Requirements
WHAT ARE THE KEY POINTS ON ISO 17025?
International Organization for Standardization is a world federation of standards which prescribes variety of standards which advances your work system and company methodology. ISO 17025 is a globally acclaimed standard, which is applicable to all or any sorts of relevant bodies whether the company is big or small. It makes your organization or agency more compliant with international methods of the management system. Below here are a number of the key points you would like to understand about ISO/IEC 17025:2017. As ISO 17025 is a basic necessity within the organizations that produce tests and calibration results, it’s considered together of the aspects of the standard Management System, which is restricted to a particular point though. Under the quality, a number of the fundamentally significant steps include the stress on senior management being skilled and requirements of continual improvement of the Management system involving proper communication with customers.
The five basic elements of ISO 17025 in Michigan are normative references, scope, terms and definitions, technical requirements and management requirements. Although, Technical and management requirements have more significance because the elements.
An accredited ISO 17025 laboratory QMS will provide:
Your assurance of meeting all customer quality requirements
Improving your laboratory’s competitiveness.
Eliminate waste
Reduce risk
Control laboratory methods variations.
OUR ISO 17025 SERVICES:
ISO 17025 Documentation Preparing all the specified ISO 17025 documents are often an intimidating feat. The ISO 17025 standard is extremely hospitable interpretation when it involves meeting its documentation requirements. Therefore, organizations seeking to implement the ISO 17025 standard can find themselves struggling early in their ISO journey. The reality of the matter is, since no two companies are an equivalent, ISO standards helps each organization prepares documents, forms, policies, and procedures that best fit their unique businesses.
At Expert Certifier, we acknowledge the differences between companies and also understand what the standards mean to realize through their various clauses. We rest on years of experience so as to organize tailored ISO 17025 documents that are bound to suits all the clauses of the ISO 17025 standard for any sort of organization.
Our customer service professionals are going to be happy to elucidate more about our ISO 17025 documentation services for companies in Norway.
How to get ISO 17025:2017 certification-Consultation in Michigan?
Our masters have more than 10 plus years of global experience, with hands-on experience in the field of ISO certification, assessment and training.
With Expert certifier you’re Business and process excellence is very well guaranteed.
Reach us at: contact@expertcertifier .com
0 notes