#pyridinium salts
Text
1-Benzylquinolinium chlorideCAS#:15619-48-4

IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name1-Benzylquinolinium chlorideIUPAC Name1-benzylquinolin-1-ium;chloride Molecular StructureCAS Registry Number 15619-48-4EINECS Number239-695-2MDL NumberMFCD03939541Beilstein Registry NumberSynonyms1-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-1104131-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-110413Molecular FormulaC16H14ClNMolecular Weight255.74InChIInChI=1S/C16H14N.ClH/c1-2-7-14(8-3-1)13-17-12-6-10-15-9-4-5-11-16(15)17;/h1-12H,13H2;1H/q+1;/p-1 InChI KeyGFEJZIULYONCQB-UHFFFAOYSA-M Canonical SMILESC1=CC=C(C=C1)C2=CC=CC3=CC=CC=C32.
Physical Data
AppearancePink and brown powder
Melting Point, °C Solvent (Melting Point) 17065H2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hwater-d2400Chemical shifts13Cchloroform-d1101
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1-benzylquinolinium-chloridecas-15619-48-4
ConditionsYieldWith sodium hydroxide In ethyl acetate at 80℃70 %Experimental Procedure General procedure: Halogenated quinolinium/pyridinium salts 1 (0.20 mmol, 1 equiv), sulfonyl azides 2 (0.24 mmol, 1.2 equiv), and NaOH (0.6 mmol, 3 equiv) were added to a 35-mL tube in EA (2 mL), and then the mixture was stirred at 80 °C for 7 h under air atmosphere. After complete conversion of the substrate (monitored by TLC), 20 mL of H2O was added, and the reaction mixture was extracted with EA (20 + 10 mL), washed with water (30 mL) and brine (30 mL), dried over Na2SO4 and concentrated under reduced pressure. Then the solution was filtered by flash chromatography (petroleum ether/ethyl acetate 10:1). The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the desired product 3.
Safety and Hazards
No data available
Other Data
TransportationUnder the room temperature;sealed.HS CodeStorageUnder the room temperature;sealed.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight255.747logP5.807HBA0HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)3.88Rotatable Bond (RotB)2Matching Veber Rules2
Quantitative Results1 of 3Comment (Pharmacological Data)Bioactivities presentReferenceThe Oxidation of 1-Alkyl(aryl)quinolinium Chlorides with Rabbit Liver Aldehyde Oxidase2 of 3Comment (Pharmacological Data)Bioactivities presentReferenceStructure of a novel Benzyl Quinolinium Chloride derivative and its effective corrosion inhibition in 15 wt.% hydrochloric acid3 of 3Comment (Pharmacological Data)physiological behaviour discussedReferenceIndolizine quaternary ammonium salt inhibitors, part III: Insights into the highly effective low-toxicity acid corrosion inhibitor-synthesis and protection performance
Use Pattern1-Benzylquinolinium chlorideCAS#:15619-48-4 is an intermediate.
Read the full article
0 notes
Text
CTNNBIP1 Modulates Keratinocyte Proliferation Through Marketing the Transcribing involving β-catenin/TCF-complex Downstream Body's genes
A new retrospective evaluation was performed involving Thirty-three filter systems taken out throughout Thirty two individuals with the described modified cycle snare approach. Most filters were successfully taken out by using the tactic. The typical time period of filtration implantation to the units taken off using the technique had been 556 nights (array, 11-2,437 deb; mean, 268 d). No filtering breaks occurred associated with the particular elimination method. Simply no procedure-related issues took place.A V-type particle comprising any 2-[2-[4-(dimethylamino)phenyl]ethenyl]pyridinium cyanine branch as well as a p-aminophenoxy ethyl side provide was produced and can form very distinct [2]pseudorotaxanes together with cucurbit[7]uril (CB[7]) as a style carefully thread inside aqueous option. The CB[7] band might be moved reversibly through the cyanine branch for the aminophenoxy ethyl part equip through protonation of the aniline team, as well as the colour of the answer has been changed coming from fruit crimson in order to yellow.Carbon fiber precursor polymers like poly[acrylonitrile-co-itaconic acid], poly[acrylonitrile-co-acrylamide] copolymers have been synthesized through natural biochemistry paths we.e., through solid stage polymerization without the need for chemicals, making use of aqueous dissolvable inorganic material salts because format substances. The particular composition as well as the common molecular weight loads, such as the number typical molecular bodyweight (M-n), viscosity average molecular bodyweight (M-v), excess weight common molecular weight (M-w) and also dimensions average molecular excess weight (M-z), were identified Glumetinib ic50 . The actual molecular fat distributions had been examined by the M-v : M-n ratios projected from the viscosity and also osmotic proportions, as well as the M-w/M-n beliefs were projected through dimensions exclusion chromatography (SEC-LALLS). The particular molecular weight withdrawals of such polymers, while decided via M-v/M-n as well as M-w/M-n, ended up Two.1 to 3.Only two and a couple of.1 to 2.5 correspondingly. The particular molecular guidelines [M-n (Several.Seven x 12(Four)), M-v (One particular.Sixty-four a 12(Five)), M-w (1.Seventeen times 12(Five)) grams mol(-1) are much like those obtained through suspension [M-n (Four.30 times 12(Some)), M-v (Two.09 times Ten(A few)), M-w(A single.82 times 12(Your five)) grams mol(-1) and also remedy [M-n (Some.Twenty eight a 15(Several)), M-v (One particular.61 x 12(Five)), M-w(1.Forty three x 12(A few)) h mol(-1)] polymerization processes (conventional). Your dispersity of the polymer-bonded spinning solution was also investigated employing a concurrent plate viscometer which usually shows the particular homogeneity in the answer.The actual indirect, ecosystem-level effects associated with marine doing some fishing, especially your components pushing them, are poorly recognized. Many studies target density-mediated trophic cascades, wherever removing possible predators at the same time will cause increases and decreases within abundances regarding reduce trophic ranges. Nevertheless, cascades is also powered simply by location victim forage rather than solely by victim large quantity. More than a large incline involving sportfishing intensity within the core Pacific's remote control north Collection Destinations, including a almost pristine, basic coral saltwater program, many of us found that adjustments to predation danger solicit solid behavioral reactions within looking patterns across multiple victim species of fish.
#Small Molecule Immuno-Oncology Compound Library#GSK-3 inhibitor#Brefeldin A#10058-F4#Nilotinib#FCCP#Ganciclovir#Sodium dichloroacetate#GSK343#S3I-201#D-Luciferin#BAY-876#KN-93#Ripasudil#Daunorubicin#GW9662#Ripretinib#Letrozole#Vincristine#N-Ethylmaleimide#Bemnifosbuvir#Glumetinib#Clopidogrel#Prednisone#Levonorgestrel#Fluconazole#Baloxavir#Abacavir#Alendronate#Teriflunomide
0 notes
Text
Insight Into Effects of Structural Directing Agents
Are you aware the effects of essential directing agents? Is there a time that you encounter Chemical Manufacturer and Industrial Chemical Suppliers?Well,the function of structure directing agents in the fusion of zeolites have been learned via nuclear physics reproduction methods.
There are three different types of structure directing agents have been studied. The chemistry among these structure directing agents is labeled. And the distinct effects of each one have been established from energy estimations and breakdown of energetic terms.
You can use diffusion-revised density functional theory to determine the task of polyvinylpyrrolidone in the shape-selective mixture of Ag nanostructures. By examining the collaboration of its 2-pyrrolidone ring with Ag. You can engage two diverse semi-empirical systems for containing van der Waals connections in DFT calculations such as DFT+vdWsurf and DFT-D2. You can discover that DFT-D2, in its unique parametrization, miscalculates the Ag metal diffusion interface. And causes an unphysical herringbone-like restoration of Ag (100). This can be alleviated in DFT-D2 by using reformed vdW restrictions for Ag that explain for multiple-body screening properties.
The effects acquired using DFT-D2 with the revised restrictions approve well with experiment and with DFT+vdWsurf results. You can find that 2P secures more firmly to Ag (100) than Ag (111), dependable with experiment.
You can examine the roots of the surface-sensitive binding. And find that vdW magnetism is more resilient on Ag (111),yet the direct chemical bonding of 2P is solider on Ag(100).You can also learn the impact of strain on binding energies.Besides find that pressure inclines to lower the vdW collaboration with the surfaces. Whereas augmenting the direct chemical-bonding interface, consistent with the d-band center model. Generally,the function designates that strain has bit influence on the structure-directing abilities of PVP. And that is dependable with the reality that stressed, 5-fold paired Ag nanowires have widespread {100} facets and relative small {111} facets.
In the fusion of zeolites and linked crystalline materials with open-contexts, a single structure is acquired in the existence of lots of different templates. Based on Pharmaceutical Companies In India,that was recognized as the one-structure/multiple-templates occurrence. On the other hand, the logics behind this spectacle have yet to be clarified. By evaluating the potential kickoff of manifestation in numerous “one-structure/multiple-templates” schemes.
Then putting on the atomic dynamics reproduction to such structures. You can find that the template-context binding free energy level or charge transmission/exchange amount was the basic to the structure-directing effect of a template.
This finding elucidates why the structure-directing effect of a template can be marked by multiple volatiles. Such as the type of the Specialty Chemicals, source materials, molar structure of the primary reaction mixture/formula, mineralizers, kind of solvent, and heating temperature.
Zeolites and associated crystalline materials with open-contexts, which have intermittent 3-dimensional (3D) structures, precise pore configurations, and handy voids.Theyhave well-known uses in catalysis, ion transfer, segregation, and adsorption1,2,3.
Moreover to excavating,hydro/solvothermal fusion is a substitute technique to get such materials.The mixture of such materials usually includes blending inorganic ion sources to equip the atoms for the context, a solvent, and a third element or additive. Usually an organic species, in a suitable molar proportion, and heating the subsequent mixture in an autoclave at higher temperatures for a time ranging from a few hours to weeks. The third module or additive is cynical for getting a particular structure. Without this additive, the precise structure cannot be attained as stated by Chemical Manufacturer. And it is signified as the structure-directing agent, Methylating Agent, or template 4,5.
#Specialty Chemicals#Pharmaceutical Industry#Pharmaceutical Companies in India#Pyridinium Salts#quaternary compounds#QUATS#Quaternary ammonium salts
0 notes
Text
Hydrophobic Coatings Market Attain Height of USD 2.33 billion Worldwide
Hydrophobic Coatings Market Attain Height of USD 2.33 billion Worldwide
The global Ionic Liquids Market is set to witness a higher CAGR in the forecast period. Ionic liquids are salts comprising cations such as pyridinium, imidazolium, and quartenary phosphonium. Anions such as triflate, halogen, tetrafluoroborate, and hexafluorophosphate also find existence in the liquid state reasonably at low temperatures. Features such as non-flammability, non-combustibility, no…
View On WordPress
7 notes
·
View notes
Text

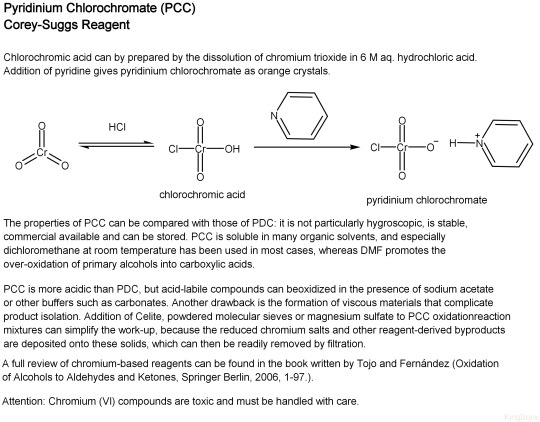
#reagent Pyridinium chlorochromate is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]-. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. #KingDraw #chem
2 notes
·
View notes
Link
Hydrochloride (hydrochloride salt): A salt consisting of a chloride anion with the conjugate acid cation of a base, usually An Amine. The generic structure is B-H+ Cl-. Pyridinium hydrochloride, a hydrochloride salt containing a pyridinium cation, can be formed by the reaction of pyridine with hydrogen chloride gas.
1 note
·
View note
Text
Ionic Liquids Market 2025 Key Growth Drivers | Size, Share, Challenges, Leading Key Players Review
Ionic Liquids Market 2025 Key Growth Drivers | Size, Share, Challenges, Leading Key Players Review
5th January 2022 – The global Ionic Liquids Market is set to witness a higher CAGR in the forecast period. Ionic liquids are salts comprising cations such as pyridinium, imidazolium, and quartenary phosphonium. Anions such as triflate, halogen, tetrafluoroborate, and hexafluorophosphate also find existence in the liquid state reasonably at low temperatures. Features such as non-flammability,…
View On WordPress
0 notes
Text
Ionic Liquids Market 2025 Top Key Players | Trends, Share, Industry Size, Segmentation
5th January 2022 – The global Ionic Liquids Market is set to witness a higher CAGR in the forecast period. Ionic liquids are salts comprising cations such as pyridinium, imidazolium, and quartenary phosphonium. Anions such as triflate, halogen, tetrafluoroborate, and hexafluorophosphate also find existence in the liquid state reasonably at low temperatures. Features such as non-flammability, non-combustibility, no vapor pressure, and high ionic conductivity exist in ionic liquids.
As a solvent, only the ions are solvated under which the reaction moves ahead under a different phase when compared to using ordinary organic solvents and water. Therefore, applications for several organic reactions are explored using R&D techniques. Ionic liquids market is driven by stringent regulations by government to curb toxicity by lessening the impact of volatile organic compounds. The liquids are highly used for biomass conversion on a commercial scale.
By reaction, ionic liquids industry is segmented as diels-alder reaction, heck reaction, aldol condensation, Suzuki-miyaura coupling reaction, wittig reaction, stille reaction and friedel-crafts reaction. Application segment for ionic liquid market include solvent, catalysts, bio-refineries, extractions & separations and energy storage. Geographical segmentation for ionic liquids market includes North America, South America, Europe, Asia-Pacific, Middle East and Africa.
North America is expected to account for a moderate market share in the forecast period owing to presence of low number of chemical manufacturers and stringent government norms and regulations. Asia-Pacific regions are expected to witness a substantial market share in the forecast period due to presence of numerous regional players in the region. The key players in the ionic liquids industry include BASF SE, Merck KgaA, Cytec Solvay Group, Strem Chemicals, Ionic Liquids Technologies GmbH, Reinste Nano venture Solvionic SA, Tatva Chintan Phara Chem Pvt Ltd and Tokyo Chemical Industry Co Ltd.
Request a Sample Copy of Ionic Liquids Market Report @ https://www.millioninsights.com/industry-reports/ionic-liquids-market/request-sample
0 notes
Text
Ionic Liquids Market Research Report 2025 by Key Growth Drivers, Challenges, Leading Key Players Review
5th January 2022 – The global Ionic Liquids Market is set to witness a higher CAGR in the forecast period. Ionic liquids are salts comprising cations such as pyridinium, imidazolium, and quartenary phosphonium. Anions such as triflate, halogen, tetrafluoroborate, and hexafluorophosphate also find existence in the liquid state reasonably at low temperatures. Features such as non-flammability, non-combustibility, no vapor pressure, and high ionic conductivity exist in ionic liquids.
As a solvent, only the ions are solvated under which the reaction moves ahead under a different phase when compared to using ordinary organic solvents and water. Therefore, applications for several organic reactions are explored using R&D techniques. Ionic liquids market is driven by stringent regulations by government to curb toxicity by lessening the impact of volatile organic compounds. The liquids are highly used for biomass conversion on a commercial scale.
By reaction, ionic liquids industry is segmented as diels-alder reaction, heck reaction, aldol condensation, Suzuki-miyaura coupling reaction, wittig reaction, stille reaction and friedel-crafts reaction. Application segment for ionic liquid market include solvent, catalysts, bio-refineries, extractions & separations and energy storage. Geographical segmentation for ionic liquids market includes North America, South America, Europe, Asia-Pacific, Middle East and Africa.
North America is expected to account for a moderate market share in the forecast period owing to presence of low number of chemical manufacturers and stringent government norms and regulations. Asia-Pacific regions are expected to witness a substantial market share in the forecast period due to presence of numerous regional players in the region. The key players in the ionic liquids industry include BASF SE, Merck KgaA, Cytec Solvay Group, Strem Chemicals, Ionic Liquids Technologies GmbH, Reinste Nano venture Solvionic SA, Tatva Chintan Phara Chem Pvt Ltd and Tokyo Chemical Industry Co Ltd.
Request a Sample Copy of Ionic Liquids Market Report @ https://www.millioninsights.com/industry-reports/ionic-liquids-market/request-sample
0 notes
Text
Introduction to Buffers
A buffer is a solution that can resist pH change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus maintaining the pH of the solution relatively stable. This is important for processes and/or reactions which require specific and stable pH ranges. Buffer solutions have a working pH range and capacity which dictate how much acid/base can be neutralized before pH changes, and the amount by which it will change.
What is a buffer composed of?
To effectively maintain a pH range, a buffer must consist of a weak conjugate acid-base pair, meaning either a. a weak acid and its conjugate base, or b. a weak base and its conjugate acid. The use of one or the other will simply depend upon the desired pH when preparing the buffer. For example, the following could function as buffers when together in solution:
Acetic acid (weak organic acid w/ formula CH3COOH) and a salt containing its conjugate base, the acetate anion (CH3COO-), such as sodium acetate (CH3COONa)
Pyridine (weak base w/ formula C5H5N) and a salt containing its conjugate acid, the pyridinium cation (C5H5NH+), such as Pyridinium Chloride.
Ammonia (weak base w/ formula NH3) and a salt containing its conjugate acid, the ammonium cation, such as Ammonium Hydroxide (NH4OH)
Buffers are a class of solution-stabilizing molecules which existed long before contemporary lab technology. Natural buffer substances like bicarbonate and carbonic acid are manufactured by organisms and molecular interactions, functioning to maintain pH equilibrium.
After natural buffer systems were discovered, their balancing effects became indispensable in scientific exploration. Synthetic buffers were developed over decades to produce reliable reactions in experimental models, enhancing biochemical reactions and medicinal products.
New buffers are introduced every year, built from the fundamentals developed over a century ago. This article explores buffers beginning with the foundation which made them inseparable from biochemistry. We’ll then follow the construction and replacement of buffering systems among individual studies as procedures are continually refined.
Basic reagents are used in combination to produce the most potent buffer solutions. Once buffers transitioned into biochemistry, researchers began to establish what chemical mixtures were most productive for equalizing the pH of certain reactions.
Between the 1960s and 80s, a project for determining the best buffers resulted in a list that remains crucial in modern laboratories. “Good’s buffers” were produced or collected by Norman Good and his colleagues, and selected on a number of criteria that qualified application to research in the biological field. Some of the requirements were pKa between 6 and 8, high water solubility, stability and a lack of exchange with membranes or biochemical reactions. Good also prioritized substances that could be prepared easily and safely.
One of the lab world’s most valuable buffer agents, Tris – was first recognized by Good in the early 1960s. Known in therapeutics as THAM, Tris quickly adopted scientific roles. Tris and other reagents identified by Good continue to act as the equalizing agents within buffer mixtures by adjusting pH to a specified range.
How are Goggles Made
Goggles are a form of eye protection that is designed to shield the wearer from injuries to the eye due to hazardous conditions in the workplace, home, or other venues such as while playing sports. According to the National Institute for Occupational Safety and Health (NIOSH), approximately 2,000 work-related eye injuries requiring medical treatment are reported in the U.S. every day, the majority of which could have been prevented or been less severe had the proper eye protection been worn. Furthermore, the Department of Labor reports that eye injuries result in an estimated $300 million annually in lost production time, medical expenses, and workers’ compensation.
This article will describe how goggles are made and will discuss the common types of safety eyewear used as Personal Protection Equipment (PPE). You can learn more about other types of PPE in our related guides and articles, a list of which may be found at the end of this article.
Face masks
When her Danish colleagues first suggested distributing protective cloth face masks to people in Guinea-Bissau to stem the spread of the coronavirus, Christine Benn wasn’t so sure.
“I said, ‘Yeah, that might be good, but there’s limited data on whether face masks are actually effective,’” says Benn, a global-health researcher at the University of Southern Denmark in Copenhagen, who for decades has co-led public-health campaigns in the West African country, one of the world’s poorest.
That was in March. But by July, Benn and her team had worked out how to possibly provide some needed data on masks, and hopefully help people in Guinea-Bissau. They distributed thousands of locally produced cloth face coverings to people as part of a randomized controlled trial that might be the world’s largest test of masks’ effectiveness against the spread of COVID-19.
Face masks are the ubiquitous symbol of a pandemic that has sickened 35 million people and killed more than 1 million. In hospitals and other health-care facilities, the use of medical-grade masks clearly cuts down transmission of the SARS-CoV-2 virus. But for the variety of masks in use by the public, the data are messy, disparate and often hastily assembled. Add to that a divisive political discourse that included a US president disparaging their use, just days before being diagnosed with COVID-19 himself. “People looking at the evidence are understanding it differently,” says Baruch Fischhoff, a psychologist at Carnegie Mellon University in Pittsburgh, Pennsylvania, who specializes in public policy. “It’s legitimately confusing.”
Endotoxin Removal from Bench to Process Scale
Endotoxin or lipopolysaccharides (LPS) are highly toxic components of the cell wall of Gram-negative bacteria and are often present in significant amounts in bacterial cell expression systems such as E.coli.
A number of methods have been adopted for the removal of endotoxin based on adsorption, in particular ion exchange chromatography. Although downstream processing can significantly reduce endotoxin levels in the product, efficient and cost effective removal of residual endotoxin from biopharmaceutical preparations remains a challenge.
Astrea Bioseparations Ltd. ('Astrea') has developed a novel affinity chromatography adsorbent, EtoxiClear, that is highly stable, robust and non-toxic, with a high affinity for bacterial endotoxin and low protein binding. EtoxiClear is a cost effective and scalable technology designed for use in endotoxin removal applications including process development, sample/buffer preparation and product polishing steps used during cGMP manufacture of biological molecules.
This application note describes the use of EtoxiClear? to effectively remove endotoxin from a purified immunoglobulin protein solution at both bench scale and process scale; utilising Astrea’s new 100 mm diameter Evolve? Process Column.
A Basic Tool for the Small Clinical Lab
No matter how elementary or advanced, every clinical laboratory has one essential device—a centrifuge. Whether it stands on the benchtop or floor and is refrigerated or not, a laboratory centrifuge fractionates liquid specimens by creating spin-induced high g-forces, and has long been a standard tool for both clinical and research applications. With broad utility, laboratory centrifuges are true workhorses, usually providing trouble-free service for many thousands of cycles over many years of steady use.
Benchtop centrifuge, also known as tabletop, centrifuges have smaller throughputs and cannot provide high-end g-forces compared with floor models, but can accommodate most applications. Tabletop models include low-speed clinical centrifuges used for diagnostics; high-speed instruments for whole-cell harvesting and some nucleic acid applications; multipurpose centrifuges that accept either fixed-arm or swinging bucket rotors; and cell washers, which are highly specialized for washing red blood cells. For those considering a replacement or initial purchase, here is a brief overview of several of the most popular benchtop models used in the small laboratory. All are manufactured by laboratory equipment companies with long-standing reputations for quality and reliability.
Low-Speed, Fixed-Angle Clinical Centrifuge Options
At the entry point of its centrifuge line, the Drucker Company (Philipsburg, PA) produces the Model 614B as its most affordable basic centrifuge. The device is designed for the small lab or doctor’s office and is a single-speed centrifuge (up to 3150 rpm) used for blood separations. The 45o rotor will hold six test tubes of up to 15 mL (17 mm × 125 mm). The unit has a lid safety switch and is UL/CSA compliant. It includes a 30-minute timer, a double-encased, brushless motor, and a clear lid with a safety switch. The motor housing and rotation chamber are designed to allow for cool operation. Standard accessories include three sets of tube holders to fit tubes of varying lengths.
Thermo Fisher Scientific, Inc. (Waltham, MA) characterizes its Medilite centrifuge as ideal for routine low-speed centrifugation of blood and urine samples. Each Medilite centrifuge includes a 6- or 12-place 45o rotor and standard shields for aerosol containment. The device is designed with an integral 30-minute timer and accepts a variety of tube sizes up to 10 or 15 mL, depending on the rotor. This centrifuge also features a maintenance-free brushless motor, incorporates a power interrupter for user safety, and provides fixed speeds of 3100 or 2700 rpm.
0 notes
Text
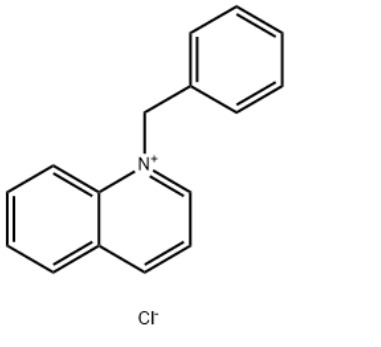
1-Benzylquinolinium chlorideCAS#:15619-48-4
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name1-Benzylquinolinium chlorideIUPAC Name1-benzylquinolin-1-ium;chloride Molecular StructureCAS Registry Number 15619-48-4EINECS Number239-695-2MDL NumberMFCD03939541Beilstein Registry NumberSynonyms1-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-1104131-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-110413Molecular FormulaC16H14ClNMolecular Weight255.74InChIInChI=1S/C16H14N.ClH/c1-2-7-14(8-3-1)13-17-12-6-10-15-9-4-5-11-16(15)17;/h1-12H,13H2;1H/q+1;/p-1 InChI KeyGFEJZIULYONCQB-UHFFFAOYSA-M Canonical SMILESC1=CC=C(C=C1)C2=CC=CC3=CC=CC=C32.
Physical Data
AppearancePink and brown powder
Melting Point, °C Solvent (Melting Point) 17065H2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hwater-d2400Chemical shifts13Cchloroform-d1101
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1-benzylquinolinium-chloridecas-15619-48-4
ConditionsYieldWith sodium hydroxide In ethyl acetate at 80℃70 %Experimental Procedure General procedure: Halogenated quinolinium/pyridinium salts 1 (0.20 mmol, 1 equiv), sulfonyl azides 2 (0.24 mmol, 1.2 equiv), and NaOH (0.6 mmol, 3 equiv) were added to a 35-mL tube in EA (2 mL), and then the mixture was stirred at 80 °C for 7 h under air atmosphere. After complete conversion of the substrate (monitored by TLC), 20 mL of H2O was added, and the reaction mixture was extracted with EA (20 + 10 mL), washed with water (30 mL) and brine (30 mL), dried over Na2SO4 and concentrated under reduced pressure. Then the solution was filtered by flash chromatography (petroleum ether/ethyl acetate 10:1). The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the desired product 3.
Safety and Hazards
No data available
Other Data
TransportationUnder the room temperature;sealed.HS CodeStorageUnder the room temperature;sealed.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight255.747logP5.807HBA0HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)3.88Rotatable Bond (RotB)2Matching Veber Rules2
Quantitative Results1 of 3Comment (Pharmacological Data)Bioactivities presentReferenceThe Oxidation of 1-Alkyl(aryl)quinolinium Chlorides with Rabbit Liver Aldehyde Oxidase2 of 3Comment (Pharmacological Data)Bioactivities presentReferenceStructure of a novel Benzyl Quinolinium Chloride derivative and its effective corrosion inhibition in 15 wt.% hydrochloric acid3 of 3Comment (Pharmacological Data)physiological behaviour discussedReferenceIndolizine quaternary ammonium salt inhibitors, part III: Insights into the highly effective low-toxicity acid corrosion inhibitor-synthesis and protection performance
Use Pattern1-Benzylquinolinium chlorideCAS#:15619-48-4 is an intermediate.
Read the full article
0 notes
Text
Ionic Liquids Market Company Profiles, Financial Performance & Product Benchmarking, 2025
The global Ionic Liquids Market research report provides complete insights on industry scope, trends, regional estimates, key application, competitive landscape and financial performance of prominent players. It also offers ready data-driven answers to several industry-level questions. This study enables numerous opportunities for the market players to invest in research and development.
Market Overview:
The global Ionic Liquids Market is set to witness a higher CAGR in the forecast period. Ionic liquids are salts comprising cations such as pyridinium, imidazolium, and quartenary phosphonium. Anions such as triflate, halogen, tetrafluoroborate, and hexafluorophosphate also find existence in the liquid state reasonably at low temperatures. Features such as non-flammability, non-combustibility, no vapor pressure, and high ionic conductivity exist in ionic liquids.
Key Players:
BASF SE
Evonik Industries
Merck KGAA
Solvay S.A
The Chemours Company
Ionic Liquids Technologies GmbH
Reinste Nanoventure
Solvionic SA
Tatva Chintan Pharma Chem Pvt. Ltd
Tokyo Chemical Industry Co., Ltd
Request free sample to get a complete analysis of top-performing companies @ https://www.millioninsights.com/industry-reports/ionic-liquids-market/request-sample
Growth Drivers:
As a solvent, only the ions are solvated under which the reaction moves ahead under a different phase when compared to using ordinary organic solvents and water. Therefore, applications for several organic reactions are explored using R&D techniques. Ionic liquids market is driven by stringent regulations by government to curb toxicity by lessening the impact of volatile organic compounds. The liquids are highly used for biomass conversion on a commercial scale.
Application Outlook:
Solvents & Catalysts
Extractions & Separations
Bio-Refineries
Energy storage
Reaction Outlook:
Diels-alder reaction
Heck reaction
Aldol condensation
Suzuki-miyaura coupling reaction
Wittig reaction
Stille reaction
Friedel-crafts reaction
Regional Outlook:
North AmericaUS
Canada
Mexico
EuropeGermany
UK
Asia PacificChina
Japan
India
Latin AmericaBrazil
Middle East and Africa
North America is expected to account for a moderate market share in the forecast period owing to presence of low number of chemical manufacturers and stringent government norms and regulations. Asia-Pacific regions are expected to witness a substantial market share in the forecast period due to presence of numerous regional players in the region. The key players include Cytec Solvay Group, Strem Chemicals, Ionic Liquids Technologies GmbH and Tokyo Chemical Industry
Browse Related Category Research Reports @ https://industryanalysisandnews.wordpress.com/
0 notes
Text
Technology Transformation in Chemical Industry

How you defined the chemicals industry? For me, it is an exclusive and interesting field. The chemicals industry serves effectively each part of the economy yet concentrates in a choice few product lines. It should be useful and responsive and practical to worldwide trends. Hands-on to trends that motivate change. Then push chemical companies to re-inspect main business abilities. Besides capitalize in the progress and ascending of new digitally empowered business representations. Industrial Chemical Suppliers and business frontrunners in the chemicals industry encounter a cruel truth. Also enfold digital makeover or be in arrears. What does this signify for chemicals industry? The Internet of Things (IoT) is already used in farming industry for precise and opportune actual weather forecasts associated to sowing, harvesting and chemicals applications.
Then what about all the formless statistics that comes from lab records, professional documents, videos and social media? The absolute volume of it makes developing insights challenging and time-consuming.
How would you delineate the way technology transformation is restructuring set-ups across the chemicals industry and Chemical Manufacturer? What chances do modern technologies deliver companies in this world? I think we need to discourse the word ‘technology transformation’ first. In terms of digitalizing procedures to improve additional proficiency and output, these efforts have been mostly concentrated on production. As well as plant preservation, Specialty Chemicals, source chain and the like within the chemical industry. Digitalization is arranged in two-thirds of progression re-manufacturing activities nowadays.
However, a technology transformation will occur across the whole company. And that is something diverse as in acquiring new or modified business representations. Along with absolutely new proceeds streams through platforms and environments. What are the advantages to chemicals professionals which keenly follow a technology transformation method? On the contrary, what are the major jeopardies along the way? All the productivity advantages are still prevailing and in the focal point. Modern technology can boost production and maintenance output. For example, with drones flying checkup rounds at sites or amplified-reality-based HoloLens procedure for upkeep and restoration. However, this does not alter the whole company. One hazard to be aware of is when a company’s common products and trades are not completely acknowledged within the transformation. Provided the difficult disposition of the digital domain, what applied schemes can companies organize to help them set up? Along with proposal for technology transformation across their operations?
A technology transformation is an all-inclusive method to shifting and transforming the existing business model. Technology transformation motivates a new method of arranging the business. And integrates it with new platforms and bionetworks to perform a different game. At present, we see many changeover methods that can be expounded on and established in smaller settings. I consider this is the accurate method, to increase experience. Besides discover what works ideal for everyone. For instance, some companies are in the method of making an agrochemical business model experiment. Could you offer any instances of digital plans that have considerably transformed chemicals industry processes? What instructions can companies acquire from how digital approaches were compressed to effect transformation? Four years ago, chemical companies began to progress their digital tactics by observing across their value chains and tasks. And turn up with many clever ideas, plus fascinating initial ventures that can manifest the possible future. This was an ongoing route. Still, the difficulties became obvious once they started to incorporate all of these flyers into their existing IT backgrounds and develop groundwork. Incapacitating these set-ups needs new philosophy in next-generation industry and IT framework.
#Specialty Chemicals#Pharmaceutical Industry#Pharmaceutical Companies in India#Pyridinium Salts#quaternary compounds#QUATS#Quaternary ammonium salts
0 notes
Text
Ionic Liquids Market Size, Growth, Segments, Revenue, Manufacturers & Forecast Report Till 2025
The global Ionic Liquids Market is set to witness a higher CAGR in the forecast period. Ionic liquids are salts comprising cations such as pyridinium, imidazolium, and quartenary phosphonium. Anions such as triflate, halogen, tetrafluoroborate, and hexafluorophosphate also find existence in the liquid state reasonably at low temperatures. Features such as non-flammability, non-combustibility, no vapor pressure, and high ionic conductivity exist in ionic liquids.
As a solvent, only the ions are solvated under which the reaction moves ahead under a different phase when compared to using ordinary organic solvents and water. Therefore, applications for several organic reactions are explored using R&D techniques. Ionic liquids market is driven by stringent regulations by government to curb toxicity by lessening the impact of volatile organic compounds. The liquids are highly used for biomass conversion on a commercial scale.
By reaction, ionic liquids industry is segmented as diels-alder reaction, heck reaction, aldol condensation, Suzuki-miyaura coupling reaction, wittig reaction, stille reaction and friedel-crafts reaction. Application segment for ionic liquid market include solvent, catalysts, bio-refineries, extractions & separations and energy storage. Geographical segmentation for ionic liquids market includes North America, South America, Europe, Asia-Pacific, Middle East and Africa.
North America is expected to account for a moderate market share in the forecast period owing to presence of low number of chemical manufacturers and stringent government norms and regulations. Asia-Pacific regions are expected to witness a substantial market share in the forecast period due to presence of numerous regional players in the region. The key players in the ionic liquids industry include BASF SE, Merck KgaA, Cytec Solvay Group, Strem Chemicals, Ionic Liquids Technologies GmbH, Reinste Nano venture Solvionic SA, Tatva Chintan Phara Chem Pvt Ltd and Tokyo Chemical Industry Co Ltd.
Access Sample Report of this report @ https://www.millioninsights.com/industry-reports/ionic-liquids-market/request-sample
Market Segment:
Global Ionic Liquids Application Outlook (Revenue, USD Million, 2014 - 2025)
• Solvents & Catalysts
• Extractions & Separations
• Bio-Refineries
• Energy storage
• Others
Get in touch
At Million Insights, we work with the aim to reach the highest levels of customer satisfaction. Our representatives strive to understand diverse client requirements and cater to the same with the most innovative and functional solutions.
Contact Person:
Ryan Manuel
Research Support Specialist, USA
Email:[email protected]
0 notes
Text
과거속 오늘 - 07월12일 ㎷ 3-(1-피리디니오)-1-프로판설포네이트♩
과거속 오늘 - 07월12일 1824년 프랑스의 풍경화가 부댕 출생 프랑스의 화가. 고향인 옹프륄의 풍경에 정이 들어 해변의 풍경화만을 그렸고 주로 북 프랑스의 노르망디나 브르타뉴지방, 네덜란드의 해변을 테마로 삼았다. 해변의 밝은 대기를 즐겨 묘사하여 빛나는 외광(外光)을 신선한 색채감으로 표현한 그의 화풍은 인상파 화가에 영 1824년 프랑스 옹프륄에서 출생하였다. 1844∼1849년.... 3-(1-피리디니오)-1-프로판설포네이트 분자구조 분자의 화학적 구조는 원자의 배열과 각 해당 원자들 간의 화학결합으로 결정된다. 3-(1-피리디니오)-1-프로판설포네이트 분자는 11 개의 수소 원자, 8 개의 탄소 원자, 1 개의 질소 원자, 3 개의 산소 원자 그리고 1 개의 황 원자로 구성되어 총 24 개의 원자로 형성된다. 3-(1-피리디니오)-1-프로판설포네이트 분자에는 총 24 개의 화학결합이 있으며, 이는 13 개의 비수소결합, 8 개의 다중결합, 4 개의 단일결합, 2 개의 이중결합, 6 개의 방향족결합, 1 개의 6원자 고리, 1 개의 양전하를 띠는 질소, 1 개의 술폰산염(티오-/다이티오-) 그리고 1 개의 피리딘로 구성되어 있다. 3-(1-피리디니오)-1-프로판설포네이트의 구조 이미지는 아래와 같다.3-Pyridin-1-ium-1-yl-propane-1-sulfonate 2차원 구조3-Pyridin-1-ium-1-yl-propane-1-sulfonate 3차원 구조 3차원 분자 모형 및 구조 데이터 3-Pyridin-1-ium-1-yl-propane-1-sulfonate 3차원 분자모형분자 모형을 회전, 확대, 축소 등 다양한 기능을 통해서 보다 상세하게 살펴볼 수 있다. 추가 메뉴를 통해 반 데르 발스 표면 등을 시각화 하고 이미지 파일로 내보내는 등의 옵션을 활용할 수 있다. 반응형 3차원 구조 모형 바로가기 ]분자의 원자, 화학 결합, 연결성 및 좌표에 관한 정보 등이 포함된 구조 데이터 파일을 다운로드 할 수 있다. 대부분의 주요 화학 소프트웨어에서 지원된다. 구조 데이터 파일 다운로드 바로가기 ] 다른 이름(동의어) 또는 등록번호 S0391 S0813 15471-17-7 3-(Pyridin-1-ium-1-yl)propane-1-sulfonate 1-(3-Sulphonatopropyl)pyridinium더보기NDSB-201 3-(1-Pyridinio)-1-propanesulfonate 1-(3-Sulfopropyl)pyridinium hydroxide, inner salt 3-pyridin-1-ium-1-ylpropane-1-sulfonate Pyridinium, 1-(3-sulfopropyl)-, inner salt 1-(3-sulfonatopropyl)pyridin-1-ium 3-(1-Pyridino)-1-propane Sulfonate 3-[1-Pyridino]-1-propane Sulfonate Pyridinium, 1-(3-sulfopropyl)-, hydroxide, inner salt 1-(3-Sulfopropyl)pyridinium hydroxide inner salt W-108028 3-(1-Pyridinio)propanesulfonate 3-PYRIDINIUM-1-YLPROPANE-1-SULFONATE N-3-Sulfopropylpyridinium betaine PYRIDINE DRONESALT 1PS QN4I6AI9EK NDSB 201 7771AA ANW-71602 MFCD00064468 Sulfopropylpyridiniumhydroxideinner salt RTR-006448 3-(Pyridinium-1-yl)propane-1-sulfonate 1-(3-SULFOPROPYL) PYRIDINIUM, PPS 3-(1-pyridin-1-iumyl)-1-propanesulfonate Pyridinium,1-(3-sulfopropyl)-, inner salt 1,3-Sulfopropyl pyridinium hydroxide inner salt A809565 I14-4743 3-(1-Pyridinio)-1-propanesulfonate, >=97.0% (N) 1547-17-7 3-(Pyridin-1-ium-1-yl)propane-1-sulfonate 1-(3-Sulphonatopropyl)pyridinium NDSB-201 3-(1-Pyridinio)-1-propanesulfonate 1-(3-Sulfopropyl)pyridinium hydroxide, inner salt 3-pyridin-1-ium-1-ylpropane-1-sulfonate Pyridinium, 1-(3-sulfopropyl)-, inner salt 1-(3-sulfonatopropyl)pyridin-1-ium 3-(1-Pyridino)-1-propane Sulfonate 3-[1-Pyridino]-1-propane Sulfonate Pyridinium, 1-(3-sulfopropyl)-, hydroxide, inner salt 1-(3-Sulfopropyl)pyridinium hydroxide inner salt W-108028 3-(1-Pyridinio)propanesulfonate 3-PYRIDINIUM-1-YLPROPANE-1-SULFONATE N-3-Sulfopropylpyridinium betaine PYRIDINE DRONESALT 1PS QN4I6AI9EK NDSB 201 7771AA ANW-71602 MFCD00064468 Sulfopropylpyridiniumhydroxideinner salt RTR-006448 3-(Pyridinium-1-yl)propane-1-sulfonate 1-(3-SULFOPROPYL) PYRIDINIUM, PPS 3-(1-pyridin-1-iumyl)-1-propanesulfonate Pyridinium,1-(3-sulfopropyl)-, inner salt S0391 S0813 1,3-Sulfopropyl pyridinium hydroxide inner salt A809565 I14-4743 3-(1-Pyridinio)-1-propanesulfonate, >=97.0% (N)
0 notes
Photo

The rate that an amine transfer reaction proceeds at is boosted when it takes place in a glass vessel, as opposed to plastic one.1 The findings suggest that glass particles catalyse the Katritzky reaction by acting as a base, potentially offering a reusable green catalyst for this and possibly other reactions. Apparatus and experimental set-ups have been known to inadvertently affect reactions before. Last year researchers found that cleaned stirrer bars are often still contaminated with metal catalysts which can alter experiments. Meanwhile, molecular nitrogen – often used as an inert carrier gas – was found to accelerate reactions that use ruthenium-based catalysts. Katritzky reaction of pyrylium salts (TPP, TTP, TMP) and p-anisidine to form pyridinium salts. It was already known that glass containers are not inert and can influence certain reactions by shedding particles. For example, soda-lime glass, the most widely used for bottles and windows, has been shown to catalyse the polymerisation of methyl methacrylate.2 Borosilicate glass, such as Pyrex, can also catalyse the ionisation of xenon difluoride.3 Now, Yangjie Li and colleagues in Graham Cooks’ lab at Purdue University, US, have observed a new phenomenon. They found that glass containers and particles accelerate the Katritzky transamination reaction – which converts primary amines into pyridinium salts – at the solid–solution phase. #research #researchers #researchanddevelopment #womeninscience #liftedlife #phdstudentlife #phdchat #phdstudentsofinstagram #sciencecommunication #urbanresearch #science #phd #chemistry #scientist #harvard #sciences #scienceart (at National Institute of Technology Tiruchirappalli) https://www.instagram.com/p/CIPZJykjEyu/?igshid=1xmln0g33wfy1
#research#researchers#researchanddevelopment#womeninscience#liftedlife#phdstudentlife#phdchat#phdstudentsofinstagram#sciencecommunication#urbanresearch#science#phd#chemistry#scientist#harvard#sciences#scienceart
0 notes