#companion diagnostics market
Text

Testiwol is an online aggregator platform, a digital marketplace for diagnostic labs in India. Patients can book lab tests from their preferred lab at their convenient time and place. Testiwol provides a time-bound, one stop solution for home sample collection of all lab tests.
Testiwol's tagline "Your Lab @ Your Doorstep
Visit Us @ testiwol.com
@testiwol #testiwol
# #labtestapproved #testiwol #testiwollove #india #brand #yourlabatyourdoorstep #bloodtestonlinereport
#companion diagnostics market#diagnostic platforms#diagnostics company#health#in vitro diagnostics market#self diagnosed autism#testiwol#may allah cure testiwol @testiwol
2 notes
·
View notes
Link
#market research future#companion diagnostics market#companion diagnostics industry#companion diagnostics size#companion diagnostics
0 notes
Text
https://www.shtfsocial.com/blogs/142854/Companion-Diagnostics-Market-Analysis-Size-Share-and-Forecast-2031
The Companion Diagnostics Market in 2023 is US$ 6.83 billion, and is expected to reach US$ 16.22 billion by 2031 at a CAGR of 11.40%.
#Companion Diagnostics Market#Companion Diagnostics Market Report#Companion Diagnostics Market Research
0 notes
Text
https://www.htfmarketintelligence.com/report/global-companion-diagnostics-market
1 note
·
View note
Link
#adroit market research#companion diagnostics market#companion diagnostics 2020#companion diagnostics trends
0 notes
Link
North America dominated the global companion diagnostics market in 2018, however Asia Pacific is expected to grow at a highest pace through 2025...
#adroit market research#companion diagnostics market#companion diagnostics 2020#companion diagnostics trends
0 notes
Text
Global Companion Diagnostics Market is Anticipated to Progress at a CAGR of 12.84% by 2028

Triton Market Research presents the Global Companion Diagnostics Market report segmented by Consumer (Reference Laboratories, Pharmaceutical and Biopharmaceutical Companies, Other Consumers), Mechanism (Polymerase Chain Reaction, Next Generation Sequencing, In-Situ Hybridization, Immunohistochemistry, Other Mechanisms), Indicator (Neurology, Oncology, Infectious Disease, Other Indicators), Commodity (Software & Services, Assay Kits & Reagents), and Regional Outlook (Europe, Asia-Pacific, North America, Latin America, Middle East and Africa).
The report further discusses the Market Summary, Industry Outlook, Impact of COVID-19, Key Insights, Porter’s Five Forces Analysis, Market Attractiveness Index, Vendor Scorecard, Drivers, Challenges, Opportunities, Competitive Landscape, Research Methodology & Scope, Global Market Size, Forecasts & Analysis (2022-2028).
As per Triton’s research report, the global companion diagnostics market is estimated to witness growth at a CAGR of 12.84% during the forecast period 2022-2028.
A companion diagnostics device helps attain crucial data to efficiently match a drug or therapy. It is a personalized treatment that includes drug testing, clinical trials, and research.
Genome sequencing has emerged as a significant step in the gene therapy process. This has increased investments in next generation gene sequencing. Moreover, the rising research in the space is expected to promote the companion diagnostics market’s growth. Furthermore, the growing investments and research in precision medicine are also anticipated to elevate the demand for companion diagnostics tests, thereby opening new avenues for the studied market.
However, a weak reimbursement framework and common cases of leakage in oncology companion diagnostics restrict the companion diagnostics market’s growth.
Globally, the Asia-Pacific is expected to emerge as the fastest-growing region over the forecast period. The region is witnessing growth owing to the increasing development of precision medicine technology. Moreover, an initiative undertaken by regulatory authorities and the pharmaceutical industry to increase precision medicine development drives the demand for companion diagnostics tests. Above all, the increase in healthcare awareness and economic growth are expected to open new avenues for the companion diagnostics market.
Danaher Corporation, GE Healthcare, Roche Diagnostics, Arup Laboratories, Almac Group, Thermo Fisher Scientific, Genomic Health, Myriad Genetics, Illumina Inc, bioMerieux, Qiagen, Biocartis, Sysmex Corporation, Agilent, and Abbott Laboratories are leading companies in the companion diagnostics market.
Companies in the market offer relatively similar products with small variations in terms of efficiency and capabilities. As a result, establishments are seeking to develop products with new features. The new entrants are required to invest a significant amount in order to adopt new technology and establish workstations. On the other hand, companies active in the market allocate significant resources, covering the majority of initial expenditures. Hence, these factors indicate the threat of new entrants to be medium.
#companion diagnostics market#companion diagnostics#Lifescience industry#diagnostics devices#triton market research#market research reports
0 notes
Link
Adroit Market Research report on global companion diagnostics market gives a holistic view of the market from 2015 to 2025, which includes factors such as market drivers, restraints, opportunities and challenges.
#adroit market research#companion diagnostics market#companion diagnostics 2020#companion diagnostics trends
0 notes
Text
Impact of Companion Diagnostics on Healthcare in 2030
1. What is Companion Diagnostics: What are they and how have they evolved?Companion diagnostics are laboratory tests used to identify which patients are most likely to benefit from a certain drug or therapy. They allow physicians to tailor treatments to individuals based on the genetic or molecular features of their disease. They have evolved over the past decade as the technology used to analyze…
View On WordPress
#Companion Diagnostics#Companion Diagnostics Market#Impact of Companion Diagnostics on Healthcare#Impact of Companion Diagnostics on Healthcare in 2030
0 notes
Link
#market research future#companion diagnostics market#companion diagnostics industry#companion diagnostics size#companion diagnostics
0 notes
Text

Locate us in your neighborhood
Testiwol are now just a stone's throw away!
Book now your test
#testdoc #testiwol #testpharma #testdoc #Testlo #testscan
#companion diagnostics market#diagnostic platforms#diagnostics company#health#in vitro diagnostics market#testiwol#self diagnosed autism#may allah cure testiwol @testiwol
0 notes
Text
Companion Diagnostics Market Opportunities, Production Cost Analysis, Market Development and Market Dynamics Forces
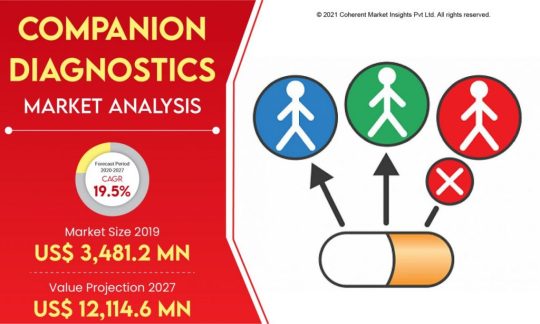
A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a corresponding drug or biological product. The test helps a health care professional determine whether a particular therapeutic product’s benefits to patients will outweigh any potential serious side effects or risks.
Companion diagnostics can:
• identify patients who are most likely to benefit from a particular therapeutic product;
• identify patients likely to be at increased risk for serious side effects as a result of treatment with a particular therapeutic product; or
• monitor response to treatment with a particular therapeutic product for the purpose of adjusting treatment to achieve improved safety or effectiveness.
Know More: https://globaltrendingorientedreports.blogspot.com/2022/09/growing-prevalence-of-cancer-to-augment.html
0 notes
Text
The Companion Diagnostics Market in 2023 is US$ 6.83 billion, and is expected to reach US$ 16.22 billion by 2031 at a CAGR of 11.40%.
#Companion Diagnostics Market#Companion Diagnostics Market Overview#Companion Diagnostics Market Report
0 notes
Link
The growing incidences of cancer cases are likely to surge the demand for companion diagnostic cancer biomarkers that can generate significant revenue in the coming period. The rising need for targeted therapy is recognized as another salient cause that may strengthen the industry. In addition, the growing number of clinical trials is anticipated to present various growth opportunities, which is expected to develop trade during the assessment period.
0 notes
Text
Companion Diagnostics Market: What’s New?
With rising health consciousness and expenditure, precision medicine has emerged as a major solution to treating chronic conditions. Estimates indicate that the personalized medicine segment is expected to account for around 40% by the end of 2022, with oncology being a major therapeutic area. As a result, this growth is expected to support the expansion of the companion diagnostics market as CDx tests identify the right drug for a specific patient pool.
Triton Market Research’s report infers that the global companion diagnostics market is expected to progress at a CAGR of 12.84% during the forecast period 2022-2028.
Presently, medical treatments are typically designed using uniform patterns, with patients with the same disease undergoing matching treatments. This trial-and-error style often becomes time-consuming and ineffective. Hence, the development of precision medicine with the help of CDx widens the studied market’s scope.

CDx Development: Technological Advancements Backs Regional Growth
In addition to a growing focus on precision medicine, the expansion of the companion diagnostics market is also fueled by technological advancements. Top trends that support the market’s growth globally, especially in the Asia-Pacific, include:
Predictive Biomarkers have proven vital in calculating responses to certain therapeutic interventions. For instance, the Oncotype Dx and MammaPrint tests and KRAS employ predictive biomarkers, providing the most effective treatment to breast and colorectal cancer patients. Estimates suggest that the oncology category secures the majority of shares in the market in terms of indication. As a result, biomarkers have gained significant prominence among oncologists across nations, including India. For instance, Tata Memorial Hospital, NIMHANS, and AIIMS are striving to identify new genes and biomarkers, ultimately generating opportunities for the Asia-Pacific companion diagnostics market.
Polymerase Chain Reaction (PCR) leads the mechanism segment in terms of revenue share in 2022. It is projected to evolve at a CAGR of 13.13% during the forecasted period. Factors such as cost-efficient, faster turnaround time, and ease of application have supported its dominance in neurology and oncology applications. Besides this, PCR-based CDx has witnessed substantial growth due to approvals such as BRACAnalysis CDx by Myriad Genetics. Such developments are expected to influence market players to expand their PCR capabilities, augmenting the companion diagnostics market’s development.
Next Generation Sequencing is another major technology fueling the CDx market development. Since NGS permits rapid sequencing, it has supported major advances in determining the molecular basis of various chronic conditions. NGS is expected to become the fastest-growing segment in the mechanism category. In this regard, PCR techniques are expected to support the success of NGS technology in the upcoming years. To illustrate, NGS diagnosis and PCR techniques offer high-precision care with less turnaround time. The rising competency in genome sequencing has also generated substantial opportunities for vendors like Shuwen Biotech and Simcere Diagnostics in the Chinese companion diagnostics market.
Competitive Landscape: Strategic Highlights & Latest CDx Approvals
The inclusion of analytics and big data has helped identify various biomarker-based illness indications regularly. This has triggered businesses to enter the market through innovations. Moreover, since it’s easy to shift from one product to another, the competition among existing players has elevated over the years. Such developments have thus led to the advent of new strategies to launch and gain approvals for their CDx devices.
In August 2022, Roche Diagnostics gained approval from FDA for VENTANA MMR RxDx Panel, the first immunohistochemistry CDx test. Based on cancer biomarkers, it helps detect solid tumor patients.
Foundation Medicine’s FoundationOne CDx and Liquid CDx were authorized with various companion diagnostics claims by the FDA in June 2022.
In May 2022, BioMerieux announced approval from FDA for its BIOFIRE Joint Infection Panel. The panel tests for 31 pathogens implicated in acute joint infections and contains 8 AMR genes to optimize therapy.
Evotec SE and BioMeriux, in July 2022, declared their partnership to create the next generation of antimicrobial and actionable diagnostics to prevent antimicrobial resistance.
In July 2021, Illumina expanded its oncology partnerships with Bristol Myer Squibb, Kura Oncology, Myriad Genetics, and Merck. This joint venture aims to develop tools for precision oncology.
Growth Prospects: CDx for Targeted Cancer Medication
Earlier, the commercial success of Herceptin and Gleevec revolutionized companion diagnostics globally. However, the increased prevalence of cancer cases has led to a dramatic shift in the drug development process, from uniform to precision medicine approach, increasing the need for new CDx solutions. Hence, the demand for predictive biomarkers for targeted cancer therapeutics is expected to take center stage, thereby creating opportunities for the companion diagnostics market.
#Companion Diagnostics Market#companion diagnostics#healthcare#diagnostic#biotechnology#life sciences#market research report#market research reports#triton market research
0 notes