#ebastine indication
Text
Ebastine Drug
Medical information for Ebastine on Pediatric Oncall including Mechanism, Indication, Contraindications, Dosing, Adverse Effect, Interaction, Hepatic Dose.
#Ebastine#medication#medications#medicine#drug#drugs#drug information#medical information#drug index#drug center#pediatric dose#Antishistamine#ebastine mechanism#ebastine indication#ebastine contraindications
0 notes
Text
Skin Allergy: Main Causes And How To Treat
Skin allergy is an inflammatory reaction that can manifest itself in different regions of the skin, such as hands, feet, face, arms, armpits, neck, legs, back or belly, causing symptoms such as redness, itching and white or red spots on the skin. Also, in some cases skin allergy can lead to other problems such as allergic swelling, for example.
Skin allergy can have different causes, such as allergy to deodorant, medication, food, sun, insect bites or even an allergy to sunscreen, and its treatment can be done with the use of antihistamine drugs such as desloratadine or ebastine, for example, indicated by the allergy specialist doctor in Kolkata.
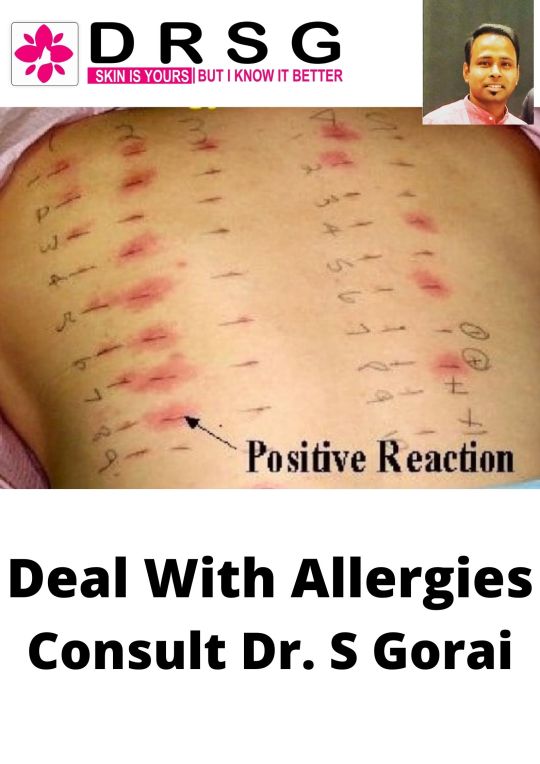
Main symptoms
The main symptoms of skin allergy include:
· Itch
· Redness
· Peeling
· Irritation
· Presence of spots or pimples (reddish or white spots).
These symptoms may appear within minutes of contact with the allergen, but may also take several hours and even days to fully develop. Thus, you should try to remember the objects or substances that were in contact with the region in the last 3 days, or the medicines or foods you ate, to try to find a cause.
What to do when symptoms arise
It is crucial to act quickly when allergy symptoms start. Wash affected areas with lots of water and soap that has a neutral pH. To relieve irritation and reduce discomfort, use hypoallergenic products that have soothing agents, such as creams or lotions that contain chamomile or lavender to wash these areas. This will also help to maintain the skin's hydration.
In addition, thermal Water is also an excellent option to use in these situations, as it hydrates the skin and reduces itching and irritation.
If the symptoms persist after 2 hours of washing and moisturizing, or if they become more severe or bothersome over time, you should consult a doctor to get medication.
0 notes
Link
Ebastine is an anti-histaminic medicine that is being used to treat allergic rhinitis, chronic urticaria, and common allergies. These allergies include runny nose, skin rashes, watery eyes, insect […]
0 notes
Text
Ddu College–China’s Import and Export Market Report of Antiallergic Drugs
Antiallergic/ anti-allergy pharmaceutical drugs are used to treat allergic diseases (allergies) and can be divided into the following categories:
1. Antihistamine drugs: loratadine, promethazine and chlorpheniramine etc.
2. Allergic medium blockers: sodium cromoglycate, ketotifen etc.
3. Others: ① calcium salts, such as calcium chloride, calcium gluconate; ② herbal desensitizer, such as injectiondermatophagoides farinae; ③ glucocorticoid etc.
Allergies, also known as allergic diseases, are a number of conditions caused by hypersensitivity of the immune system to something in the environment that usually causes little or no problems to most people. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing and a runny nose, shortness of breath or swelling. Food intolerances and food poisoning are separate conditions.
Today, Ddu, the leading global pharmaceutical and medical device B2B online platform, will share China’s Import and Export Market Report of Antiallergic Drugs with you.
I. General situation of global market of antiallergic drugs
According to the statistics of WHO, the global antiallergic drugs market was 5.215 billion USD in 2016, of which anti-histamine drugs accounted for 66%, allergic mediator products accounted for 20% and other anti-allergic drugs accounted for 14%.
Antihistamine drugs, the most widely used pharmaceutical drugs in clinical application, treat allergic rhinitis and other allergies. Antihistamines provides relief from nasal congestion, sneezing or hives caused by pollen, dust mites or animal allergies.
In 2016 the top 5 antihistamines used in the world were desloratadine citrate disodium tablets, loratadine, ebastine, levocetirizine and cetirizine.
In the past few years most transnational pharmaceutical companies have devoted themselves to the development of antitumor drugs, antivirus drugs, rare drugs and targeted drugs rather than the development of antiallergic drugs resulting in a sluggish market.
As indicated by the statistics above, eight out of ten of antiallergic drug sales were flat or falling and no sales exceeded one million USD in 2016.
In addition to this, the sales for Singulair, the blockbuster medication from MSD, topped the list with 0.9 billion USD. Singulair was approved for the treatment of anaphylactic rhinitis and asthma, becoming a highly recommended drug for treatment guidelines in many countries.
Currently, the key antiallergic drug markets are the United States, France, Germany, Italy, Spain, the United Kingdom, Japan, China and India and this is expected to grow.
Foreign-funded brands take up major shares in the global antiallergic drugs market. Loratadine, antiallergic drug from Schering-Plough in the United States, is currently being sold in over 110 countries and regions as a non–prescription drug, becoming the best-selling antiallergic drug in the world.
II. China’s antiallergic drugsmarket
According to the statistics of PDB, China’s overall antiallergic drug sales were 5.2 billion RMB in 2016, with an increase of 5.1% over the previous year.
The top ten companies in the antiallergic drugs market are mostly domestic funded companies and account for 51.6% of market shares with relatively high market concentration.
There were four foreign-funded companies. Bayer HealthCare, with its products being very popular in the retail market, ranked first for two consecutive years , which shows an increasing trend in 2017.
There were six domestic-funded companies. Yangtze River Pharmacy and Hainan Poly Pharmaceutical ranked second and third with market shares of 8.1% and 6.4% , which also shows an increasing trend in 2017.
Claritin (loratadine tablets) held dominant position and ranked first for two consecutive years with a stable market share of 10.3% in 2015 and 10.5% in 2016. Desloratadine citrate disodium tablets from Yangtze River Pharmacy, ranked second for two consecutive years with a market share of 5.1% in 2015 and 6.8% in 2016. There was fierce competition among the other top ten brands.
III. General situation of China’s antiallergic drug imports
Loratadine tablets took up most of China’s imported antiallergic drug shares with the main products being Claritin from Byers Healthcare and Astemizole from Johnson & Johnson.
According to the statistics of China customs, China mainly imported antiallergic drugs from France, the United States and Switzerland as indicated on the chart below.
IV. General situation of China’s antiallergic drug exports
As the Chinese pharmaceutical drugs market is integrating with the world market, the product structure of antihistamines, especially piperidines, monoethanolamides, alkyl amine, phenothiazines and piperazine, have gradually improved. What’s more, with product upgrades, first generation antihistamines have been developed to third generation antihistamines, accelerating the export of these drugs.
According to the statistics of China customs, China’s antihistamine drugs were mainly exported to Spain, the United States and Belgium in 2016 and export sales of top 10 counties totaled 21.06 million USD.
V. Leading antiallergic drug companies
Hainan Poly Pharm., Ltd.
Hainan Poly Pharm. Co., Ltd. is a leading international Chinese pharmaceutical preparation company.
Representative product: Desloratadine tablets
Indication: Anaphylactic rhinitis
Main exporting countries: Germany, Holland, France
Yangze River Pharmaceutical Group
Founded in 1971, YRPG is a giant national cross-regional pharmaceutical group, integrating research, manufacturing and trading. It is listed as one of the first innovative enterprises by China MIIT.
Representative product: Desloratadine citrate disodium tablets
Indication: Anaphylactic rhinitis
Main exporting countries: EU, US
Zhejiang Wolw bio-pharmaceutical Co., Ltd
Zhejiang Wolw bio-pharmaceutical Co., Ltd has the largest allergen drug development base in Asia and is China's largest desensitization drug manufacturer.
Representative product: Dermatophagoides farinae drops
Indication: Dermatophagoides farinae allergy
Main exporting countries: Asian-Pacific region
VI. OTC antiallergic drugs are to be the next blockbuster.
With more investment in the market and an increasing awareness by users, the OTC market has become favored by more and more pharmaceutical manufacturers.
In terms of the domestic market, antiallergic drugs are rarely seen in the OTC market and manufacturers still need to step up their consumption guidance efforts. However, with the improvement of self-treatment, antiallergic pharmaceutical drugs are expected to take up a greater share in the OTC market and become the new main force within the next two to three years.
Thus, Ddu, the leading global pharmaceutical and medical device B2B online platform, advises domestic companies to adapt to market demands with diversified products and seize the opportunity to upgrade the structure of products and devote themselves to the development of drugs that help adjust the immune system and alleviates allergy symptoms.
Source: http://medicalhealthinformation.31722.n8.nabble.com/Ddu-College-China-s-Import-and-Export-Market-Report-of-Antiallergic-Drugs-td2.html
1 note
·
View note
Text
Ebatin Fast 10mg 10pcs
Ebatin Fast 10mg 10pcs
Indications Ebastine is indicated for the symptomatic treatment of: Seasonal and perennial allergic rhinitis, Idiopathic chronic urticaria. Therapeutic Class Non-sedating antihistamines Pharmacology Ebastine, a piperidine derivative, is a long-acting, nonsedating, second-generation histamine receptor antagonist that binds preferentially to peripheral H1 receptors. It is metabolised to active…
View On WordPress
0 notes
Text
Validation of Assay for Simultaneous Estimation of Ebastine and Montelukast in Tablet Dosage Forms by RP-HPLC Method
INTRODUCTION
Ebastine is a second-generation H1 receptor antagonist that is indicated mainly for allergic rhinitis and chronic idiopathic urticaria. It is chemically known as 1-(4-tert-butylphenyl)-4- butan-1-one. Figure 1. It is soluble in methanol, chloroform, and dimethyl sulfoxide. Ebastine and its active metabolite is selective peripheral histamine H1 receptor antagonist. Thus it prevents the attachment of histamine on receptors and its activation (Activation of receptors of histamine on various tissues produce various allergic symptoms e.g. a Runny nose). Ebastine also has a specific inhibitory effect on Th2-type cytokine production and inhibit T cell migration and pro-inflammatory cytokine production by T cells and macrophages.
Montelukast is a leukotriene receptor antagonist (LTRA) used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies. It is chemically known as Sodium; 2- phenyl]-3- propyl] sulfanylmethyl] cyclopropyl] acetate. Figure 2. It is freely soluble in ethanol, methanol, and water. Montelukast blocks the action of leukotriene D4 on the cysteinyl leukotriene receptor CysLT1 in the lungs and bronchial tubes by binding to it. This reduces the bronchoconstriction otherwise caused by the leukotriene and results in less inflammation.
A detailed survey of the literature for Ebastine and Montelukast reveals that the available analytical methods are costly and with more retention time. Hence we developed a rapid and sensitive RP-HPLC method with UV detection (244 nm) for routine analysis of montelukast sodium and ebastine in a pharmaceutical formulation (Ebast-M). A literature review revealed few methods on method development and validation of Ebastine and Montelukast by RP-HPLC. So now the main aim is to develop a method with less run time and retention time compared to those methods.1-7
MATERIAL AND METHOD
Instruments
HPLC from Waters with model No HPLC 2965 system with Empower 2 software.
Materials
Ebastine and Montelukast (API) were received from spectrum lab, Combination Ebastine and Montelukast (EBAST M TABLET) tablets were obtained from Micro Labs, Distilled water (HPLC grade), acetonitrile, ammonium acetate buffer, methanol, Potassium dihydrogen phosphate buffer, Triethylamine, orthophosphoric acid (HPLC grade) were obtained from MERCK.
Methods
Diluent
Based upon the solubility of the drugs, diluent was selected, Methanol and Water were taken in the ratio 50:50.
Preparation of Standard Stock Solutions
Accurately Weighed and transferred 10mg and 10mg of Ebastine and Montelukast working Standards into 10ml and 10ml clean dry volumetric flasks separately, add 3/4th volume of diluent, sonicated for 30 minutes and makeup to the final volume with diluents.
Preparation of Standard Working Solutions (100% solution)
From the above each stock solution, 1 ml was pipetted out into a 10ml volumetric flask and then makeup to the final volume with diluent.
Preparation of Sample Stock Solutions
20 tablets were weighed and calculate the average weight of each tablet then the tablet powder weight equivalent to 10 mg of Ebastine and 7.5 mg of Montelukast was transferred into a 10ml volumetric flask, 7ml of diluent added and sonicated for 30 min, further the volume made up with diluent and filtered.
Preparation of Sample Working Solutions (100% solution)
From the filtered solution, 1ml was pipetted out into a 10 ml volumetric flask and made up to 10ml with diluent.
Preparation of Buffer
1ml of OPA was taken in 1000 ml volumetric flask and makeup to the mark with milli-Q water.
Preparation of Buffer: 0.01N Potassium dihydrogen orthophosphate (pH 4.8)
Accurately weighed 1.36gm of Potassium dihydrogen orthophosphate in a 1000ml of Volumetric flask add about 900ml of milli-Q water added and degas to sonicate and finally make up the volume with water the pH was adjusted to 4.8 with Orthophosphoric acid.
RESULTS AND DISCUSSION
Method Development
Table 1: Different trials were performed by changing Mobile phase and buffer
Trials
Column Used
Mobile phase
Buffer
Flow rate
Wave length
Temperature
Injection Volume
Trial: 1
Discovery 250 x 4.6 mm, 5m.
Water: Methanol (50:50)
1ml/min
244nm
25 ͦ C
10µl
Trial: 2
Discovery 250 x 4.6 mm, 5m.
Water: Acetonitrile (50:50)
Water
1ml/min
244nm
30 ͦ C
10µl
Trial: 3
Discovery 250 x 4.6 mm, 5m.
buffer: ACN (60:40)
0.1%OPA
1ml/min
244nm
30 ͦ C
10µl
Trial: 4
Discovery 250 x 4.6 mm, 5m.
buffer: Acetonitrile (60:40)
0.01N KH2PO4 (4.8) solution
1ml/min
244nm
30 ͦ C
10µl
Trial: 5
buffer: Acetonitrile (70:30A)
buffer: Acetonitrile (70:30)
0.01N KH2PO4 (4.8) solution
1ml/min
244nm
30 ͦ C
10µl
Optimized Method
Kromosil 250 x 4.6 mm, 5m.
Buffer: Acetonitrile (60:40)
0.01N KH2PO4 (4.8) solution
Diluent : Water: ACN: (50:50)
1.0ml/min
244nm
30 ͦ C
10µl
Table 2: Optimization of chromatographic conditions
Trials
Observation
Trial: 1
Ebastine peak was eluted but Montelukast peak was not eluted and peak shape also not good so further trial is carried out.
Trial: 2
Peaks were eluted but peak shape was not good and baseline disturbances hump, USP plate count were not good so further trial is carried out.
Trial: 3
Both peaks were eluted but resolution was less so further trial is carried out.
Trial: 4
Retention time is more and ebastin eluted at void range so further trial is carried out.
Trial: 5
Increasing buffer ratio montelukast retention time is more and ebastine eluted at void range so further trial is carried out.
Optimized Method
Drugs were eluted with good retention time, resolution; all the system suitable parameters like Plate count and Tailing factor were within the limits. Peak shape and retention time is good so, further process is carried out.
Method Validation
The present study was carried method was validated based on ICH (Q2B) parameters.8
The following parameters were validated for the proposed method.
System Suitability
All the system suitability parameters are within range and satisfactory as per ICH guidelines. Table 3
Discussion: According to ICH guidelines plate count should be more than 2000, tailing factor should be less than 2 and resolution must be more than 2. All the system suitable parameters were within the limits.
Discussion: Retention times of Ebastine and Montelukast were 2.447 min and 3.436 min respectively. We did not find any interfering peaks in blank and placebo at retention times of these drugs in this method. So this method was said to be specific.
Linearity
Six Linear concentrations of Ebastine (25-
150ppm) and Montelukast (20-120ppm) were prepared and injected. Regression equation of the Ebastine and Montelukast were found to be, y = 19263x +1149, and y = 19946x + 1095 and the regression coefficient was 0.999. Table 4 Figure 4 & 5
Precision
Intraday precision (Repeatability): Intraday Precision was performed and % RSD for Ebastine and Montelukast were found to be 0.2% and 0.2% respectively. Table 5
Inter-day precision: Inter-day precision was performed with 24 hrs time lag and the %RSD Obtained for Ebastine and Montelukast were 0.3% and 0.2%. Table 6
Accuracy
Three concentrations 50%, 100%, 150%, were injected in a triplicate manner and amount Recovered and % Recovery was displayed in Table 7. Figure 6-8
Robustness
Small deliberate changes in a method like Flow rate, mobile phase ratio, and temperature are made but there were no recognized change in the result and are within range as per ICH Guidelines. Table 6 Figure 9 & 10
Discussion: Robustness conditions like Flow minus (0.9ml/min), Flow plus (1.1ml/min), mobile phase minus (65B:35A), mobile phase plus (55B:45A), temperature minus (25°C) 3and temperature plus (35°C) was maintained and samples were injected in a duplicate manner. System suitability parameters were not much affected and all the parameters were passed. %RSD was within the limit.
Assay
Standard preparations are made from the API and Sample Preparations are from Formulation (EBAST M TABLET). Both sample and standards are injected six homogeneous samples. The drug in the formulation was estimated by taking the standard as the reference. The Average % assay was calculated and found to be 99.05% and 99.20% for Ebastine and Montelukast respectively. Table 7
Degradation Studies
Standards and degraded samples are injected and calculated the percentage of drug degraded in solution by applying different conditions like acid, alkali, and oxidative, photolytic, thermal and neutral analysis. Table 8 Figure 11-15
Table 3: System Suitability Studies of Ebastine and Montelukast
Property
Ebastine
Montelukast
Retention time (tR)
2.447min
3.436min
Theoretical plates (N)
8019 ± 63.48
10040 ± 63.48
Tailing factor (T)
1.37 ± 0.117
1.33 ± 0.117
Table 4: Calibration Data of Ebastine and Montelukast Method
S.No
Concentration
Ebastine (µg/ml)
Response
Concentration
Montelukast (µg/ml)
Response
1
0
0
0
0
2
25
533631
20
478732
3
50
987156
40
956442
4
75
1467357
60
1501069
5
100
1976938
80
1885033
6
125
2503069
100
2386656
7
150
3011189
120
2913242
Table 5: Repeatability results for Ebastine and Montelukast
Sl. No.
Ebastine
Montelukast
1
1958452
1868712
2
1957170
1870107
3
1952368
1865840
4
1959570
1870800
5
1953581
1865343
6
1952026
1859653
Mean
1955528
1866743
S.D.
3274.9
4115.7
%RSD
0.2
0.2
*Average of six determinations
Table 6: Inter-Day Precision Results for Ebastine and Montelukast
S. No.
Ebastine
Montelukast
1
2159276
1882066
2
2168976
1884258
3
2165538
1892454
4
2158679
1885947
5
2162743
1875128
6
2157355
1882066
Mean
2162095
1883653
S.D
4510.1
5671.0
%RSD
0.2
0.3
Table 7: Table of Accuracy
Sample
Concentration (%) (µg/ml)
Recovery (%)
Mean % Recovery
%RSD
Ebastine
50
101.07
99.93%
0.07
100
98.93
0.33
150
99.81
0.30
Montelukast
50
100.8
99.69%
0.08
100
99.63
0.74
150
98.64
0.52
Table 8: Robustness Data of Ebastine and Montelukast
S. No
Robustness condition
Ebastine
%RSD
Montelukast
%RSD
1
Flow minus (0.9ml/min)
0.1
0.2
2
Flow Plus (1.1ml/min)
0.4
0.5
3
Mobile phase minus (65:35)
0.3
0.3
4
Mobile phase Plus (55:45)
0.2
0.1
5
Temperature minus (250c)
0.2
0.2
6
Temperature Plus (300c)
0.3
0.1
Table 9: Assay of Tablet
S. No.
Ebastine %Assay
Montelukast % Assay
1
99.15
98.91
2
99.23
99.19
3
99.00
98.10
4
99.26
99.84
5
98.97
99.46
6
98.67
99.70
AVG
99.05
99.20
S.D
0.2184
0.6339
% RSD
0.2
0.64
Table 10: Different Types of Degradation Studies
Types of Degradation
EBASTINE
Area
%
Recovered
%
Degraded
Acid
1800991
95.56
4.44
Base
1833505
97.28
2.72
Peroxide
1853657
98.35
1.65
Thermal
1871146
99.28
0.72
UV
1868750
99.15
0.85
Water
1867367
99.08
0.92
MONTELUKAST
Acid
1867882
95.28
4.72
Base
1910567
97.45
2.55
Peroxide
1930866
98.49
1.51
Thermal
1950620
99.50
0.50
UV
1951371
99.54
0.46
Water
1947871
99.36
0.64
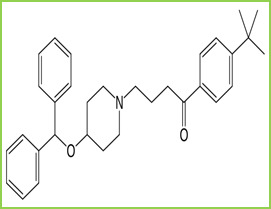
Figure 1: Structure of Ebastine
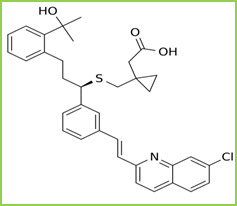
Figure 2: Structure of Montelukast
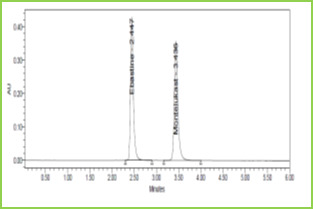
Figure 3: Typical chromatogram of Ebastine and Montelukast
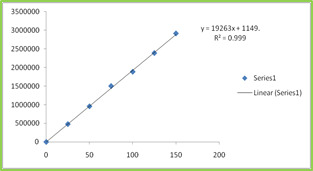
Figure 4: Calibration curve of Ebastine
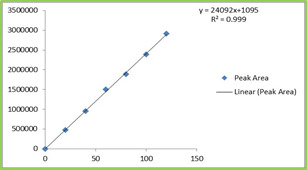
Figure 5: Calibration curve of Montelukast
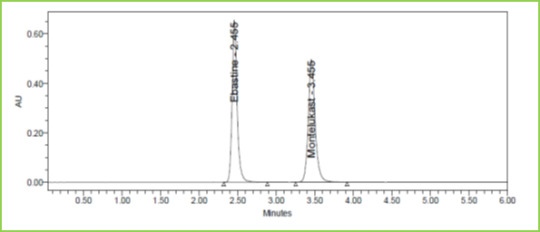
Figure 6: Accuracy 50% Chromatogramof Ebastine and Montelukast
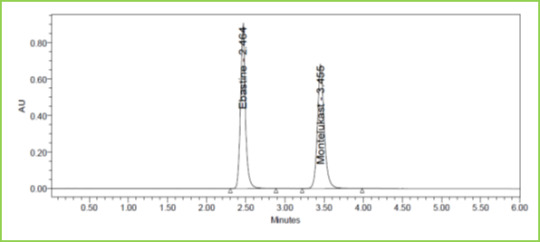
Figure 7: Accuracy 100% Chromatogram of Ebastine and Montelukast
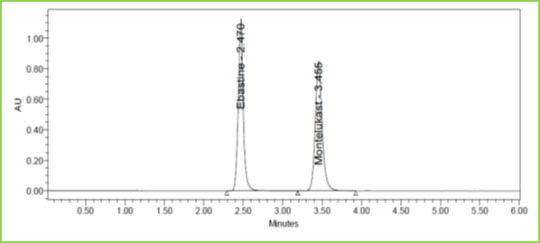
Figure 8: Accuracy 150% Chromatogram of Ebastine and Montelukast
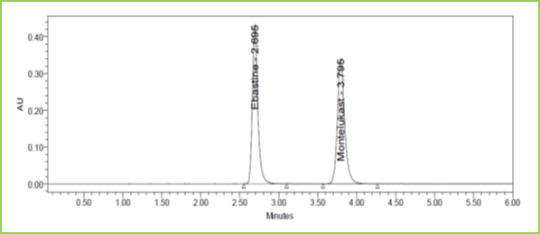
Figure 9: Flow minus Chromatogram of Ebastine and Montelukast
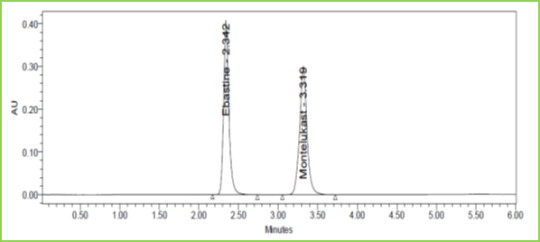
Figure 10: Flow plus Chromatogram of Ebastine and Montelukast
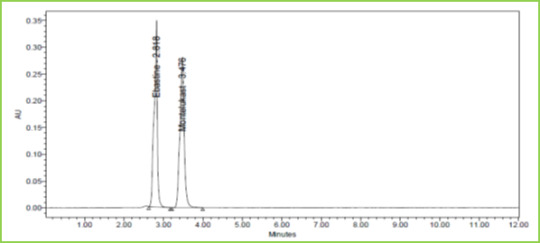
Figure 11: Chromatogram showing Acid degradation
CONCLUSION
A new method was established for simultaneous estimation of Ebastine and Montelukast by RP-HPLC method. The proposed HPLC method was found to be simple, specific, precise, accurate, rapid and economical for simultaneous estimation of Ebastine and Montelukast in the pharmaceutical dosage form. The developed method was validated in terms of accuracy, precision, linearity, robustness, and ruggedness, and results will be validated statistically according to ICH guidelines. The Sample recoveries in all formulations were in good agreement with their respective label claims. Hence the suggested RP-HPLC method can be used for routine analysis of Ebastine and Montelukast in API and Pharmaceutical dosage form.
ACKNOWLEDGMENTS
I am very much thankful to the management of Seven Hills College of Pharmacy for providing the necessary facilities to carry out the research work and for their encouragement and support.
REFERENCES
Rana, N. S., Rajesh, K. S., Patel, N. N., Patel, P. R., Limbachiya, U., & Pasha, T. Y. (2013). Development and validation of an RP-HPLC method for the simultaneous estimation of montelukast sodium and ebastine in tablet dosage form. Indian Journal of Pharmaceutical Sciences, 75(5), 599.PMid:24403662, PMCid:PMC3877523
Singh, R. M., Saini, P. K., Mathur, S. C., Singh, G. N., & Lal, B. (2010). Development and validation of an RP-HPLC method for estimation of montelukast sodium in bulk and in tablet dosage form. Indian Journal of Pharmaceutical Sciences, 72(2), 235.https://doi.org/10.4103/0250-474X.65023 ,PMid:20838530 , PMCid:PMC2929785
Yadav, O. M., & Jain, H. K. (2014). RP-HPLC Method Development and Validation for Simultaneous Estimation of Phenylephrine Hydrochloride and Ebastine in Tablet Dosage Form. International Journal of Pharmacy and Pharmaceutical Sciences, 6(8), 466-470.
Savsani, J. J., Goti, P. P., & Patel, P. B. (2012). Development and validation of simultaneous equation method for estimation of ebastine and montelukast sodium in combined tablet dosage form. Der Pharmacia Sinica, 3(6), 690-698.
Shrikrishna, B., & Nisharani, R. (2015). Analytical Method Development and Validation for Simultaneous Estimation of Montelukast and Ebastine by HPLC. Research Journal of Pharmacy and Technology, 8(1), 1. https://doi.org/10.5958/0974-360X.2015.00001.3
Singh, K., Bagga, P., Shakya, P., Kumar, A., Khalid, M., Akhtar, J., & Arif, M. (2015). Validated UV Spectroscopic Method for Estimation of Montelukast Sodium. International Journal of Pharmaceutical Sciences and Research, 6(11), 4728-4732.
Thakor K. A, Pasha, T. Y., Patel P. U., Chauhan R. J., Patel N. H. (2014). Development and validation of analytical method for simultaneous estimation of ebastine and phenylephrine hydrochloride in tablet dosage form. International Bulletin of Drug Research, 4(7): 16-40, 2014.
8. ICH Harmonised Tripartite Guideline, validation of analytical procedures: Text methodology, Q2 (R1) (2005). International Conference on Harmonization, Geneva, pp: 1-13.
Read the full article
0 notes
Text
Validation of Assay for Simultaneous Estimation of Ebastine and Montelukast in Tablet Dosage Forms by RP-HPLC Method
INTRODUCTION
Ebastine is a second-generation H1 receptor antagonist that is indicated mainly for allergic rhinitis and chronic idiopathic urticaria. It is chemically known as 1-(4-tert-butylphenyl)-4- butan-1-one. Figure 1. It is soluble in methanol, chloroform, and dimethyl sulfoxide. Ebastine and its active metabolite is selective peripheral histamine H1 receptor antagonist. Thus it prevents the attachment of histamine on receptors and its activation (Activation of receptors of histamine on various tissues produce various allergic symptoms e.g. a Runny nose). Ebastine also has a specific inhibitory effect on Th2-type cytokine production and inhibit T cell migration and pro-inflammatory cytokine production by T cells and macrophages.
Montelukast is a leukotriene receptor antagonist (LTRA) used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies. It is chemically known as Sodium; 2- phenyl]-3- propyl] sulfanylmethyl] cyclopropyl] acetate. Figure 2. It is freely soluble in ethanol, methanol, and water. Montelukast blocks the action of leukotriene D4 on the cysteinyl leukotriene receptor CysLT1 in the lungs and bronchial tubes by binding to it. This reduces the bronchoconstriction otherwise caused by the leukotriene and results in less inflammation.
A detailed survey of the literature for Ebastine and Montelukast reveals that the available analytical methods are costly and with more retention time. Hence we developed a rapid and sensitive RP-HPLC method with UV detection (244 nm) for routine analysis of montelukast sodium and ebastine in a pharmaceutical formulation (Ebast-M). A literature review revealed few methods on method development and validation of Ebastine and Montelukast by RP-HPLC. So now the main aim is to develop a method with less run time and retention time compared to those methods.1-7
MATERIAL AND METHOD
Instruments
HPLC from Waters with model No HPLC 2965 system with Empower 2 software.
Materials
Ebastine and Montelukast (API) were received from spectrum lab, Combination Ebastine and Montelukast (EBAST M TABLET) tablets were obtained from Micro Labs, Distilled water (HPLC grade), acetonitrile, ammonium acetate buffer, methanol, Potassium dihydrogen phosphate buffer, Triethylamine, orthophosphoric acid (HPLC grade) were obtained from MERCK.
Methods
Diluent
Based upon the solubility of the drugs, diluent was selected, Methanol and Water were taken in the ratio 50:50.
Preparation of Standard Stock Solutions
Accurately Weighed and transferred 10mg and 10mg of Ebastine and Montelukast working Standards into 10ml and 10ml clean dry volumetric flasks separately, add 3/4th volume of diluent, sonicated for 30 minutes and makeup to the final volume with diluents.
Preparation of Standard Working Solutions (100% solution)
From the above each stock solution, 1 ml was pipetted out into a 10ml volumetric flask and then makeup to the final volume with diluent.
Preparation of Sample Stock Solutions
20 tablets were weighed and calculate the average weight of each tablet then the tablet powder weight equivalent to 10 mg of Ebastine and 7.5 mg of Montelukast was transferred into a 10ml volumetric flask, 7ml of diluent added and sonicated for 30 min, further the volume made up with diluent and filtered.
Preparation of Sample Working Solutions (100% solution)
From the filtered solution, 1ml was pipetted out into a 10 ml volumetric flask and made up to 10ml with diluent.
Preparation of Buffer
1ml of OPA was taken in 1000 ml volumetric flask and makeup to the mark with milli-Q water.
Preparation of Buffer: 0.01N Potassium dihydrogen orthophosphate (pH 4.8)
Accurately weighed 1.36gm of Potassium dihydrogen orthophosphate in a 1000ml of Volumetric flask add about 900ml of milli-Q water added and degas to sonicate and finally make up the volume with water the pH was adjusted to 4.8 with Orthophosphoric acid.
RESULTS AND DISCUSSION
Method Development
Table 1: Different trials were performed by changing Mobile phase and buffer
Trials
Column Used
Mobile phase
Buffer
Flow rate
Wave length
Temperature
Injection Volume
Trial: 1
Discovery 250 x 4.6 mm, 5m.
Water: Methanol (50:50)
1ml/min
244nm
25 ͦ C
10µl
Trial: 2
Discovery 250 x 4.6 mm, 5m.
Water: Acetonitrile (50:50)
Water
1ml/min
244nm
30 ͦ C
10µl
Trial: 3
Discovery 250 x 4.6 mm, 5m.
buffer: ACN (60:40)
0.1%OPA
1ml/min
244nm
30 ͦ C
10µl
Trial: 4
Discovery 250 x 4.6 mm, 5m.
buffer: Acetonitrile (60:40)
0.01N KH2PO4 (4.8) solution
1ml/min
244nm
30 ͦ C
10µl
Trial: 5
buffer: Acetonitrile (70:30A)
buffer: Acetonitrile (70:30)
0.01N KH2PO4 (4.8) solution
1ml/min
244nm
30 ͦ C
10µl
Optimized Method
Kromosil 250 x 4.6 mm, 5m.
Buffer: Acetonitrile (60:40)
0.01N KH2PO4 (4.8) solution
Diluent : Water: ACN: (50:50)
1.0ml/min
244nm
30 ͦ C
10µl
Table 2: Optimization of chromatographic conditions
Trials
Observation
Trial: 1
Ebastine peak was eluted but Montelukast peak was not eluted and peak shape also not good so further trial is carried out.
Trial: 2
Peaks were eluted but peak shape was not good and baseline disturbances hump, USP plate count were not good so further trial is carried out.
Trial: 3
Both peaks were eluted but resolution was less so further trial is carried out.
Trial: 4
Retention time is more and ebastin eluted at void range so further trial is carried out.
Trial: 5
Increasing buffer ratio montelukast retention time is more and ebastine eluted at void range so further trial is carried out.
Optimized Method
Drugs were eluted with good retention time, resolution; all the system suitable parameters like Plate count and Tailing factor were within the limits. Peak shape and retention time is good so, further process is carried out.
Method Validation
The present study was carried method was validated based on ICH (Q2B) parameters.8
The following parameters were validated for the proposed method.
System Suitability
All the system suitability parameters are within range and satisfactory as per ICH guidelines. Table 3
Discussion: According to ICH guidelines plate count should be more than 2000, tailing factor should be less than 2 and resolution must be more than 2. All the system suitable parameters were within the limits.
Discussion: Retention times of Ebastine and Montelukast were 2.447 min and 3.436 min respectively. We did not find any interfering peaks in blank and placebo at retention times of these drugs in this method. So this method was said to be specific.
Linearity
Six Linear concentrations of Ebastine (25-
150ppm) and Montelukast (20-120ppm) were prepared and injected. Regression equation of the Ebastine and Montelukast were found to be, y = 19263x +1149, and y = 19946x + 1095 and the regression coefficient was 0.999. Table 4 Figure 4 & 5
Precision
Intraday precision (Repeatability): Intraday Precision was performed and % RSD for Ebastine and Montelukast were found to be 0.2% and 0.2% respectively. Table 5
Inter-day precision: Inter-day precision was performed with 24 hrs time lag and the %RSD Obtained for Ebastine and Montelukast were 0.3% and 0.2%. Table 6
Accuracy
Three concentrations 50%, 100%, 150%, were injected in a triplicate manner and amount Recovered and % Recovery was displayed in Table 7. Figure 6-8
Robustness
Small deliberate changes in a method like Flow rate, mobile phase ratio, and temperature are made but there were no recognized change in the result and are within range as per ICH Guidelines. Table 6 Figure 9 & 10
Discussion: Robustness conditions like Flow minus (0.9ml/min), Flow plus (1.1ml/min), mobile phase minus (65B:35A), mobile phase plus (55B:45A), temperature minus (25°C) 3and temperature plus (35°C) was maintained and samples were injected in a duplicate manner. System suitability parameters were not much affected and all the parameters were passed. %RSD was within the limit.
Assay
Standard preparations are made from the API and Sample Preparations are from Formulation (EBAST M TABLET). Both sample and standards are injected six homogeneous samples. The drug in the formulation was estimated by taking the standard as the reference. The Average % assay was calculated and found to be 99.05% and 99.20% for Ebastine and Montelukast respectively. Table 7
Degradation Studies
Standards and degraded samples are injected and calculated the percentage of drug degraded in solution by applying different conditions like acid, alkali, and oxidative, photolytic, thermal and neutral analysis. Table 8 Figure 11-15
Table 3: System Suitability Studies of Ebastine and Montelukast
Property
Ebastine
Montelukast
Retention time (tR)
2.447min
3.436min
Theoretical plates (N)
8019 ± 63.48
10040 ± 63.48
Tailing factor (T)
1.37 ± 0.117
1.33 ± 0.117
Table 4: Calibration Data of Ebastine and Montelukast Method
S.No
Concentration
Ebastine (µg/ml)
Response
Concentration
Montelukast (µg/ml)
Response
1
0
0
0
0
2
25
533631
20
478732
3
50
987156
40
956442
4
75
1467357
60
1501069
5
100
1976938
80
1885033
6
125
2503069
100
2386656
7
150
3011189
120
2913242
Table 5: Repeatability results for Ebastine and Montelukast
Sl. No.
Ebastine
Montelukast
1
1958452
1868712
2
1957170
1870107
3
1952368
1865840
4
1959570
1870800
5
1953581
1865343
6
1952026
1859653
Mean
1955528
1866743
S.D.
3274.9
4115.7
%RSD
0.2
0.2
*Average of six determinations
Table 6: Inter-Day Precision Results for Ebastine and Montelukast
S. No.
Ebastine
Montelukast
1
2159276
1882066
2
2168976
1884258
3
2165538
1892454
4
2158679
1885947
5
2162743
1875128
6
2157355
1882066
Mean
2162095
1883653
S.D
4510.1
5671.0
%RSD
0.2
0.3
Table 7: Table of Accuracy
Sample
Concentration (%) (µg/ml)
Recovery (%)
Mean % Recovery
%RSD
Ebastine
50
101.07
99.93%
0.07
100
98.93
0.33
150
99.81
0.30
Montelukast
50
100.8
99.69%
0.08
100
99.63
0.74
150
98.64
0.52
Table 8: Robustness Data of Ebastine and Montelukast
S. No
Robustness condition
Ebastine
%RSD
Montelukast
%RSD
1
Flow minus (0.9ml/min)
0.1
0.2
2
Flow Plus (1.1ml/min)
0.4
0.5
3
Mobile phase minus (65:35)
0.3
0.3
4
Mobile phase Plus (55:45)
0.2
0.1
5
Temperature minus (250c)
0.2
0.2
6
Temperature Plus (300c)
0.3
0.1
Table 9: Assay of Tablet
S. No.
Ebastine %Assay
Montelukast % Assay
1
99.15
98.91
2
99.23
99.19
3
99.00
98.10
4
99.26
99.84
5
98.97
99.46
6
98.67
99.70
AVG
99.05
99.20
S.D
0.2184
0.6339
% RSD
0.2
0.64
Table 10: Different Types of Degradation Studies
Types of Degradation
EBASTINE
Area
%
Recovered
%
Degraded
Acid
1800991
95.56
4.44
Base
1833505
97.28
2.72
Peroxide
1853657
98.35
1.65
Thermal
1871146
99.28
0.72
UV
1868750
99.15
0.85
Water
1867367
99.08
0.92
MONTELUKAST
Acid
1867882
95.28
4.72
Base
1910567
97.45
2.55
Peroxide
1930866
98.49
1.51
Thermal
1950620
99.50
0.50
UV
1951371
99.54
0.46
Water
1947871
99.36
0.64
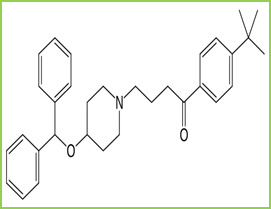
Figure 1: Structure of Ebastine
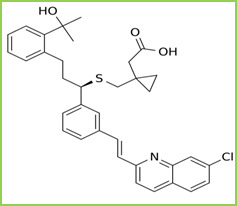
Figure 2: Structure of Montelukast
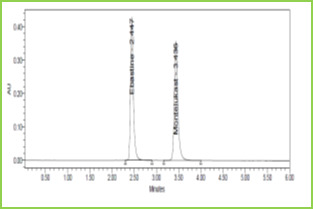
Figure 3: Typical chromatogram of Ebastine and Montelukast
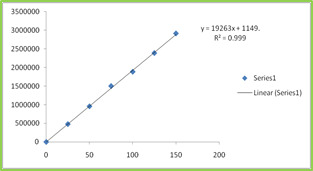
Figure 4: Calibration curve of Ebastine
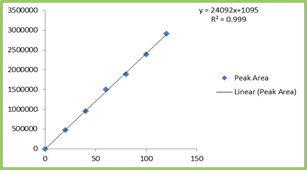
Figure 5: Calibration curve of Montelukast
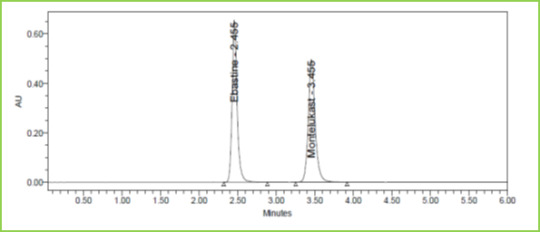
Figure 6: Accuracy 50% Chromatogramof Ebastine and Montelukast
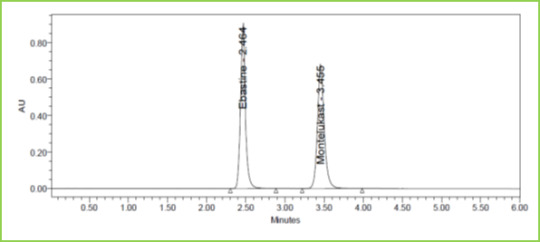
Figure 7: Accuracy 100% Chromatogram of Ebastine and Montelukast
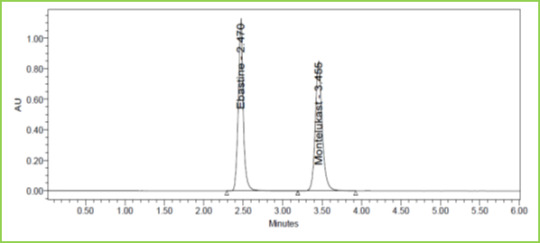
Figure 8: Accuracy 150% Chromatogram of Ebastine and Montelukast
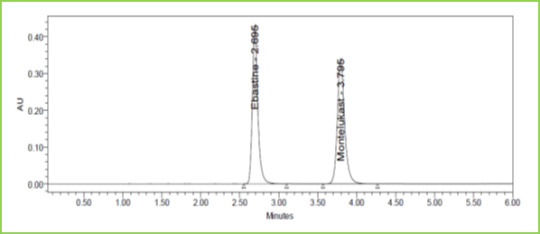
Figure 9: Flow minus Chromatogram of Ebastine and Montelukast
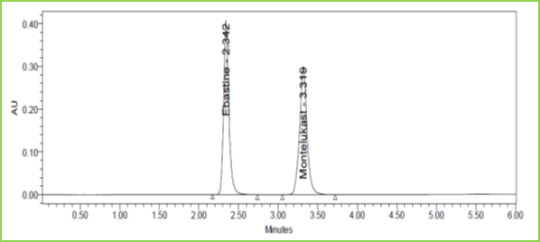
Figure 10: Flow plus Chromatogram of Ebastine and Montelukast
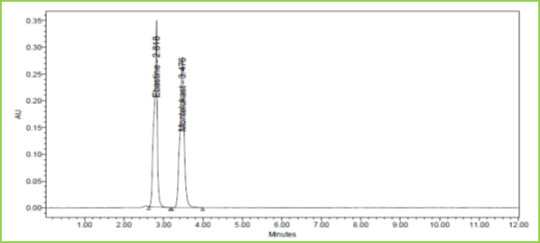
Figure 11: Chromatogram showing Acid degradation
CONCLUSION
A new method was established for simultaneous estimation of Ebastine and Montelukast by RP-HPLC method. The proposed HPLC method was found to be simple, specific, precise, accurate, rapid and economical for simultaneous estimation of Ebastine and Montelukast in the pharmaceutical dosage form. The developed method was validated in terms of accuracy, precision, linearity, robustness, and ruggedness, and results will be validated statistically according to ICH guidelines. The Sample recoveries in all formulations were in good agreement with their respective label claims. Hence the suggested RP-HPLC method can be used for routine analysis of Ebastine and Montelukast in API and Pharmaceutical dosage form.
ACKNOWLEDGMENTS
I am very much thankful to the management of Seven Hills College of Pharmacy for providing the necessary facilities to carry out the research work and for their encouragement and support.
REFERENCES
Rana, N. S., Rajesh, K. S., Patel, N. N., Patel, P. R., Limbachiya, U., & Pasha, T. Y. (2013). Development and validation of an RP-HPLC method for the simultaneous estimation of montelukast sodium and ebastine in tablet dosage form. Indian Journal of Pharmaceutical Sciences, 75(5), 599.PMid:24403662, PMCid:PMC3877523
Singh, R. M., Saini, P. K., Mathur, S. C., Singh, G. N., & Lal, B. (2010). Development and validation of an RP-HPLC method for estimation of montelukast sodium in bulk and in tablet dosage form. Indian Journal of Pharmaceutical Sciences, 72(2), 235.https://doi.org/10.4103/0250-474X.65023 ,PMid:20838530 , PMCid:PMC2929785
Yadav, O. M., & Jain, H. K. (2014). RP-HPLC Method Development and Validation for Simultaneous Estimation of Phenylephrine Hydrochloride and Ebastine in Tablet Dosage Form. International Journal of Pharmacy and Pharmaceutical Sciences, 6(8), 466-470.
Savsani, J. J., Goti, P. P., & Patel, P. B. (2012). Development and validation of simultaneous equation method for estimation of ebastine and montelukast sodium in combined tablet dosage form. Der Pharmacia Sinica, 3(6), 690-698.
Shrikrishna, B., & Nisharani, R. (2015). Analytical Method Development and Validation for Simultaneous Estimation of Montelukast and Ebastine by HPLC. Research Journal of Pharmacy and Technology, 8(1), 1. https://doi.org/10.5958/0974-360X.2015.00001.3
Singh, K., Bagga, P., Shakya, P., Kumar, A., Khalid, M., Akhtar, J., & Arif, M. (2015). Validated UV Spectroscopic Method for Estimation of Montelukast Sodium. International Journal of Pharmaceutical Sciences and Research, 6(11), 4728-4732.
Thakor K. A, Pasha, T. Y., Patel P. U., Chauhan R. J., Patel N. H. (2014). Development and validation of analytical method for simultaneous estimation of ebastine and phenylephrine hydrochloride in tablet dosage form. International Bulletin of Drug Research, 4(7): 16-40, 2014.
8. ICH Harmonised Tripartite Guideline, validation of analytical procedures: Text methodology, Q2 (R1) (2005). International Conference on Harmonization, Geneva, pp: 1-13.
Read the full article
0 notes
Text
Exler 10mg 1pcs
Indications Ebastine is indicated for the symptomatic treatment of: Seasonal and perennial allergic rhinitis, Idiopathic chronic urticaria. PharmacologyEbastine, a piperidine derivative, is a long-acting, nonsedating, second-generation histamine receptor antagonist that binds preferentially to peripheral H1 receptors. It is metabolised to active metabolite, carebastine. It has antihistaminic,…
View On WordPress
0 notes
Text
Ebast 20mg Tablet
Ebast 20mg Tablet is for the most part given to understanding who have had an unfavorably susceptible response to a specific sustenance or medication. Known to be a piperidine subordinate, Ebast 20mg Tablet is non-quieting and also long-acting hostile to histamine. In this way, the medication basically hinders the activity of histamine in the body, decreasing the side effects of a sensitivity.
It is a class B sedate, and despite the fact that examination has neglected to show on the off chance that it has a negative effect on the hatchling or another conceived, expecting ladies and lactating moms ought to talk about the positive and negative impacts of Ebast 20mg Tablet before utilizing it. People who are known to be excessively touchy to Ebast 20mg Tablet ought not take the medication. Patients who are as of now experiencing kidney or liver debilitation, ought to be careful when utilizing Ebast 20mg Tablet.
Specialists for the most part recommend 10 mg to 20 mg of the medication every day. It can be taken either with sustenance or even without. Ebast 20mg Tablet can be put away in a sterile space at a temperature of around 25 degree Celsius.
A couple of reactions that you may involvement of Ebast 20mg Tablet are dry mouth, cerebral pain, seeping of the nose, advancement of sinus, torment in the stomach, queasiness, heartburn, shortcoming and languor.
What is Ebast?
Ebast Tablet is utilized for Seasonal unfavorably susceptible conditions, Chronic tingling, Allergic rhinitis, Chronic idiopathic urticaria, Nasal hypersensitivity, Nasal sensitivities and different conditions. Ebast Tablet may likewise be utilized for purposes not recorded in this medicine manage.
Ebast Tablet contains Ebastine as a dynamic fixing.
Ebast Tablet works by blocking activities of endogenous histamine.
Small scale Labs (B And B) produces Ebast Tablet.
Ebast Tablet Uses
Ebast Tablet is utilized for the treatment, control, anticipation, and change of the accompanying ailments, conditions and indications:
Occasional unfavorably susceptible conditions
Endless tingling
Hypersensitive rhinitis
Endless idiopathic urticaria
Nasal hypersensitivity
Nasal hypersensitivities
Ebast Tablet may likewise be utilized for purposes not recorded here.
In Depth Information on Ebast Tablet
Master guidance for Ebast Tablet
Try not to begin or proceed with the ebastine tablets:
In the event that you are unfavorably susceptible (easily affected) to ebastine or any of alternate elements of ebastine tablet.If you are pregnant or bosom sustaining.
Specialist's recommendation ought to be considered in the event of following ailment conditions: liver impedance, kidney deficiency, QTc interim prolongation.
0 notes
Text
Ddu College–China’s Import and Export Market Report of Antiallergic Drugs
Antiallergic/ anti-allergy pharmaceutical drugs are used to treat allergic diseases (allergies) and can be divided into the following categories:
1. Antihistamine drugs: loratadine, promethazine and chlorpheniramine etc.
2. Allergic medium blockers: sodium cromoglycate, ketotifen etc.
3. Others: ① calcium salts, such as calcium chloride, calcium gluconate; ② herbal desensitizer, such as injectiondermatophagoides farinae; ③ glucocorticoid etc.
Allergies, also known as allergic diseases, are a number of conditions caused by hypersensitivity of the immune system to something in the environment that usually causes little or no problems to most people. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing and a runny nose, shortness of breath or swelling. Food intolerances and food poisoning are separate conditions.
Today, Ddu, the leading global pharmaceutical and medical device B2B online platform, will share China’s Import and Export Market Report of Antiallergic Drugs with you.
I. General situation of global market of antiallergic drugs
According to the statistics of WHO, the global antiallergic drugs market was 5.215 billion USD in 2016, of which anti-histamine drugs accounted for 66%, allergic mediator products accounted for 20% and other anti-allergic drugs accounted for 14%.
Antihistamine drugs, the most widely used pharmaceutical drugs in clinical application, treat allergic rhinitis and other allergies. Antihistamines provides relief from nasal congestion, sneezing or hives caused by pollen, dust mites or animal allergies.
In 2016 the top 5 antihistamines used in the world were desloratadine citrate disodium tablets, loratadine, ebastine, levocetirizine and cetirizine.
In the past few years most transnational pharmaceutical companies have devoted themselves to the development of antitumor drugs, antivirus drugs, rare drugs and targeted drugs rather than the development of antiallergic drugs resulting in a sluggish market.
As indicated by the statistics above, eight out of ten of antiallergic drug sales were flat or falling and no sales exceeded one million USD in 2016.
In addition to this, the sales for Singulair, the blockbuster medication from MSD, topped the list with 0.9 billion USD. Singulair was approved for the treatment of anaphylactic rhinitis and asthma, becoming a highly recommended drug for treatment guidelines in many countries.
Currently, the key antiallergic drug markets are the United States, France, Germany, Italy, Spain, the United Kingdom, Japan, China and India and this is expected to grow.
Foreign-funded brands take up major shares in the global antiallergic drugs market. Loratadine, the antiallergic drug from Schering-Plough in the United States, is currently being sold in over 110 countries and regions as a non–prescription drug, becoming the best-selling antiallergic drug in the world.
II. China’s antiallergic drugs market
According to the statistics of PDB, China’s overall antiallergic drug sales were 5.2 billion RMB in 2016, with an increase of 5.1% over the previous year.
The top ten companies in the antiallergic drugs market are mostly domestic funded companies and account for 51.6% of market shares with relatively high market concentration.
There were four foreign-funded companies. Bayer HealthCare, with its products being very popular in the retail market, ranked first for two consecutive years , which shows an increasing trend in 2017.
There were six domestic-funded companies. Yangtze River Pharmacy and Hainan Poly Pharmaceutical ranked second and third with market shares of 8.1% and 6.4% , which also shows an increasing trend in 2017.
Claritin (loratadine tablets) held dominant position and ranked first for two consecutive years with a stable market share of 10.3% in 2015 and 10.5% in 2016. Desloratadine citrate disodium tablets from Yangtze River Pharmacy, ranked second for two consecutive years with a market share of 5.1% in 2015 and 6.8% in 2016. There was fierce competition among the other top ten brands.
III. General situation of China’s antiallergic drug imports
Loratadine tablets took up most of China’s imported antiallergic drug shares with the main products being Claritin from Byers Healthcare and Astemizole from Johnson & Johnson.
According to the statistics of China customs, China mainly imported antiallergic drugs from France, the United States and Switzerland as indicated on the chart below.
IV. General situation of China’s antiallergic drug exports
As the Chinese pharmaceutical drugs market is integrating with the world market, the product structure of antihistamines, especially piperidines, monoethanolamides, alkyl amine, phenothiazines and piperazine, have gradually improved. What’s more, with product upgrades, first generation antihistamines have been developed to third generation antihistamines, accelerating the export of these drugs.
According to the statistics of China customs, China’s antihistamine drugs were mainly exported to Spain, the United States and Belgium in 2016 and export sales of top 10 counties totaled 21.06 million USD.
V. Leading antiallergic drug companies
Hainan Poly Pharm., Ltd.
Hainan Poly Pharm. Co., Ltd. is a leading international Chinese pharmaceutical preparation company.
Representative product: Desloratadine tablets
Indication: Anaphylactic rhinitis
Main exporting countries: Germany, Holland, France
Yangze River Pharmaceutical Group
Founded in 1971, YRPG is a giant national cross-regional pharmaceutical group, integrating research, manufacturing and trading. It is listed as one of the first innovative enterprises by China MIIT.
Representative product: Desloratadine citrate disodium tablets
Indication: Anaphylactic rhinitis
Main exporting countries: EU, US
Zhejiang Wolw bio-pharmaceutical Co., Ltd
Zhejiang Wolw bio-pharmaceutical Co., Ltd has the largest allergen drug development base in Asia and is China’s largest desensitization drug manufacturer.
Representative product: Dermatophagoides farinae drops
Indication: Dermatophagoides farinae allergy
Main exporting countries: Asian-Pacific region
VI. OTC antiallergic drugs are to be the next blockbuster.
With more investment in the market and an increasing awareness by users, the OTC market has become favored by more and more pharmaceutical manufacturers.
In terms of the domestic market, antiallergic drugs are rarely seen in the OTC market and manufacturers still need to step up their consumption guidance efforts. However, with the improvement of self-treatment, antiallergic pharmaceutical drugs are expected to take up a greater share in the OTC market and become the new main force within the next two to three years.
Thus, Ddu, the leading global pharmaceutical and medical device B2B online platform, advises domestic companies to adapt to market demands with diversified products and seize the opportunity to upgrade the structure of products and devote themselves to the development of drugs that help adjust the immune system and alleviates allergy symptoms.
Source:https://wordpress.com/post/medicaldeviceandequipmentinformation.wordpress.com/24
0 notes
Text
Ddu College–China’s Import and Export Market Report of Antiallergic Drugs
Antiallergic/ anti-allergy
pharmaceutical drugs
are used to treat allergic diseases (allergies) and can be divided into the following categories:
1. Antihistamine drugs: loratadine, promethazine and chlorpheniramine etc.
2. Allergic medium blockers: sodium cromoglycate, ketotifen etc.
3. Others: ① calcium salts, such as calcium chloride, calcium gluconate; ② herbal desensitizer, such as injectiondermatophagoides farinae; ③ glucocorticoid etc.
Allergies, also known as allergic diseases, are a number of conditions caused by hypersensitivity of the immune system to something in the environment that usually causes little or no problems to most people. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing and a runny nose, shortness of breath or swelling. Food intolerances and food poisoning are separate conditions.
Today, Ddu, the leading global pharmaceutical and medical device B2B online platform, will share China’s Import and Export Market Report of Antiallergic Drugs with you.
I. General situation of global market of antiallergic drugs
According to the statistics of WHO, the global antiallergic drugs market was 5.215 billion USD in 2016, of which anti-histamine drugs accounted for 66%, allergic mediator products accounted for 20% and other anti-allergic drugs accounted for 14%.
Antihistamine drugs, the most widely used pharmaceutical drugs in clinical application, treat allergic rhinitis and other allergies. Antihistamines provides relief from nasal congestion, sneezing or hives caused by pollen, dust mites or animal allergies.
In 2016 the top 5 antihistamines used in the world were desloratadine citrate disodium tablets, loratadine, ebastine, levocetirizine and cetirizine.
In the past few years most transnational pharmaceutical companies have devoted themselves to the development of antitumor drugs, antivirus drugs, rare drugs and targeted drugs rather than the development of antiallergic drugs resulting in a sluggish market.
As indicated by the statistics above, eight out of ten of antiallergic drug sales were flat or falling and no sales exceeded one million USD in 2016.
In addition to this, the sales for Singulair, the blockbuster medication from MSD, topped the list with 0.9 billion USD. Singulair was approved for the treatment of anaphylactic rhinitis and asthma, becoming a highly recommended drug for treatment guidelines in many countries.
Currently, the key antiallergic drug markets are the United States, France, Germany, Italy, Spain, the United Kingdom, Japan, China and India and this is expected to grow.
Foreign-funded brands take up major shares in the global antiallergic drugs market. Loratadine, antiallergic drug from Schering-Plough in the United States, is currently being sold in over 110 countries and regions as a non–prescription drug, becoming the best-selling antiallergic drug in the world.
II. China’s antiallergic drugsmarket
According to the statistics of PDB, China’s overall antiallergic drug sales were 5.2 billion RMB in 2016, with an increase of 5.1% over the previous year.
The top ten companies in the antiallergic drugs market are mostly domestic funded companies and account for 51.6% of market shares with relatively high market concentration.
There were four foreign-funded companies. Bayer HealthCare, with its products being very popular in the retail market, ranked first for two consecutive years , which shows an increasing trend in 2017.
There were six domestic-funded companies. Yangtze River Pharmacy and Hainan Poly Pharmaceutical ranked second and third with market shares of 8.1% and 6.4% , which also shows an increasing trend in 2017.
Claritin (loratadine tablets) held dominant position and ranked first for two consecutive years with a stable market share of 10.3% in 2015 and 10.5% in 2016. Desloratadine citrate disodium tablets from Yangtze River Pharmacy, ranked second for two consecutive years with a market share of 5.1% in 2015 and 6.8% in 2016. There was fierce competition among the other top ten brands.
III. General situation of China’s antiallergic drug imports
Loratadine tablets took up most of China’s imported antiallergic drug shares with the main products being Claritin from Byers Healthcare and Astemizole from Johnson & Johnson.
According to the statistics of China customs, China mainly imported antiallergic drugs from France, the United States and Switzerland as indicated on the chart below.
IV. General situation of China’s antiallergic drug exports
As the Chinese pharmaceutical drugs market is integrating with the world market, the product structure of antihistamines, especially piperidines, monoethanolamides, alkyl amine, phenothiazines and piperazine, have gradually improved. What’s more, with product upgrades, first generation antihistamines have been developed to third generation antihistamines, accelerating the export of these drugs.
According to the statistics of China customs, China’s antihistamine drugs were mainly exported to Spain, the United States and Belgium in 2016 and export sales of top 10 counties totaled 21.06 million USD.
V. Leading antiallergic drug companies
Hainan Poly Pharm., Ltd.
Hainan Poly Pharm. Co., Ltd. is a leading international Chinese pharmaceutical preparation company.
Representative product: Desloratadine tablets
Indication: Anaphylactic rhinitis
Main exporting countries: Germany, Holland, France
Yangze River Pharmaceutical Group
Founded in 1971, YRPG is a giant national cross-regional pharmaceutical group, integrating research, manufacturing and trading. It is listed as one of the first innovative enterprises by China MIIT.
Representative product: Desloratadine citrate disodium tablets
Indication: Anaphylactic rhinitis
Main exporting countries: EU, US
Zhejiang Wolw bio-pharmaceutical Co., Ltd
Zhejiang Wolw bio-pharmaceutical Co., Ltd has the largest allergen drug development base in Asia and is China's largest desensitization drug manufacturer.
Representative product: Dermatophagoides farinae drops
Indication: Dermatophagoides farinae allergy
Main exporting countries: Asian-Pacific region
VI. OTC antiallergic drugs are to be the next blockbuster.
With more investment in the market and an increasing awareness by users, the OTC market has become favored by more and more pharmaceutical manufacturers.
In terms of the domestic market, antiallergic drugs are rarely seen in the OTC market and manufacturers still need to step up their consumption guidance efforts. However, with the improvement of self-treatment, antiallergic pharmaceutical drugs are expected to take up a greater share in the OTC market and become the new main force within the next two to three years.
Thus, Ddu, the leading global pharmaceutical and medical device B2B online platform, advises domestic companies to adapt to market demands with diversified products and seize the opportunity to upgrade the structure of products and devote themselves to the development of drugs that help adjust the immune system and alleviates allergy symptoms.
By Ddu
Source:http://media.drugdu.com/market/ddu-college-chinas-import-and-export-market-report-of-antiallergic-drugs.html?dtag=1001310078
0 notes
Text
Stiba 30ml
Indications Ebastine is indicated for the symptomatic treatment of: Seasonal and perennial allergic rhinitis, Idiopathic chronic urticaria. Therapeutic Class Non-sedating antihistamines Pharmacology Ebastine, a piperidine derivative, is a long-acting, nonsedating, second-generation histamine receptor antagonist that binds preferentially to peripheral H1 receptors. It is metabolised to active…
View On WordPress
0 notes
Text
Ebahist
Indications Ebastine is indicated for the symptomatic treatment of: Seasonal and perennial allergic rhinitis, Idiopathic chronic urticaria. Therapeutic Class Non-sedating antihistamines Pharmacology Ebastine, a piperidine derivative, is a long-acting, nonsedating, second-generation histamine receptor antagonist that binds preferentially to peripheral H1 receptors. It is metabolised to active…
View On WordPress
0 notes
Text
Ebasten 10mg 1Pcs 8
Ebasten 10mg 1Pcs 8
Indications Ebastine is indicated for the symptomatic treatment of: Seasonal and perennial allergic rhinitis, Idiopathic chronic urticaria. Therapeutic Class Non-sedating antihistamines Pharmacology Ebastine, a piperidine derivative, is a long-acting, nonsedating, second-generation histamine receptor antagonist that binds preferentially to peripheral H1 receptors. It is metabolised to active…
View On WordPress
0 notes
Text
Stiba 10mg 1pcs
Indications Ebastine is indicated for the symptomatic treatment of: Seasonal and perennial allergic rhinitis, Idiopathic chronic urticaria. Therapeutic Class Non-sedating antihistamines Pharmacology Ebastine, a piperidine derivative, is a long-acting, nonsedating, second-generation histamine receptor antagonist that binds preferentially to peripheral H1 receptors. It is metabolised to active…
View On WordPress
0 notes