#remdesivir injection
Photo



For treatment of suspected or laboratory confirmed corona virus disease 2019 (COVID-19) in adults and children hospitalized with moderate to severe disease.
Remdesivir for Injection 100 mg/vial
Lyophilized Powder for Injection for IV Infusion
Lyophilized Powder
Remdesivir for injection, 100 mg, is a sterile, preservative-free lyophilized powder that is to be reconstituted with 19 mL of Sterile Water for Injection and diluted into 0.9% saline prior to administration by intravenous infusion. Remdesivir for injection, 100 mg, is supplied in a single-dose clear glass vial. The appearance of the lyophilized powder is white to off-white to yellow.
Inactive Ingredients
The inactive ingredients are sulfobutylether-β-cyclodextrin sodium salt (SBECD), Water for Injection, USP, and may include hydrochloric acid and/or sodium hydroxide for pH adjustment. Remdesivir for injection, 100 mg, contains 3 g SBECD.
General Information
The optimal dosing and duration of treatment are unknown. The suggested dose and duration may be updated as data from clinical trials become available.
Adult and pediatric patients (>28 days old) must have an eGFR determined and full-term neonates (≥7 days to ≤28 days old) must have serum creatinine determined before dosing of remdesivir.
Hepatic laboratory testing should be performed in all patients before starting remdesivir and daily while receiving remdesivir.
Remdesivir should be administered via intravenous (IV) infusion only. Do not administer as an intramuscular (IM) injection.
Adult Patients
The dose of the drug for adults should be a single dose of 200 mg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 100 mg, infused intravenously over 30-120 minutes for 4 days.
Remdesivir is to be administered via intravenous infusion in a total volume of up to 250 mL 0.9% saline over 30 to 120 minutes [For further information see Dosage and Administration].
Extension of administration of the drug beyond 5 days to 10 days is not recommended.
All adult patients must have creatinine clearance determined before dosing [For further information see Dosage and Administration].
Hepatic laboratory testing should be performed in all patients before starting remdesivir and daily while receiving remdesivir dosing [For further information see Dosage and Administration].
Pediatric Patients
Dosing in pediatric patients is based upon physiologically-based (PBPK) modeling and simulation of pharmacokinetic data from healthy adult subjects. The recommended pediatric dose for pediatric patients weighing between 3.5 kg and <40 kg should be calculated using the mg/kg dose according to the patient’s weight [For further information see Dosage and Administration];
The dose of the drug for pediatric patients weighing more than 40 kg should be a single dose of 200 mg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 100 mg, infused intravenously over 30-120 minutes for 4 days.
The dose for pediatric patients with bodyweight between 3.5 kg and less than 40 kg should be a single dose of 5 mg/kg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 2.5 mg/kg, infused intravenously over 30-120 minutes for 4 days.
Extension of administration of the drug beyond 5 days to 10 days is not recommended.
Pediatric patients (>28 days old) must have an eGFR determined and full-term neonates (≥7 days to ≤28 days old) must have serum creatinine determined before dosing [For further information see Dosage and Administration].
COVID-19: coronavirus disease 2019
0 notes
Text
INSANITY: The WHO Are Tyrants “In the World Health Organization's founding charter, they wrote an absolute amnesty for all criminal acts conducted by themselves, including murder by the way. By law, not a single one of them cannot only be tried and prosecuted, they can't even be investigated.
Now I wonder if there are examples in the history of the WHO of where that amnesty has been implemented or effective or where its boundaries might likely be tested.
Let's keep it really current. Remember that in 2018, during the Ebola clinical trials run by the World Health Organization in Africa, it was very clear that remdesivir, the drug that was promoted by doctor Deborah Birx and doctor Anthony Fauci as a drug for the treatment of COVID. The World Health Organization and its infinite wisdom and high morality decided that remdesivir was actually too lethal to inject into Africans. And that is because the fatality rate of people exposed to remdesivir in the Ebola trials was 53%. And the bad news about that number is that Ebola doesn't kill 53%.
You get over Ebola. You don't get over remdesivir.
16 notes
·
View notes
Text
There is a thing going around claiming the FDA recalled a covid vaccine due to it causing health issues.
This is false.
There is exactly one covid-related drug that has ever been recalled, and it was an injectable treatment for hospitalised patients with covid. It was recalled in December 2021 (over 2 years ago) because it had glass particulates.
That's it.
You can check here for yourself:
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts
Here's the specific drug recall I talked about:
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/gilead-issues-voluntary-nationwide-recall-two-lots-vekluryr-remdesivir-due-presence-glass
4 notes
·
View notes
Text
“It was the remdesivir”
—see haslam on nipah
#self spreading vaccines#overwhelming propaganda dominance#SARS cov-2 is a SARS1 bat vaccine#remdesivir
5 notes
·
View notes
Text
0 notes
Text
In the US, unvaxxed people are being segregated on admission to hospitals – the unvaxxed are deprived of all sustenance, treated with Remdesivir, injected with fentanyl and put on ventilators
https://peterhalligan.substack.com/p/in-the-us-unvaxxed-people-are-being
View On WordPress
0 notes
Text
ECC refuses to increase price of Remdesivir injection
Pharmacy employees wearing facemasks as a preventive measure against the coronavirus attend to customers in Islamabad, Pakistan, on March 23, 2020. — AFP
The Economic Coordination Committee (ECC) of the federal cabinet Monday decided not to increase the price of Remdesivir injection, used for the treatment of COVID-19, Radio Pakistan reported.
The ECC, under the chair of Finance Minister Ishaq…

View On WordPress
0 notes
Text
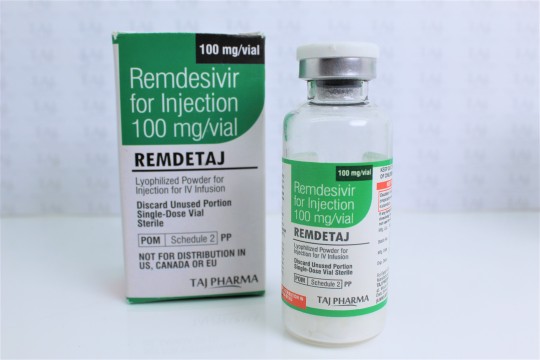
#Remdesivir 100 mg #injections which is very essential #LifeSavingDrug in the #hospitalized #ICU #COVID #patients. Our company carries expertise in the production of Remdetaj Injection #OmicronVariant
Lyophilized.#tajpharma #EmergencySupplies. 👉More: https://bit.ly/3BsZTqj.
Ready stock available.
#manufacturing#tajpharma#injection#emergencyresponse#product#pharmaceutical#supplier#exporter#india#maldives#oman#bahrain#cambodia#saudiarabia#lebanon#uganda#africa#iran#middleeast#country
0 notes
Text
Tyldesley mum fundraising for beloved cat's covid FIP treatment
New Post has been published on https://petnews2day.com/pet-news/cat-news/tyldesley-mum-fundraising-for-beloved-cats-covid-fip-treatment/
Tyldesley mum fundraising for beloved cat's covid FIP treatment
A DEVASTATED mum is fighting to keep her beloved pet alive after it contracted a strain of coronavirus.
Samantha Howard, from Tyldesley, bought a British Shorthair kitten from a local breeder in the summer, to the delight of her three kids.
Calling her Nala, the family fell in love with their new pet, but in a devastating turn of events, the kitten quickly became unwell and was later diagnosed with Feline Infectious Peritonitis (FIP).
FIP is a rare mutation from a strain of feline coronavirus (FCoV) and is usually declared as fatal and the animal has to be put down.
READ > Family share heartbreaking TV appeal in first Christmas without daughter
The British Shorthair was bought by Samantha in the summer (Image: Samantha Howard)
However, not prepared to give up on Nala, NHS worker Samantha did some research and found vets who are trialling treatment for FIP.
The treatment, which has shown successful results in the US, consists of fortnightly Remdesivir injections over a 90-day period.
However, as the trial treatment is not currently covered by pet insurance, it will cost Samantha between £6,000 and £9,000 for the treatment and frequent visits to the Bolton Animal Trust.
Desperate to save their “beautiful girl”, Samantha sold the family Mercedes to help fund the treatment and is now fundraising to give Nala the best chance of survival.
Samantha and the kids were devastated when Nala became ill (Image: Samantha Howard)
Samantha, 36, said: “Me and the kids all fell in love with Nala but once she got ill, she deteriorated rapidly.
“I had accepted that I was going to have to put her down, which would have devastated the kids, when I found out about the research and trials that are going on.
“It’s amazing because the vets said she was on the verge of dying, but the medication has made a massive difference already – she’s like a brand new cat.”
Nala has been responding well to the trial treatment (Image: Samantha Howard)
Around a month into the 90-day treatment, Samantha said Nala is re-gaining strength and has been able to get rid of much of the fluid that was building in her organs.
However, after selling her car and using all the savings she had, the 36-year-old is now appealing for help to ensure Nala can finish the so-far successful treatment.
Samantha is fundraising to ensure Nala can finish her treatment (Image: Samantha Howard)
Thankful for the support she has received so far, Samantha added: “I’m fundraising because of how expensive the treatment is and I’ve done everything I can to contribute towards it.
“But I’m also sharing the story to let pet owners know about the virus because it’s so deadly and there is no treatment that is covered by pet insurance – and I had no idea about it.
“Everyone who has a pet understands that you will do everything you can for them, so I hope this treatment can save Nala.”
To support Samantha’s fundraiser and help to fund Nala’s treatment, you can visit the donation page here.
Samantha and Nala (Image: Samantha Howard)
0 notes
Photo
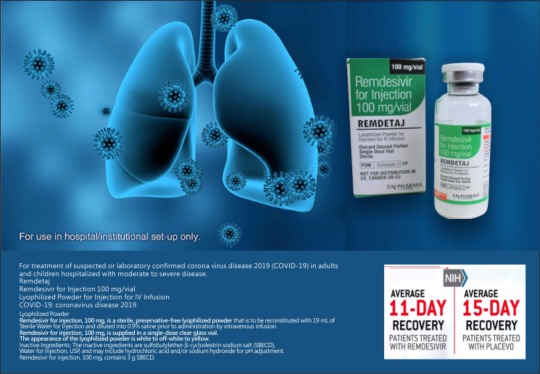
For treatment of suspected or laboratory confirmed corona virus disease 2019 (COVID-19) in adults and children hospitalized with moderate to severe disease. #Remdesivir for Injection 100 mg/vial Lyophilized Powder for #Injection for IV Infusion #COVID-19: coronavirus disease 2019
0 notes
Text
Best timing synonym

Best timing synonym for free#
“Paxlovid is usually very well-tolerated,” he says.īut people should stop taking Paxlovid and call a health care provider right away if they experience any of the following signs of an allergic reaction: Most people who take Paxlovid should not experience serious side effects, explains Dr. It’s important to note that although health care providers can write a prescription, pharmacists may also provide Paxlovid (with certain limitations) if they’ve opted to do so, provided you can share your electronic or printed medical records, including a list of medications you are already taking, and blood test results from the last 12 months. If you could become pregnant, it’s recommended that you use effective barrier contraception or do not have sexual activity while taking Paxlovid. If you are pregnant or breastfeeding, the FDA recommends discussing your options and specific situation with your health care provider, since there is no experience using the drug in these populations. The FDA granted the EUA in December, just as a staggering number of people were infected with Omicron and the need for care skyrocketed, leading to supply issues. The hope is that the restrictions on who can take Paxlovid will be relaxed over time. The more underlying medical conditions a person has, the higher their risk for developing a severe case of COVID-19, according to the CDC. That means you must either have certain underlying conditions (including cancer, diabetes, obesity, or others) or be 65 or older (more than 81% of COVID-19 deaths occur in in this group). But in order to qualify for a prescription, you must also have had a positive COVID-19 test result and be at high risk for developing severe COVID-19. The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. We asked Yale Medicine infectious diseases experts common questions about Paxlovid. The FDA also granted an EUA in December to a pill from Merck called molnupiravir (Lagevrio), but some studies suggest that molnupiravir has only a 30% reduction in the risk for hospitalization and death from COVID-19.Īnd as far as convenience, this medication is considered an improvement over treatments like remdesivir (approved by the FDA in October 2020), which is administered by intravenous (IV) injection. It’s important to note that Paxlovid (the brand name for the drug, which is made up of two generic medications-nirmatrelvir and ritonavir) isn’t the only pill available to treat COVID-19. It shows clear benefit, and it really can prevent hospitalization and death in people who are at high risk.” “It's really our first efficacious oral antiviral pill for this virus. “I think it is the beginning of a ‘game-changer,’” says Scott Roberts, MD, a Yale Medicine infectious diseases specialist. government while there is a public health emergency) and, perhaps most reassuring, it is expected to work against the Omicron variant.
Best timing synonym for free#
The drug, developed by Pfizer, has a lot of positives: It had an 89% reduction in the risk of hospitalization and death in the clinical trial that supported the EUA, a number that was high enough to prompt the National Institutes of Health (NIH) to prioritize it over other COVID-19 treatments and it’s cheaper than many other COVID-19 drugs (it’s provided for free by the U.S. So, if you test positive for the coronavirus and you are eligible to take the pills, you can take them at home and lower your risk of going to the hospital. Paxlovid is an oral antiviral pill that can be taken at home to help keep high-risk patients from getting so sick that they need to be hospitalized. The drug was granted an emergency use authorization (EUA) by the Food and Drug Administration (FDA) in December for anyone ages 12 and older who weighs at least 88 pounds, and is at high risk for severe disease. Paxlovid is the latest COVID-19 treatment that’s been all over the news. Because information about COVID-19 changes rapidly, we encourage you to visit the websites of the Centers for Disease Control & Prevention (CDC), World Health Organization (WHO), and your state and local government for the latest information. Note: Information in this article was accurate at the time of original publication.

0 notes
Text
Important updates by the US Food and Drug Administration (FDA) to two important COVID-19 medications
COVID-19 Treatment Medication Remdesivir (Veklury) Update
Remdesivir (Veklury) is a COVID-19 treatment medication injected or given through infusion once a day for 3–10 days. The 3–10-day range is based on whether a patient is hospitalized and whether they need mechanical ventilation or oxygen (Extracorporeal Membrane Oxygenation).
As of April 25, 2022, the FDA expanded who can get Remdesivir (Veklury) to include anyone at least 1 month old, weighing at least 3kg (about 7 pounds). This makes Remdesivir (Veklury) the first COVID-19 treatment medication approved for people younger than age 12, weighing less than 40kg (about 88 pounds).
COVID-19 Preventative Medication Evusheld Updates
Evusheld is a COVID-19 prevention medication for people ages 12 and older, weighing at least 40kg (about 88 pounds), who do not have COVID-19 and either:
● are moderately to severely immunocompromised, or
● have had a severe allergic reaction to a COVID-19 vaccine(s)
As of February 24, 2022, the US FDA raised the recommended initial dose of Evusheld from 300mg total to 600mg total.
As of June 29, 2022, the US FDA expanded the Evusheld recommendations to include additional 600mg doses every six months as needed for ongoing protection from COVID-19.
What does this mean for me?
● If you got an first lower dose of Evusheld (300mg total), talk to your doctor about getting another 300mg dose soon. You can get your next 600mg Evusheld dose 6 months from when you get this additional 300mg dose. Then, you can begin getting 600mg Evusheld doses every 6 months.
● If you are eligible for Evusheld but have not gotten it yet, you should talk to your doctor about getting your first 600mg dose soon. You can get additional 600mg doses at 6-month periods as needed.
Ask your doctor if you have any questions about Remdesivir or Evusheld.
Sources:
· FDA: COVID-19 Treatment for Young Children
· Gilead Press: Remdesivir for Patients under 12
· Gilead: Veklury COVID-19 Medication
· Gilead: Veklury COVID-19 Patients
· FDA: Drug Safety Evusheld Dosing Authorization
· FDA: Evusheld Factsheet
· FDA: FAQ about Evusheld Emergency Use
0 notes
Text
Remdesivir
Remdesivir, sold under the brand name Veklury, is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein.
0 notes
Text
Launch of sukuk plan approved
Launch of sukuk plan approved
ISLAMABAD: The Cabinet on Tuesday approved the launch of the Local and International Monopoly Sukuk Programme on the recommendation of the Finance Division. The Cabinet also approved reducing the price of 100mg Remdesivir injections used in the treatment of Coronavirus from Rs2,308.63 to Rs1,892.
Briefing the mediapersons about the decisions taken in the Federal Cabinet meeting along with Prime…

View On WordPress
0 notes
Text
Vaccination for 18-44 age group to begin from tomorrow in Madhya Pradesh
Vaccination for 18-44 age group to begin from tomorrow in Madhya Pradesh
Image Source : PTI/ REPRESENTATIONAL.
Vaccination for 18-44 age group to begin from May 5 (Wednesday) in Madhya Pradesh.
Vaccination against COVID-19 for people in the 18-44 age group will begin in Madhya Pradesh from Wednesday in a phased manner as per the availability of doses, a senior official said.
State IEC Bureau deputy director Archana Mundir said vaccination will be on the basis of…
View On WordPress
#Bhopal#coronavirus pandemic#COVID 19#covid pandemic fear#Madhya Pradesh#Remdesivir injection#second wave#vaccination#VACCINE drive on wednesday
1 note
·
View note
Text
तीस दिन में एक भी रेमडेसिविर की बिक्री नहीं, गोदामों में 73 लाख के इंजेक्शन डंप
तीस दिन में एक भी रेमडेसिविर की बिक्री नहीं, गोदामों में 73 लाख के इंजेक्शन डंप
प्रतीकात्मक तस्वीर।
– फोटो : Social media
ख़बर सुनें
ख़बर सुनें
कोरोना की दूसरी लहर में संक्रमितों के लिए संजीवनी माने गए रेमडेसिविर इंजेक्शन की बिक्री अब ठप है। बीते तीस दिनों में एक भी रेमडेसिविर इंजेक्शन की बिक्री नहीं हुई है। स्टाकिस्टों के गोदामों में करीब 73 लाख के इंजेक्शन डंप हैं। कंपनियां सौदे के मुताबिक इंजेक्शन की वापसी लेने को तैयार नहीं हैं। ऐसे में थोक दवा कारोबारियों को इंजेक्शनों…
View On WordPress
#corona symptoms#Corona vaccine#COVID vaccine#Latest Prayagraj News in Hindi#medical store#news today#Prayagraj#Prayagraj Hindi Samachar#prayagraj news#Prayagraj News in Hindi#remdesivir injection#remdesivir injection price#remdesivir uses
0 notes