#vosevi
Text
Why I Do This


It´s kind of been a whirlwind of thoughts and feelings since I gotten to the first project that I’ve been assigned to fly into. I´ve had to change my perspective on my goals and objectives here in this project and my time overall with MSF. I was assigned to improve the HIV/TB program in each of the projects I visit, but along with that, I have also been making myself helpful by working in the pediatric wards, which has been taking up the majority of my day.
The town is called Kalolele, there are no major roads to get here, and it is only accessible via helicopter or airplane. There are no vehicles in sight, only motorbikes. There is a road supposedly that one can use through motorbikes that would take one back to the capital, but it is rarely used and expensive. Kalole is a very young place, I’ve read in a project briefing that the median age here is about 19 years old. The majority of hospital admissions are children, which is why I am always in the pediatric wards in the usual morning. I feel like I’ve assimilated well in their medical team here and I look forward to helping and rounding with the local doctors. I´ve seen some difficult tropical medicine cases, such as cerebral malaria and many vaccine-preventable cases such as tetanus and measles. I have gotten a handful of new TB related cases every week, pulmonary and extrapulmonary TB. The majority of extrapulmonary TB I have seen are TB lymphadenopathy cases and one probable peritoneal TB case.
As for HIV, I am seeing a new diagnosis at least every two to three days. They have usually been in children, a few very young children below the age of two, usually presenting for fevers and having a positive malaria test, but due to their malnourish state, get tested for HIV. It made me more alert to re-present the guidelines for MSF to prevent mother to child transmission of HIV, which I will be presenting with one of the local nurses next week Tuesday. The local HIV nurse in charge of their program is great; she is hungry for knowledge, capable and tries to be as organized as she can in this kind of setting. Mind you though, there is no Wifi in the hospital and all of her records are hand-written which can be very tedious work. We´ve also made it a habit almost every weekday to meet at 4 PM to discuss an HIV/TB topic and talk about it so that she learns something new.
There is one case that I´ve been thinking about and is a constant reminder for me why I should and will always make the time to work in these settings in the future. There was a 15-year-old boy presenting about 10 days ago for a large swollen abdomen. Family says that his belly has been gradually getting bigger for the past 1 year, parents have tried traditional local medicine with no relief. They come from a town about 2 hours away using motorbike. On presentation the boy is in moderate distress, emaciated and appears about 10 years older than his stated age. Due to the high prevalence of tuberculosis in this setting, our first instinct as a team was to discuss the probability of TB-related peritoneal/abdominal disease. My first project in South Sudan had quite a number of these cases as the people living in that region drink unpasteurized milk, so Mycobacterium Bovis infection was quite common, and the presentation was very similar. Cases that I have seen responded well to anti-tuberculosis medication, so we started him on them right away.
For all patients presenting with TB symptoms, we also run a panel of other tests, which includes the rapid antigen/antibody test for HIV and the antibody tests for both Hepatitis C and hepatitis B. To our surprise, the hepatitis C antibody test was positive. The viral hepatitis diseases are very difficult to treat at this level in the Congo. MSF, despite all the good that it aims to do for the people it wants to help, does usually have a narrow scope of aid. Treatment for Hepatitis B could be started, but Hepatitis C is a completely different story. Due to the lack of (financial) interest by pharmaceutical companies to make Hepatitis C treatment more accessible to all people, this child will likely die from liver failure secondary to hepatitis C. Now, I have no way of confirming whether this patient does have active disease or not; the hepatitis C test that I have been given to use does not confirm active disease, it just says whether the person has been exposed to Hepatitis C or not. In fact, there is a chunk of patients that will clear the virus on their own. But, if he does have active disease and the cause of his abdominal swelling (or ascites) is hepatitis C due to the liver starting to fail, he will eventually die from the disease.
This family has no means to go to the capital and seek treatment. The best we could do for this child is continue the course of TB medication, as long as can he tolerate it, treat his symptoms related to Hepatitis C with diuretics and paracenteses (abdominal puncture to let out fluid). He will be sent home this upcoming Monday with follow up at the local Ministry of Health clinic by his home. Him and his family were thankful for our help and services, but we emphasized as a team that his symptoms may continue to come back and that it may not be curable at this level. It´s very difficult to communicate this kind of news considering that this is my first French speaking project and that this family only speaks Swahili so all communication had to be dealt with a translator. Despite this saddening news, he was excited to go home and asked when he can go back to school.
It´s these kinds of cases and people that I have met in my life that leave a kind of thorn hanging on my side. It makes me uncomfortable thinking that we live in a world so capable to accepting this kind of injustice. Having a growing son and a family now, I want the best. I want my son to grow up, go to a nice school, become educated and live a life of relative comfort, but, its theses kinds of cases and situations that leaves a sense of discomfort thinking about that. It´s not like I shouldn´t want that for him either, its just that, this is the kind of world that we live in.
It´s all a roll of the dice.
It´s the luck of the draw.
It´s an unfortunate truth.
I want my son to somehow realize this as well. I want him to see that what he has, others do not have and that this life is only worth living if we can find a way to try to right these wrongs. I shouldn´t have to accept that this 15-year-old boy will die here in the Democratic Republic of Congo, but if he were to be in my family, if he were my son, he would live and reach his potential in the United States.
But… this is why I do this.
This is why I am here.
I choose to see this.
0 notes
Link
معلومات عن دواء فوسيفي VOSEVI لعلاج إلتهاب الكبد C
#فوسيفي يتركَّب من المواد الفعالة سوفوسبوفير، فيلباتاسفير، فوكسيلابريفير.
إقرأ جميع هذه الصفحة بعناية قبل البدء بتناول هذا الدواء لأنها تحتوي على معلومات هامة لك. تحتوي هذه الصفحة على معلومات أساسية حول هذا الدواء. إذا كان لديك أي أسئلة أخرى، اسأل طبيبك أو الصيدلي. لقد تم وصف هذا الدواء لك فقط. لا تعطيه لآخرين لأنه قد يضرهم، حتى لو تبدو أعراض حالتهم الطبية مشابهة لحالتك
اقرأ معلومات وتفاصيل أكثر عن #VOSEVI عبر المزيد.
0 notes
Text
Vosevi: The Hepatitis C Buster Drug
Vosevi: The Hepatitis C Buster Drug
Affirmation of Vosevi, the latest treatment for Hepatitis C was done by FDA. Adult HCV patients Genotype 1-6 without liver failure were treated with Vosevi approved by the American Food and Drug Administration. Vosevi is the first drug of choice to be used in combination with Sovaldi treatment and other medications that inhibit the production of NS5a protein. Vosevi is a mixture of Sofosbuvir and…
View On WordPress
1 note
·
View note
Text
FDA approves Vosevi for Hepatitis C
FDA approves Vosevi for Hepatitis C
FDA approves Vosevi for Hepatitis C 07/18/2017 The U.S. Food and Drug Administration today approved Vosevi to treat adults with chronic hepatitis C virus (HCV) genotypes 1-6 without cirrhosis (liver disease) or with mild cirrhosis. The U.S. Food and Drug Administration today approved Vosevi to treat adults with chronic hepatitis C virus (HCV) genotypes 1-6 without cirrhosis (liver disease) or…
View On WordPress
#Breakthrough Therapy designations.#FDA 2017#Gilead Sciences Inc#HEPATITIS B#Priority review#Sofosbuvir#VELPATASVIR#Vosevi#voxilaprevir
0 notes
Photo

Gilead Stock: Is It A Buy After Dumping Its Arthritis Drug Onto Galapagos?

Biotech company Gilead Sciences (GILD) makes an approved coronavirus treatment, but conflicting reports of the drug’s effectiveness haven’t helped Gilead stock.
X
Doctors successfully treated President Donald Trump with Gilead’s remdesivir — now known by the brand name Veklury — when he was diagnosed with Covid-19. In November, fellow drugmaker Eli Lilly (LLY) gained emergency authorization for a coronavirus treatment.
Still, in October the World Health Organization said Veklury had little or no effect on death rates of Covid-19 patients. And, last month, a top intensive care doctor said Veklury shouldn’t be used routinely in critical care wards.
In addition, its September announcement of what would be its largest acquisition ever — $21 billion for cancer drug maker Immunomedics — has weighed on Gilead stock. In December, Gilead announced it would buy MYR GmbH, a German biotech focused on hepatitis treatments.
So, should investors consider buying Gilead stock right now?
A Fundamental Look At Gilead Stock
Shares have been volatile through its remdesivir coronavirus treatment efforts. Gilead stock has fallen 9.4% in 2020 as of midday Dec. 16. Gilead stock is trading barely above seven-year lows, after touching a 21-month high of 85.97 in March.
Besides its coronavirus efforts, Gilead is trying to stake its claim in HIV treatments. It’s facing off against an outlet mostly owned by GlaxoSmithKline (GSK) called ViiV Healthcare.
Also, Gilead is pushing into cancer drugs with the takeovers of biotech companies Forty Seven, Kite Pharma and now Immunomedics. It’s also signed a collaboration deal with Arcus Biosciences (RCUS).
Gilead used to be a powerhouse in treating hepatitis C.
The drug called Sovaldi gained Food and Drug Administration approval in 2013. The following year, it raked in $10.3 billion in sales. Gilead also had success with drugs dubbed Harvoni and Epclusa. About 95% of hepatitis C patients can now be cured with treatment.
In 2016, hepatitis C drug sales tumbled 23% as the number of cures increased. That trend has persisted ever since despite the addition of a new hepatitis C drug known as Vosevi.
Gilead Sciences Earnings Expected To Rise Again
In the wake of that hepatitis C drug sales cliff, earnings have mostly painted a dim picture. That looked to turn around in the third quarter. Then, earnings popped 29% to $2.11 per share. Sales rose 17% to $6.58 billion.
But the earnings release didn’t help Gilead stock as shares lost 2.2% on the report.
Analysts polled by FactSet expect another rebound in the fourth quarter. On average, they expect Gilead to report adjusted earnings of $1.73 per share on $6.43 billion in sales, up a respective 33% and 9% year over year.
Still, Gilead stock isn’t completely lining up with CAN SLIM rules for investing. Investors should look for stocks with 20%-25% or better earnings and sales growth in the most recent quarter.
Taking into account fundamental and technical measures, Gilead stock had a weak Composite Rating of 41 as of Dec. 16, according to IBD Digital. That puts shares in the bottom half of all stocks.
Annual Metrics: What Does 2019 Tell Us?
In 2019, Gilead earnings were $6.63 per share, minus certain items. That edged down a fraction on a year-over-year basis. Operating cash flow came out to $6.69 per share — less than 1% ahead of profit. The fastest-growing companies have operating cash flow at least 20% above earnings per share.
But sales inched up 1% to $22.45 billion. That was the first time sales rose since 2015.
Less bullishly, Gilead’s annual pretax margin hit 47.6% — its lowest in six years. Pretax margin also slowed for the fourth year running. When looking for strong stocks, it’s smart to consider those with accelerating pretax margins.
In 2020, analysts polled by FactSet expect Gilead to earn $6.61 per share on $23.7 billion in sales. Earnings would climb by 4% and sales 5%.
GILD Stock Technical Analysis: No Chart Pattern
Shares of Gilead stock are currently trading below their 50-day and 200-day moving averages.
Gilead has an RS Rating of just 5, meaning its shares have outperformed just 5% of stocks over the last 12 months. Leading stocks tend to have RS Ratings of 80 or above.
Gilead stock ranks just No. 166 out of 636 publicly traded biotech stocks by Composite Rating in IBD’s Stock Checkup. Keep tabs on those scores by visiting IBD Digital.
Which Mutual Funds Are Investing In Gilead?
Slightly more funds increased their GILD stock positions last quarter vs. those that cut their holdings, 1,133 vs. 991.
For now, Gilead stock has an Accumulation/Distribution grade of B-, meaning more institutional investors are buying than selling shares.
GILD Stock News: Coronavirus Treatment Gets FDA Nod
In May, the Food and Drug Administration granted Gilead an emergency use authorization for the drug now called Veklury. Gilead’s coronavirus treatment officially gained approval in October.
Physicians can administer remdesivir intravenously each day for five days or 10 days in patients hospitalized with Covid-19.
In late June, Gilead set a $3,120 price tag for U.S. commercial patients in need of its coronavirus treatment. The price tag for patients not covered by private insurance is $2,340. Those prices undercut more bullish expectations.
The Institute for Clinical and Economic Review, which examines the value of drugs, put a fair price for the coronavirus treatment at $4,500, RBC Capital Markets analyst Brian Abrahams said in a report. A week earlier, remdesivir gained conditional marketing authorization in Europe.
Will GILD Stock Fly On Coronavirus Treatment?
In an open letter from Chief Executive Daniel O’Day on Oct. 8, Gilead reported its data on Covid-19 patients recovering on average five days faster with remdesivir, and seven days faster for the most seriously ill. Gilead sells the drug under the band name Veklury.
Moreover, O’Day wrote that remdesivir reduced the likelihood of patients progressing to more severe stages of the disease where they would require new or additional oxygen support. And he wrote that in the largest group of patients in the study, those on low-flow oxygen, there was a significant reduction in mortality in a post-hoc analysis.
That day, the company said the New England Journal of Medicine published final results from a U.S. National Institute of Allergy and Infectious Diseases Phase 3 trial showing Veklury “resulted in consistent, clinically meaningful improvements across multiple outcome assessments compared with placebo in Covid-19 patients.
“The final results demonstrate that treatment with Veklury resulted in a faster time to recovery than previously reported,” Gilead said in a news release.
But just a week later, the WHO reported that remdesivir appeared to have little or no effect on hospitalized Covid-19 patients, as indicated by overall mortality, initiation of ventilation and duration of hospital stay.
2 Million Treatment Courses Of Veklury
Also on Oct. 8, Gilead announced a new supply deal with the European Union for its remdesivir, and GILD stock edged higher. Gilead said it’s on pace to produce more than 2 million treatment courses of Veklury this year.
On Oct. 5, GILD stock rose 2.3% on the news that President Trump had received remdesivir over the previous days after contracting Covid-19.
Gilead stock also edged higher more than 1% on Sept. 14, after the company announced its $21 billion acquisition of Immunomedics. That company’s drug Trodelvy recently received accelarate approval from the FDA to treat adult patients with metastatic triple-negative breast cancer who have received at least two prior therapies.
Also helping shares on Sept. 14 was the announcement of positive clinical trials data by Eli Lilly and partner Incyte (INCY) for their Covid-19 treatment candidate combined with remdesivir. They said they would seek U.S. FDA emergency use authorization for the treatment.
Other Gilead Stock News
On Aug. 19, GILD stock fell 4.9% after the company announced the FDA’s unexpected decision to seek two more years of data for the company’s rheumatoid arthritis drug, known as filgotinib.
But Gilead stock rose 1% on Sept. 28 after European officials approved filgotinib as a treatment for rheumatoid arthritis. It’s sold under the brand name Jyseleca.
On Dec. 15, Gilead said it wouldn’t seek FDA approval for filgotinib as a rheumatoid arthritis treatment. It also transferred primary responsibility in Europe to its partner, Galapagos (GLPG).
Gilead stock jumped in early March after the biotech company announced its $4.9 billion plan to buy Forty Seven, a cancer-focused player. Forty Seven is testing an immuno-oncology drug in blood cancers and solid tumors.
In 2017, Gilead spent $11.9 billion to acquire Kite Pharma, the maker of Yescarta, a CAR-T drug. CAR-T drugs also target cancer by training the immune system.
So, Is Gilead Stock A Buy?
Based on CAN SLIM investing principles, the short answer is no. Gilead stock is not a buy right now based on the metrics that other big winning stocks showed at the beginning of major price runs.
Investors should look for stocks that beat expectations while growing sales and earnings.
Shares of GILD stock currently aren’t forming a clear pattern or consolidation, as of Dec. 16, according to MarketSmith.com.
(Related: See stocks near a buy zone.)
It will be key to watch for data from additional studies of Veklury in coronavirus treatment, including its possible use in combination with other drug therapies. Also, it’s testing an inhaled version, and other studies remain ongoing.
Is it time to buy or sell these other large-cap stocks? Keep tabs on IBD analysis.
Follow Allison Gatlin on Twitter at @IBD_AGatlin.
YOU MAY ALSO LIKE:
Stocks To Buy And Watch: Top IPOs, Big And Small Caps, Growth Stocks
Looking For Growth? Take A Look At These Biotechnology Stocks
Join IBD Live For Stock Ideas Each Morning Before The Open
Is Gilead’s Coronavirus Drug On Track To Become A Mega-Blockbuster?
IBD Stock Of The Day: See How To Find, Track And Buy The Best Stocks
0 notes
Text
Global Hepatitis C Drugs Market would be US$ 5.9 Billion by 2025 | Renub Research
According to the latest report by Renub Research, titled "Hepatitis C Drugs Market Global Forecast By Drugs, Distribution Channels, Regions, Company Analysis" Hepatitis C affects the liver of patient though viral infection caused by the hepatitis C virus. This often leads to cirrhosis which may develop complications just like liver failure, liver cancer, gastric varices and esophageal. This is a deadly disease as it doesn’t show any symptoms in the early stage. Symptoms like fever, abdominal pain, dark urine, poor appetite, headaches, nausea, vomiting, joint pain, clay colored stools, and yellow tinged skin are shown at the critical stage. Infection through HIV and people suffering from alcoholism are more prone to Hepatitis C disease. Global Hepatitis C Drugs Market is expected to be around US$ 5.9 Billion by 2025, according to Renub Research analysis.

Causes of this infection are unsterilized medical equipment, blood transfusion from infected mother to newborn baby and sharing needles. Countries like India, China and Brazil will be the major market as compared to the market in developed countries. The hepatitis C drugs market will also propel due to initiative taken by governments and NGOs around the world by raising awareness and educating people to challenge hepatitis C infection. The market for Hepatitis C drug is challenged by high drug cost and therapy along with the stringent regulatory norms are delaying advancements and arrivals of new products in the market, restraining its growth. Hepatitis C treatment requires oral injection and weekly dosage, the medicine used can lead to side effects which can damage other organs of patients permanently.
COVID-19 Impact on Hepatitis C Drug Market
Treatment of COVID-19 patients is the prime focus of healthcare facilities, and other patients are unable to get treated properly. Telehealth has become a trend during this period as most of the health experts are providing consultation through this system. Governments around the world are investing hugely in health systems to fight against COVID-19. These initiatives will be beneficial in the long term; after this pandemic, medical experts will focus on the development of vaccine and drugs for other diseases like Hepatitis C as well.
In the year 2019, numerous drugs were approved for the treatment of Hepatitis C disease. Mavyret was approved by USFDA for the treatment of all types of genotypes for Hepatitis C disease. Around 130 – 150 million people are infected due to this virus, around 700,000 people die from Hepatitis C related disease. Around 15% to 20% of infected people have liver cancer and liver cirrhosis, which is a greater concern in the treatment of this disease.
Request a free Sample copy of the report: https://www.renub.com/request-sample-page.php?gturl=hepatitis-c-market-and-forecast-hepatitis-c-pipeline-drugs-sales-and-forecast-clinical-trials-global-466-p.php
Market Summary:
Drugs: This research report has covered the market and market share of Harvoni, Mavyret, Sovaldi, Zepatier, Viekira Pak, Epclusa, Vosevi, and other drugs. According to Renub Research analysis, Mavyret will lead the global hepatitis C market.
Distribution Channels: The market and market share of Retail Pharmacies, Hospital Pharmacies, and Online Pharmacy are given in this research report. Hospital Pharmacies are dominating the market for Hepatitis C drug as compared to other distribution channels according to Renub Research analysis.
Regions: This research report has covered the market and market share analysis for Europe, Latin America, Middle East & Africa, North America, and the Asia Pacific. According to Renub Research analysis, Europe and North America have significant market share than other regions.
Companies: This research report has covered Company Overview, Recent Development or Strategy, and Revenue analysis for Gilead Sciences, Bristol-Myers Squibb, Abbvie, Merck, and other companies.
About Company:
Renub Research is a Market Research and Consulting Company. We have more than 10 years of experience especially in international Business-to-Business Researches, Surveys and Consulting. We provide wide range of business research solutions that helps companies in making better business decisions. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses. Our wide clientele comprises of major players in Life Sciences, Information Technology, Telecom, Financial Services (Banking, Insurance), Energy, Retail, Manufacturing, Automotive, and Social sector. Our clients rely on our market analysis and data to make informed knowledgeable decisions. We are regarded as one of the best providers of knowledge. Our pertinent analysis helps consultants, bankers and executives to make informed and correct decisions.
Contact Us:
Renub Research
Phone No: +1 678-302-0700 (USA) | +91-120-421-9822 (IND)
Email: [email protected]
Web: https://www.renub.com
Follow on Linkedin: https://www.linkedin.com/company/renub-research
#hepatitis c drug market#global hepatitis c drug market#hepatitis c market#hepatitis c drug market forecast#hepatitis c drug market size
0 notes
Text
The Coronavirus Coping Portfolio (QDEL, GILD, QBIO, CYDY)

The COVID-19 pandemic has forced a dramatic realignment of social, cultural, and economic agendas because, in all cases, the healthcare issue has priority. It’s not a genuine public debate – if you don’t solve the healthcare crisis, you can’t solve any of the other crises that have been boiling over in the public discourse.
That puts a ton of pressure on the vaccine race. But an effective vaccine will take at least another 4-6 months, and probably longer than that. Which brings us to the coping tools: Therapeutics and diagnostics.
For investors, the coping theme is a treasure trove. Companies able to offer real resources to help manage the situation until a viable vaccine candidate is available will reap huge rewards. We profile several stocks on this interesting playing board here: Quidel Corporation (NASDAQ:QDEL), Gilead Sciences, Inc. (NASDAQ:GILD), Q BioMed Inc. (OTCMKTS:QBIO), and CytoDyn Inc (OTCMKTS:CYDY).
Quidel Corporation (NASDAQ:QDEL) leapt onto the stage in spectacular form on Monday after news spread that the FDA approved its antigen testing method following the close of trading last Friday. An effective antigen test is a superior option to the PCR RNA testing we have been leveraging thus far in our fight against the new coronavirus.
The QDEL antigen test is expected to change the game for testing for COVID-19, with results in minutes rather than days. That’s a key part of an effective “test and trace” approach to tracking and defeating this enemy. The stock exploded higher in response to the news, but there may still be room to run.
Quidel Corporation (NASDAQ:QDEL) frames itself as a company that develops, manufactures, and markets diagnostic testing solutions for applications in infectious diseases, cardiology, thyroid, women's and general health, eye health, gastrointestinal diseases, and toxicology worldwide.
It offers Sofia and Sofia 2 fluorescent immunoassay systems; QuickVue, a lateral flow immunoassay products; and InflammaDry and AdenoPlus, a point-of-care products for the detection of infectious and inflammatory diseases and conditions of the eye.
The company also provides Triage MeterPro, a portable testing platform that enables physicians to promote enhanced health outcomes, as well as the detection of certain drugs of abuse; Triage BNP test for use on Beckman Coulter lab analyzers; and Triage TOX drug screen, which provides results for the determination of the presence of drug and/or the major metabolites in urine. In addition, the company offers traditional cell lines, specimen collection devices, media, and controls for use in laboratories that culture and test for various human viruses, including respiratory and herpes family viruses; and cell-based products comprising tubes, shell vials, and multi-well plates.
The stock has been ripping over recent days, up something like 50% in that time.
Quidel Corporation (NASDAQ:QDEL) pulled in sales of $174.7M in its last reported quarterly financials, representing top line growth of 18%. In addition, the company is battling some balance sheet hurdles, with cash levels struggling to keep up with current liabilities ($108.8M against $136.2M, respectively).
Gilead Sciences, Inc. (NASDAQ:GILD) is the leading player in the treatment, or therapeutic, domain when it comes to the pandemic. It’s ebola anti-viral asset, remdesivir, has shown legitimate promise as a weapon against COVID-19, especially when administered early in the disease progression.
That said, the results suggest this isn’t a “silver bullet” drug, and it has to be administered at a hospital, where few “early” patients ever are. But it does work, and evidence mounts that it represents a key part of our response puzzle.
Gilead Sciences, Inc. (NASDAQ:GILD) promulgates itself as a biopharmaceutical company that discovers, develops, and commercializes therapeutics in the areas of unmet medical needs in the United States, Europe, and internationally.
The company's products include Biktarvy, Descovy, Odefsey, Genvoya, Stribild, Complera/Eviplera, Atripla, Truvada, Viread, Emtriva, and Tybost for the treatment of human immunodeficiency virus (HIV) infection in adults; and Vosevi, Vemlidy, Epclusa, Harvoni, Sovaldi, Viread, and Hepsera products for treating liver diseases.
It also provides Yescarta, a chimeric antigen receptor T cell therapy for adult patients with relapsed or refractory large B-cell lymphoma; Zydelig, a PI3K delta inhibitor for certain blood cancers; Letairis, an oral formulation of an endothelin receptor antagonist for pulmonary arterial hypertension; Ranexa, a tablet to treat chronic angina; and Lexiscan, an injection for use as a pharmacologic stress agent in radionuclide myocardial perfusion imaging.
In addition, the company offers Cayston, an inhaled antibiotic for the treatment of respiratory systems in cystic fibrosis patients; Tamiflu, an oral antiviral capsule for the treatment and prevention of influenza A and B; AmBisome, an antifungal agent to treat serious invasive fungal infections; and Macugen, an anti-angiogenic oligonucleotide to treat neovascular age-related macular degeneration.
It will be interesting to see if the stock can break out of its recent sideways action. Over the past week, the stock is net flat, and looking for something new to spark continuation to the upside.
Gilead Sciences, Inc. (NASDAQ:GILD) generated sales of $5.5B, according to information released in the company's most recent quarterly financial report. That adds up to a sequential quarter-over-quarter growth rate of -5.6% on the top line. In addition, the company has a strong balance sheet, with cash levels far exceeding current liabilities ($21B against $8.9B).
Q BioMed Inc. (OTCMKTS:QBIO) is a biomedical acceleration and development company that focuses on licensing, acquiring, and providing resources to life sciences and healthcare companies.
The company’s ace offering is Strontium Chloride SR89, a radiopharmaceutical therapeutic for the treatment of bone cancer pain therapies. Q BioMed Inc. has a research partnership with Mannin Research Inc. for the development of therapeutics to treat a variety of vascular diseases, including the new coronavirus.
In addition, the company is working on adjunct treatments for complications stemming from COVID-19. The main asset under development (rapid path) through the company’s partner, Mannin, is targeting the stabilization of 'leaky vessels' that play a critical part in organ injury, a major determinant of negative outcomes in patients affected by several infectious diseases, including influenza and the current COVID-19 pandemic.
Q BioMed Inc. is a biotech acceleration and commercial stage company focused on licensing and acquiring undervalued biomedical assets in the healthcare sector. Q BioMed is dedicated to providing these target assets the strategic resources, developmental support, and expansion capital needed to ensure they meet their developmental potential, enabling them to provide products to patients in need.
The company focuses exclusively in the biotechnology and healthcare sectors, targeting a broad spectrum of biomedical products and healthcare solutions. Q’s expertise is in business & product development and the capital formation required for phased advancement of products.
Q BioMed’s team assists companies by utilizing its strategic partners and network of experts to provide public market access for private company assets. 80% of biomedical start-ups lack capital and resources to transition from incubation to development and beyond.
Q BioMed Inc. (OTCMKTS:QBIO) expects to maximize risk-adjusted returns by focusing on value-driven assets from early stage to near-revenue businesses where the technical, regulatory, and commercial risks have been mitigated or where major valuation inflections are imminent.
CytoDyn Inc (OTCMKTS:CYDY) is another treatment play. The company is currently testing the efficacy of its HIV drug, leronlimab. So far, CYDY is actually riding a wave of positive momentum in terms of the direction of this narrative.
As a result, shares of the stock are up as much as 900% in the past 3-4 months.
CytoDyn Inc (OTCMKTS:CYDY) promulgates itself as a late-stage biotechnology company that focuses on the clinical development and commercialization of humanized monoclonal antibodies to treat human immunodeficiency virus (HIV) infection.
Its lead product is PRO 140, a therapeutic anti-viral agent, which is in Phase IIb extension study for HIV as monotherapy, rollover study for HIV as a combination therapy, Phase IIb/III investigative trial for HIV, Phase Ib/II trial for triple-negative breast cancer, and Phase II trial for graft-versus-host disease.
CytoDyn Inc. has strategic agreement with Samsung BioLogics Co. Ltd. for the clinical and commercial manufacturing of leronlimab. The company was formerly known as RexRay Corporation. CytoDyn Inc. was incorporated in 2002 and is based in Vancouver, Washington.
And the stock has been acting well over recent days, up something like 12% in that time.
CytoDyn Inc (OTCMKTS:CYDY) had no reported sales in its last quarterly financial data. In addition, the company is battling some balance sheet hurdles, with cash levels struggling to keep up with current liabilities ($7.1M against $41M, respectively).
Read the full article
0 notes
Text
Anvisa aprova novo medicamento para tratamento da hepatite C em adultos
Anvisa aprova novo medicamento para tratamento da hepatite C em adultos
Vosevi®, medicamento da farmacêutica Gilead Sciences, trata todos os genótipos de infecção crônica pelo vírus da hepatite C (HCV) em adultos com apenas um comprimido ao dia.
A Agência Nacional de Vigilância Sanitária (Anvisa) acaba de aprovar o uso de Vosevi® (sofosbuvir/velpatasvir/voxilaprevir) da Gilead Sciences no Brasil. O medicamento é indicado para pacientes com infecção crônica pelo HCV,…
View On WordPress
0 notes
Text
FDA Warns of Liver Problems for Some Taking Hep C Drugs
FDA Warns of Liver Problems for Some Taking Hep C Drugs
THURSDAY, Aug. 29, 2019 — Taking the hepatitis C drugs Mavyret, Zepatier or Vosevi can trigger rare cases of severe liver problems or liver failure in patients who already have moderate-to-severe liver impairment, the U.S. Food and Drug Administration warned Wednesday.
The agency has identified 63 cases of worsening liver function, some resulting in liver failure or death, among patients taking…
View On WordPress
0 notes
Text
FDA Warns of Problems for Some Taking Hep C Drugs
Taking the hepatitis C drugs Mavyret, Zepatier or Vosevi can trigger rare cases of severe liver problems or liver failure in patients who already have moderate-to-severe liver impairment, the FDA warned Wednesday.
The post FDA Warns of Problems for Some Taking Hep C Drugs appeared first on Clean Energy Health.
from WordPress https://cleanenergyhealth.com/fda-warns-of-problems-for-some-taking-hep-c-drugs/
0 notes
Text
FDA Warns of Problems for Some Taking Hep C Drugs
Taking the hepatitis C drugs Mavyret, Zepatier or Vosevi can trigger rare cases of severe liver problems or liver failure in patients who already have moderate-to-severe liver impairment, the FDA warned Wednesday.
FDA Warns of Problems for Some Taking Hep C Drugs published first on https://wittooth.tumblr.com/
0 notes
Photo
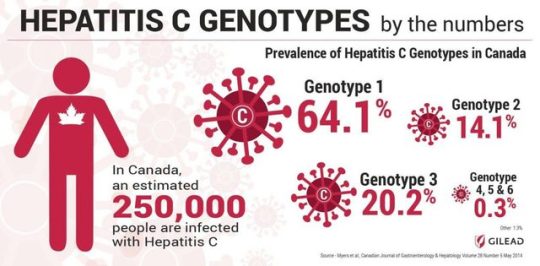
Canada Welcomes Gilead Science’s Vosevi to Treat Canadian Sufferers from HCV Disease Vosevi is the latest HCV regimen in Gilead's series of sofosbuvir-based treatments that offer people living with HCV a short course of therapy to cure their HCV infection, with the convenience associated with once-daily single-tablet regimens.
0 notes
Text
Tahukah Anda 4 Obat Baru di Bulan Juli 2017 yang Disetujui FDA
Tahukah Anda 4 Obat Baru di Bulan Juli 2017 yang Disetujui FDA
farmasetika.com – Setiap bulan selalu muncul produk obat atau perluasan indikasi baru yang disetujui oleh FDA (Food and Drug Adminsitration). Pada bulan Juli 2017, ada 4 obat baru yakni Benlysta, Lusduna Nexvue, Orencia, dan Vosevi.
1. Benlysta
FDA menyetujui formulasi subkutan dari GSK, belimumab (Benlysta) pada tanggal 21 Juli 2017.1
Benlysta, yang awalnya disetujui sebagai formulasi intravena…
View On WordPress
0 notes
Text
Hepatitis C Drugs Market Global Forecast By Distribution Channels - Renub Research
Hepatitis C is a viral infection that causes severe damage to the human body; it is an infection caused by a virus that damages the liver, which leads to inflammation. It spread through contaminated blood. The prevalence of hepatitis C infection among the global populace is escalating. This is mainly caused by sharing needles, unsterilized medical equipment, and a blood transfusion from infected mothers to newborn babies. As per Renub Research Analysis, the Global Hepatitis C drugs Market would be US$ 5.9 Billion by 2025.
In recent years, several drugs have been launched due to the high prevalence of Hepatitis C across the globe. Hepatitis C drug market is always developing, and competition is between new launches of fixed-dose combination led to a change in revenue of companies. The newly launched drug by AbbiVie i.e., Mavyret, has led to a downfall in the revenue of one of the market leader i.e. Gilead’s. Mavyret has several advantages compared to other drugs such as its treatment course is shorter, and it is much cheaper. Harvoni drugs are popular in India, and it's easier affordable among the public.

Oral injections and dosages are required weekly in Hepatitis C; at the same time, its side effects can lead to permanent damage of other organs of patients. Around the globe, many clinical trials, as well as numerous successful drug clearances, give traction to this market. Apart from that, the rising number of regulatory approvals will further boost the market during the forecast time frame.
Hepatitis C drugs have witnessed numerous drug approvals likewise, in the year 2019, USFDA approves Mavyret for the treatment of pediatric hepatitis patients. For the treatment of all genotypes of Hepatitis C, Mavyret was the first treatment.
In the North American region, rising adoption of hepatitis C drugs will favor the surging of the market. The market share of Asia – Pacific’s and Latin American regions will grow during the forecast period.
Renub Research report titled “Hepatitis C Drugs Market Global Forecast By Drugs (Mavyret, Viekira Pak, Harvoni, Epclusa, Sovaldi, Vosevi, Zepatier, Others), Distribution Channels (Hospital Pharmacies, Retail Pharmacies, Online Pharmacy), Regions (North America, Europe, Asia Pacific, Latin America, Middle East & Africa), Company Analysis (Abbvie, Gilead Sciences, Merck ,Bristol-Myers Squibb, Others)” provides a complete analysis Hepatitis C market globally.
Request a free Brochure copy of the report: https://www.renub.com/request-sample-page.php?gturl=hepatitis-c-market-and-forecast-hepatitis-c-pipeline-drugs-sales-and-forecast-clinical-trials-global-466-p.php
Mavyret is the leading drug in the global hepatitis C market
Complete analysis of 7 drugs Mavyret, Viekira Pak, Harvoni, Epclusa, Sovaldi, Vosevi, Zepatier, Others are given in the report. Mavyret is the leading drug in the global hepatitis C market.
Distribution Channel is dominated by Hospital Pharmacies in the Global Hepatitis C Drugs Market
Distribution channels like Hospital Pharmacies, Retail Pharmacies & Online Pharmacy are covered in this research report. Hospital Pharmacies is the leading distribution channel in the global hepatitis C drug market.
North America and Europe are the top two leading regions in the Global Hepatitis C Drugs Market
The report studies the hepatitis C drug market of the following regions: North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America and Europe are the top two leading regions in the global hepatitis C drug market.
All the companies have been studied from two points
• Recent Developments
• Sales Analysis
By Company - Global Hepatitis C Drugs Market
• Abbvie
• Gilead Sciences, Inc.
• Merck & Co. Inc.
• Bristol-Myers Squibb
• Others
By Regions - Global Hepatitis C Drugs Market
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East & Africa
By Drugs - Global Hepatitis C Drugs Market
• Mavyret
• Viekira Pak
• Harvoni
• Epclusa
• Sovaldi
• Vosevi
• Zepatier
• Others
By Distribution Channels - Global Hepatitis C Drugs Market
• Hospital Pharmacies
• Retail Pharmacies
• Online Pharmacy
About Us:
Renub Research is a Market Research and Consulting Company. We have more than 10 years of experience especially in international Business-to-Business Researches, Surveys, and Consulting. We provide a wide range of business research solutions that helps companies in making better business decisions. Our clients rely on our market analysis and data to make informed knowledgeable decisions. Our pertinent analysis helps consultants, bankers and executives to make informed and correct decisions.
Contact Us
Renub Research
Phone: +1-678-302-0700
Email: [email protected]
Website: www.renub.com
Follow us on LinkedIn: www.linkedin.com/company/renub-research
#Hepatitis C Drug Market#Global Hepatitis C Drug Market#Hepatitis C Market#Hepatitis C Drug Market Forecast#Hepatitis C Drug Market Size#Hepatitis C Drug Market Share
0 notes
Text
The Coronavirus Coping Portfolio (QDEL, GILD, QBIO, CYDY)

The COVID-19 pandemic has forced a dramatic realignment of social, cultural, and economic agendas because, in all cases, the healthcare issue has priority. It’s not a genuine public debate – if you don’t solve the healthcare crisis, you can’t solve any of the other crises that have been boiling over in the public discourse.
That puts a ton of pressure on the vaccine race. But an effective vaccine will take at least another 4-6 months, and probably longer than that. Which brings us to the coping tools: Therapeutics and diagnostics.
For investors, the coping theme is a treasure trove. Companies able to offer real resources to help manage the situation until a viable vaccine candidate is available will reap huge rewards. We profile several stocks on this interesting playing board here: Quidel Corporation (NASDAQ:QDEL), Gilead Sciences, Inc. (NASDAQ:GILD), Q BioMed Inc. (OTCMKTS:QBIO), and CytoDyn Inc (OTCMKTS:CYDY).
Quidel Corporation (NASDAQ:QDEL) leapt onto the stage in spectacular form on Monday after news spread that the FDA approved its antigen testing method following the close of trading last Friday. An effective antigen test is a superior option to the PCR RNA testing we have been leveraging thus far in our fight against the new coronavirus.
The QDEL antigen test is expected to change the game for testing for COVID-19, with results in minutes rather than days. That’s a key part of an effective “test and trace” approach to tracking and defeating this enemy. The stock exploded higher in response to the news, but there may still be room to run.
Quidel Corporation (NASDAQ:QDEL) frames itself as a company that develops, manufactures, and markets diagnostic testing solutions for applications in infectious diseases, cardiology, thyroid, women's and general health, eye health, gastrointestinal diseases, and toxicology worldwide.
It offers Sofia and Sofia 2 fluorescent immunoassay systems; QuickVue, a lateral flow immunoassay products; and InflammaDry and AdenoPlus, a point-of-care products for the detection of infectious and inflammatory diseases and conditions of the eye.
The company also provides Triage MeterPro, a portable testing platform that enables physicians to promote enhanced health outcomes, as well as the detection of certain drugs of abuse; Triage BNP test for use on Beckman Coulter lab analyzers; and Triage TOX drug screen, which provides results for the determination of the presence of drug and/or the major metabolites in urine. In addition, the company offers traditional cell lines, specimen collection devices, media, and controls for use in laboratories that culture and test for various human viruses, including respiratory and herpes family viruses; and cell-based products comprising tubes, shell vials, and multi-well plates.
The stock has been ripping over recent days, up something like 50% in that time.
Quidel Corporation (NASDAQ:QDEL) pulled in sales of $174.7M in its last reported quarterly financials, representing top line growth of 18%. In addition, the company is battling some balance sheet hurdles, with cash levels struggling to keep up with current liabilities ($108.8M against $136.2M, respectively).
Gilead Sciences, Inc. (NASDAQ:GILD) is the leading player in the treatment, or therapeutic, domain when it comes to the pandemic. It’s ebola anti-viral asset, remdesivir, has shown legitimate promise as a weapon against COVID-19, especially when administered early in the disease progression.
That said, the results suggest this isn’t a “silver bullet” drug, and it has to be administered at a hospital, where few “early” patients ever are. But it does work, and evidence mounts that it represents a key part of our response puzzle.
Gilead Sciences, Inc. (NASDAQ:GILD) promulgates itself as a biopharmaceutical company that discovers, develops, and commercializes therapeutics in the areas of unmet medical needs in the United States, Europe, and internationally.
The company's products include Biktarvy, Descovy, Odefsey, Genvoya, Stribild, Complera/Eviplera, Atripla, Truvada, Viread, Emtriva, and Tybost for the treatment of human immunodeficiency virus (HIV) infection in adults; and Vosevi, Vemlidy, Epclusa, Harvoni, Sovaldi, Viread, and Hepsera products for treating liver diseases.
It also provides Yescarta, a chimeric antigen receptor T cell therapy for adult patients with relapsed or refractory large B-cell lymphoma; Zydelig, a PI3K delta inhibitor for certain blood cancers; Letairis, an oral formulation of an endothelin receptor antagonist for pulmonary arterial hypertension; Ranexa, a tablet to treat chronic angina; and Lexiscan, an injection for use as a pharmacologic stress agent in radionuclide myocardial perfusion imaging.
In addition, the company offers Cayston, an inhaled antibiotic for the treatment of respiratory systems in cystic fibrosis patients; Tamiflu, an oral antiviral capsule for the treatment and prevention of influenza A and B; AmBisome, an antifungal agent to treat serious invasive fungal infections; and Macugen, an anti-angiogenic oligonucleotide to treat neovascular age-related macular degeneration.
It will be interesting to see if the stock can break out of its recent sideways action. Over the past week, the stock is net flat, and looking for something new to spark continuation to the upside.
Gilead Sciences, Inc. (NASDAQ:GILD) generated sales of $5.5B, according to information released in the company's most recent quarterly financial report. That adds up to a sequential quarter-over-quarter growth rate of -5.6% on the top line. In addition, the company has a strong balance sheet, with cash levels far exceeding current liabilities ($21B against $8.9B).
Q BioMed Inc. (OTCMKTS:QBIO) is a biomedical acceleration and development company that focuses on licensing, acquiring, and providing resources to life sciences and healthcare companies.
The company’s ace offering is Strontium Chloride SR89, a radiopharmaceutical therapeutic for the treatment of bone cancer pain therapies. Q BioMed Inc. has a research partnership with Mannin Research Inc. for the development of therapeutics to treat a variety of vascular diseases, including the new coronavirus.
In addition, the company is working on adjunct treatments for complications stemming from COVID-19. The main asset under development (rapid path) through the company’s partner, Mannin, is targeting the stabilization of 'leaky vessels' that play a critical part in organ injury, a major determinant of negative outcomes in patients affected by several infectious diseases, including influenza and the current COVID-19 pandemic.
Q BioMed Inc. is a biotech acceleration and commercial stage company focused on licensing and acquiring undervalued biomedical assets in the healthcare sector. Q BioMed is dedicated to providing these target assets the strategic resources, developmental support, and expansion capital needed to ensure they meet their developmental potential, enabling them to provide products to patients in need.
The company focuses exclusively in the biotechnology and healthcare sectors, targeting a broad spectrum of biomedical products and healthcare solutions. Q’s expertise is in business & product development and the capital formation required for phased advancement of products.
Q BioMed’s team assists companies by utilizing its strategic partners and network of experts to provide public market access for private company assets. 80% of biomedical start-ups lack capital and resources to transition from incubation to development and beyond.
Q BioMed Inc. (OTCMKTS:QBIO) expects to maximize risk-adjusted returns by focusing on value-driven assets from early stage to near-revenue businesses where the technical, regulatory, and commercial risks have been mitigated or where major valuation inflections are imminent.
CytoDyn Inc (OTCMKTS:CYDY) is another treatment play. The company is currently testing the efficacy of its HIV drug, leronlimab. So far, CYDY is actually riding a wave of positive momentum in terms of the direction of this narrative.
As a result, shares of the stock are up as much as 900% in the past 3-4 months.
CytoDyn Inc (OTCMKTS:CYDY) promulgates itself as a late-stage biotechnology company that focuses on the clinical development and commercialization of humanized monoclonal antibodies to treat human immunodeficiency virus (HIV) infection.
Its lead product is PRO 140, a therapeutic anti-viral agent, which is in Phase IIb extension study for HIV as monotherapy, rollover study for HIV as a combination therapy, Phase IIb/III investigative trial for HIV, Phase Ib/II trial for triple-negative breast cancer, and Phase II trial for graft-versus-host disease.
CytoDyn Inc. has strategic agreement with Samsung BioLogics Co. Ltd. for the clinical and commercial manufacturing of leronlimab. The company was formerly known as RexRay Corporation. CytoDyn Inc. was incorporated in 2002 and is based in Vancouver, Washington.
And the stock has been acting well over recent days, up something like 12% in that time.
CytoDyn Inc (OTCMKTS:CYDY) had no reported sales in its last quarterly financial data. In addition, the company is battling some balance sheet hurdles, with cash levels struggling to keep up with current liabilities ($7.1M against $41M, respectively).
Read the full article
0 notes
Text
FDA approves Gilead's HCV treatment as 2nd line therapy — 4 insights
The FDA approved Gilead Sciences' Vosevi as a second line therapy for chronic hepatitis C. Here's what you should know: 1. Vosevi is a fixed-dose, ...
from Google Alert - Gastroenterology http://ift.tt/2uammVS
0 notes