Photo

Stay away from this blog if you hate laughing!
2K notes
·
View notes
Photo
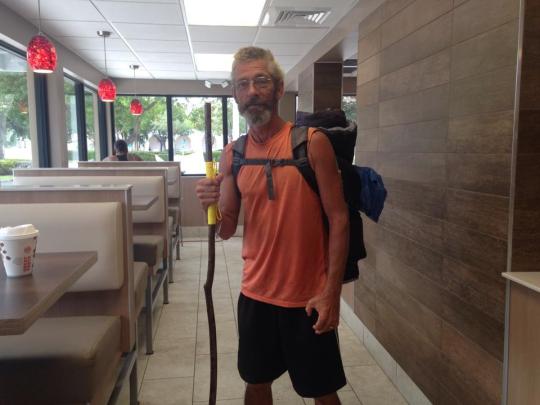
Yesterday a man came into my work, all scruffy and dirty with a big backpack on his shoulders, and as he was leaving I told him to stay dry because it’s been raining almost none stop for a few days.
He stopped and turned around and laughed, and then he showed me his new shoes that he just bought and said “I’ve been walking the perimeter of the United States; these shoes will last me another month, rain or sunshine.”
We talked for a bit and he told me about how he’s walking around the perimeter of the entire U.S. to raise awareness and money for the homeless. He told me about some things he’s seen and the places he’s been so far. Before he left, he gave me a big smile, said “God be with you,” and walked out the door with the happiest strut I’ve ever seen.
He started off in Virginia and made it all the way down here to Florida on foot, and he’s still got a long way to go. He was very kind and very optimistic.
I’ve never met someone like him before, so I want to try and spread him and his effort here on tumblr.
He isn’t very popular yet, but I really want to try to spread his word and raise awareness of the homelessness issue here in the U.S., and try to help him raise money for this cause.
His name is Leroy Bailey and if you want to follow him around the U.S. his facebook is here.
I didn’t get a photo of him so I took this one from his facebook
138K notes
·
View notes
Photo

Chemical reaction between Potassium Iodide and Lead Nitrate.
Otherwise known as ‘Golden Rain’.
[Click for more interesting science facts and gifs]
8K notes
·
View notes
Text
Hah long time no see everyone! Let's start the long being away with some good NO
...
Nitric Oxide that is.
This is a pretty cool compound since natural occurring Nitrogen and Oxygen usually come in 2. As in N2 or O2. This doesn't mean that NO isn't naturally occurring, it just means it might need to go through a chemical reaction to get to this compound.
NO is formed by electric sparks or high temperatures. This can be through natural occurrences such as lightning. A more convenient chemical reaction is using dilute nitric acid on mercury or copper.
On to the usual characteristics of the compound:
It's a colorless, toxic gas
You can make it through oxidation of nitrogen (loss of electrons)
Used in neurology (chemical/nervous signaling) in animals and humans.
Also used in medical field
Is a serious pollutant generated by automotive engines and thermal power plants.
That's quite a difference in application huh? Well I have NO more info today. (excuse my puns, didn't you miss me?)
I salute your future chemistry chronicles!
-Mona
when ur chemistry teacher asks if u understand and ur just like

114K notes
·
View notes
Photo
Happy Late 4th Everyone! Sorry for the neglect!
Happy Birthday America, keep on sparking.








What makes fireworks colorful?
It’s all thanks to the luminescence of metals. When certain metals are heated (over a flame or in a hot explosion) their electrons jump up to a higher energy state. When those electrons fall back down, they emit specific frequencies of light - and each chemical has a unique emission spectrum.
You can see that the most prominent bands in the spectra above match the firework colors. The colors often burn brighter with the addition of an electron donor like Chlorine (Cl).
But the metals alone wouldn’t look like much. They need to be excited. Black powder (mostly nitrates like KNO3) provides oxygen for the rapid reduction of charcoal (C) to create a lot hot expanding gas - the BOOM. That, in turn, provides the energy for luminescence - the AWWWW.
Aluminium has a special role — it emits a bright white light … and makes sparks!
Images: Charles D. Winters, Andrew Lambert Photography / Science Source, iStockphoto, Epic Fireworks, Softyx, Mark Schellhase, Walkerma, Firetwister, Rob Lavinsky, iRocks.com, Søren Wedel Nielsen
27K notes
·
View notes
Link
I've actually made ice cream in an ice cream maker before and that was cool and all. The basis was slowly mixing the ice cream while slowly lowering the temperature.
After reading this i researched and you could make some ice cream yourself without an ice cream maker! Which is more cost effective.
http://chemistry.about.com/cs/howtos/a/aa020404a.htm
I might try this when I have more time, but it would be really cool to try! The link even talks more in depth about what Justine was saying about rock salt which is also pretty cool itself.
Ice cream is my favorite thing to eat, but I’ve never wondered how its made. I mean you have your usual ingredients sugar, milk, and vanilla, but i would have never thought rock salt would be the main component.To begin with, the sugar is made up of carbon, oxygen, and hydrogen. Milk is mostly water with carbs, proteins, vitamins, fats, and minerals. The vanilla contains vanillin, ethyl vanillin and aqueous solvent. The rock salt is made up of crystallized sodium chloride. Each of these ingredients have a job in making the ice cream. Rock salt melts the ice to become liquidy. Ice is used to just keep the ice cream cold. Sugar comes from the glucose and fructose, and this removes the water. The vanilla comes from a vanillin plant, and milk, milk is just made up of all different components.
4 notes
·
View notes
Link
I love running shoes, especially light weight running shoes, now I know what material to thank for the Nikes I lace up before gym class and track.
Polyurethane is actually in a lot of other things other than running shoes too, it's in stuff like skateboard wheels, paints, varnishes, adhesives, and foams.
Polyester is also in a bunch of things. It can be a fabric in your clothes or part of your water bottle.
Some shoes contain cotton, leather, or natural rubber, but what you lace up before a run is probably an assortment of industrial chemicals derived from hydrocarbons. Polyester and polyurethane which are petroleum-based synthetics, make up 36 percent of the weight of the average running shoe....
4 notes
·
View notes
Link
Wow Lindsay! I never saw that people's hair turns green once out of a pool (since my hair is black) but I have heard of it. I always assumed that it was chlorine like you said, but the copper turning things green makes a lot more sense!
For example you all know how the Statue of Liberty is green now? Well the Statue of Liberty is actually made of copper and it used to be brownish gold like our copper pennies (when they're new). When copper oxidizes (Ooh chemistry!: meaning copper eventually lost electrons) it actually turns green.
So a living example of Lindsay's blog post, you learn a new thing everyday!
Don’t you hate it when your hair turns green after swimming? Ugh! It is the worst? I always wonder why that happens after I leave the pool! Being a swimmer all year round, it can definitely be frustrating!!
I did some research, and I found out what is the cause of the green hair after...
4 notes
·
View notes
Text
Oh look! Let's talk moles today!
A mole is essential in chemistry and a pain. It's the basis of most of the scientific equations we use to determine molar mass, particle numbers, numerous conversions, and a bunch of other things that are super important in determining anything in chemistry.
The definition of a mole is: a unit of measurement used in chemistry to express amounts of a chemical substance, defined as the amount of any substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12, the isotope of carbon with relative atomic mass 12.
LET'S BREAK THAT DOWN! Because that definition is scaring me as much as it is you.
A mole is actually basically a unit of measurement. To delve further in the carbon atom business basically the carbon atoms are constant. Moles defines the unit of 12 grams of carbon-12 atoms because it is something that can be measured.
The 6.0221415×10²³ guacas part of the joke is the number of atoms that are in 12 grams of carbon 12 atoms. That 6.0221415×10²³ is called avogradro's number.
That's basically what moles are, sorry if you don't get it! It's a very abstract thing to explain, This website might be able to help more!
http://chemistry.about.com/cs/generalchemistry/f/blmole.htm
Happy chemistry chronicles!
one guacamole is equal to 6.0221415×10²³ guacas
510K notes
·
View notes
Link
My mom always warned me about the chemicals in different makeup. This is partially why I barely use it ever in the first place. If anything I only use eyeliner, I usually never use concealer or foundation, so let's look at what chemicals are in eyeliner.
Eyeliner has many mediums, liquid, pencil, gel... and that's all my knowledge extends to, surprisingly all these types contain mainly the same things!
Pigments: Pigments include iron oxides to create blacks and browns, ultramarine for blue, chromium oxide for green, and titanium dioxide for white.
FIlm Formers: Film formers deposit a thin layer on your skin.
Thickeners: These are usually waxes, natural gums, and clays. This is what stabilizes the eyeliner formula and lets it stay on your eyelids.
There are a bunch of elements mentioned in the pigment alone, and chemists actually are a main component in cosmetic making, so there's a bunch of chemistry to be explored there!
To relate back to Aneesa's post, I wouldn't say eyeliner is particularly harmful, but there is some stuff in it you wouldn't want in your eye.
It’s not surprising that your concealer you put on daily maybe the reason why your skin condition is bad.
If you look deeper,dozens of harsh synthesized chemicals have made their way to approval through loopholes in US’s Federal Law and FDA.
Most of these chemicals are known to be great risk...
5 notes
·
View notes
Photo
And I bring another reblog for your entertainment!
But we all know by now how my blog works right? Let's relate it to chemistry!
Thanks to chemistry class I know exactly how a roll of film develops as well as the chemistry behind the process, right now I'll just describe what is on a roll of film. And how it works to develop pictures.
The film is made of plastic and the coating has silver halide salt that is suspended in gelatin. This silver halide is extremely light sensitive and reacts to light. When light hits the silver halides they turn into silver metal that forms the inverse of what you see in the camera and took a picture of.
The silver metal formed creates an image on the film. This means that the negatives you see on the film of old cameras (not digital everyone) is actually silver metal! Isn't that fascinating?
~In conclusion~
I guess that means that no pictures will develop in the boys' stomach from the pictureset above.
I hope you learned more about photography from this post! Also if you have anything you want me to cover just message me with the ask link on my sidebar. I'll hopefully know what to do!




278K notes
·
View notes
Link
Wow who knew there was so much behind the contact lens? I’ve wanted to get contact lenses for quite some time, but now I know what they’re exactly made out of.
My mother still acts as though they’re made from glass though, so I’m not getting any hydrogel in my eyes for a while.
Nowadays, many people’s eyesights are worsening due to television, staring at computer screens for hours, reading, and whatnot. If you’re like me and dislike glasses, you probably own contacts.
Contacts have come a long way. They used to be uncomfortable slices of glass. That’s right - glass! It...
3 notes
·
View notes
Link
I always wondered what the heck the letters on pencils meant, now I understand it's because of hardness and darkness. And darkness and hardness is determined by the graphite in pencils.
As for erasers, they are usually made of synthetic rubber, when on top of a pencil, however the blocks of eraser are usually made of vinyl.
Erasers work by being stickier than the paper, therefore the graphite clings onto the rubber rather than the paper and is removed.
A pencil is a utensil that a student like myself uses everyday especially in math, chemistry, and when I do basketball stats. Pencils used to be made of lead, however now pencils are made of graphite. This is ironic because everyone calls them lead pencils even though they’re not produced with lead anymore. Now pencils are made by grinding graphite and mixing it with clay and water. This will produce a paste substance that is then transferred into tubes to form the core of the pencil. Cedar wood is usually then used to form the casing around the graphite core, but first the core is superheated while in the metal tube so that it becomes smooth. The graphite rods are then fitted to the grooves in the pencil, and the eraser and grips are then added.
There are so many types of pencils that you have to classify one from another. To do so you have to follow certain steps. Firstly, pencils are classified for their darkness. “H” stands for hardness while “B” stands for blackness. This means that a pencil that is “9B” is lighter than a pencil labeled “9H.” The harder the graphite is the lighter the pencil shade will be. This rule applies to all pencils.
This relates to chemistry because graphite is used in the making of a pencil. Graphite has chemistry behind it as well as wood and the other parts of the pencil. Maybe for my fellow classmates, someone can find the chemistry of an eraser or a full pencil? That might be interesting! If anyone does find anything, let me know!
3 notes
·
View notes
Photo
Although I am posting this to give everyone some giggles (if you understand the joke that is)
If you don't understand, that's ok C12H22O11 is the chemical formula for table sugar. The maker of that post was funny and cubed it in order to make sugar cubes.
With that set aside, I'm going to analyze sugar today.
If you don't understand how to read chemical formulas I'll translate, Sugar has:
12 carbon atoms
22 hydrogen atoms
11 oxygen atoms
Let's start an analysis along with some chemistry math lessons...
The molar mass of this compound is 342.29 g/mol. This is found by finding the atomic weight of each element and multiplying each by their respective number of atoms.
For example, Hydrogen's atomic mass is 1, therefore it would be 1 x 22= 22. After adding all of the elements' atomic weight together you get the molar mass.
I hope you enjoyed and learned from that little analysis, bye!

431K notes
·
View notes
Link
I remember a flame lab we’ve done in school where the fire burned different colors because of different metal salts. I thought that was really cool, fire can have really vibrant colors from emerald green to magenta, it was really pretty.
Now I found out that blue fire is relatively easy to make too, but you should always take safety precautions when doing this kind of stuff. First of all you should roll up your sleeves and tie back your hair if it’s long. You should also clear an area for the experiment (you don’t want anything catching on fire!). Lastly, you should have water or a fire extinguisher nearby just in case anything bad happens.
Just for my followers to try if they wish! Be safe guys!
When everyone sees the word “fire” they usually think about the colors yellow, orange, or red. But many people do not realize that blue fire is also common and easy to make. Easy with the help of a little chemistry! And this article explains it! ...
6 notes
·
View notes

