#Coronary Microvascular Dysfunction
Text
Day 11 - O Mexiofrio - October 8
I slept well at the Albergue Camino Real. Which isn’t to say I didn’t still get up at 6 am. I think most of the pilgrims were out the door before I was.
This morning I left alone, yet still was part of a group of peregrinos. Most of them passed me, it’s true. But a set of 5 or 6 people stayed at my hiking speed, though we passed each other numerous times.
I walked past a small barn that…

View On WordPress
#Angina#camino#camino-de-santiago#CMD#Coronary Microvascular Disorders#Coronary Microvascular Dysfunction#El Camino#El Camino Ingles#El Camino Trail#heart#heart disease#Heart Failure#Heart Health#Hiking#IBERIA#Spain#Travel#Walking#women#Women of a certain age#Women&039;s Health#women&039;s heart
0 notes
Text
What is it:
Small vessel disease is a condition in which the walls of the small arteries in the heart aren't working properly. This reduces the flow of oxygen-rich blood to the heart, causing chest pain (angina), shortness of breath, and other signs and symptoms of heart disease.
Small vessel disease may also be called:
Coronary microvascular disease
Microvascular endothelial dysfunction
Small vessel disease is treatable but may be difficult to detect. The condition is typically diagnosed after a health care provider finds little or no narrowing in the main arteries of the heart despite the presence of symptoms that suggest heart disease.
Small vessel disease is more common in women and in people who have diabetes or high blood pressure.
Symptoms:
Small vessel disease signs and symptoms include:
Chest pain, squeezing or discomfort (angina), which may get worse with activity or emotional stress
Discomfort in the left arm, jaw, neck, back or abdomen along with chest pain
Shortness of breath
Tiredness and lack of energy
1 note
·
View note
Text
Coronary Microvascular Dysfunction Market is Expected to Expand at a Healthy Growth Rate During the Forecast Period (2023-2032), States DelveInsight | Caladrius Biosciences, AbbVie, Coroventis, Akcea
http://dlvr.it/T3Qpdc
0 notes
Text
Coronary Microvascular Dysfunction Market is Expected to Expand at a Healthy Growth Rate During the Forecast Period (2023-2032), States DelveInsight | Caladrius Biosciences, AbbVie, Coroventis, Akcea
http://dlvr.it/T3QZwS
0 notes
Text
Angina
Angina happens when your heart isn't getting enough blood, usually because of narrowed coronary arteries. Your heart may try to improve its blood supply by beating harder and faster. This causes symptoms of angina and is a sign that your heart needs to rest.
The key difference between angina and a heart attack is that angina is the result of narrowed (rather than blocked) coronary arteries. This is why, unlike a heart attack, angina does not cause permanent heart damage.
Some people experience episodes of angina before having a heart attack and may continue to experience it afterwards. Other people never experience angina before or after a heart attack.
Symptoms of Angina
Angina symptoms differ from person to person, but can include:
Discomfort, heaviness or tightness of the chest which may spread to the back, shoulders, neck or jaw. Other people describe it as a dull ache
Discomfort in the arm, neck or jaw with no chest discomfort
The discomfort can range from mild or dull to severe
What causes angina?
Angina is usually caused by coronary heart disease (also called atherosclerosis), which is the build-up of plaque in the walls of your arteries. This build-up can narrow one or more of the coronary arteries that feed blood to your heart and makes it harder for your heart to pump blood around your body.
How to treat angina
There are treatments that can be used to help control angina long-term. These include:
Opening up your arteries with a special balloon and stent (angioplasty)
Making a new way for blood to flow around a blocked artery (heart bypass surgery)
Medications to help prevent further angina episodes
Stable and unstable angina
Stable angina is when you get angina symptoms during moderate physical activity or when you are pushing yourself physically. These symptoms go away with rest and/or medication.
Unstable angina is when you get angina symptoms while doing very little or resting. This can happen to people who have never experienced angina before.
Stable angina can become unstable.
Microvascular angina
The coronary arteries supply blood to your heart muscle by routing it through a network of smaller blood vessels. When a blood flow problem occurs in one or more of the smaller blood vessels supplying blood to your heart, it is called microvascular angina, cardiac syndrome X, or coronary microvascular dysfunction.
Because of the reduced size of the blood vessels, it’s likely you will be advised to start a combination of medication and lifestyle changes rather than offered a stent or heart bypass surgery.
Women seem to be more likely than men to experience microvascular angina. The reasons for this have not yet been confirmed.
Preventing angina
Angina is a symptom of coronary artery disease or atherosclerosis. This is a process that is accelerated by a number of factors including unhealthy lifestyle choices such as smoking, poor diet, being overweight, physical inactivity and poor mental health and wellbeing.
Making changes to your risk factors can slow or stop the damage to your arteries and lower your risk of having another heart attack.
Harvard Referencing:
HEARTFOUNDATION. (N/A) Angina. [Online] Available from: https://www.heartfoundation.org.nz/your-heart/heart-conditions/angina [Accessed: 14th November 2023]
1 note
·
View note
Text
Autologous Stem Cell and Non-Stem Cell Based Therapies Market Analysis
A primary driver for this market is the worldwide increase in occurrence of diabetes and cancer, across all age groups. According to the World Health Organization (WHO) 2018 statistics, the number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014. Moreover according to the World Health Organization cancer factsheet 2018, Cancer is the second leading cause of death globally, and is responsible for an estimated 9.6 million deaths in 2018. Furthermore, increasing old age population is another driver, which is anticipated to create a high growth scenario for the market. As per the World Population Prospects 2019 revision, the number of people aged 80 years or over is projected to be triple, from 143 million in 2019 to 426 million in 2050.
Alternatively, factors restraining the market are complications and risks associated with the treatment including diarrhea, nausea, hair loss, vomiting, severe infections, infertility, and heart complications.
Increasing prevalence of cancer will drive the overall autologous stem cell and non-stem cell based therapies market
According to a report by National Cancer Institute, in 2016, around 1,685,210 new cases of cancer were diagnosed in the U.S. alone. Moreover, it was also noted that around 595,690 people died from the disease in the same year. Autologous stem cell and non-stem cell based therapies is likely to become one of the preferred treatment for cancer. As the prevalence of cancer is rising at a considerable rate, the market is likely to grow during the forecast period.
North America, followed by Europe accounted for the major share of the autologous stem cell and non-stem cell based therapies market. This is due to minimization of risks related with the treatment. Moreover, the demand for these treatments is high due to their ability to cure a significant number of infectious diseases. Autologous stem cell and non-stem cell based therapies do not need an outside donor; therefore the treatment is less infectious and convenient. These factors are likely to boost the growth of the market in North America. However, Asia Pacific is expected to show the maximum growth in the forecast period. The demand in this region will be led by countries such as China, India, Malaysia, and Vietnam. The demand is likely to grow as autologous stem cell and non-stem cell based therapies aid in the efficient management of cardiovascular diseases as well. Growing healthcare infrastructure as well as increasing collaboration and acquisition by market players in this region is also expected to help in the growth of the autologous stem cell and non-stem cell based therapies market in the Asia Pacific. For instance in January 2018, Vericel Corporation made a licensing agreement with Innovative Cellular Therapeutics (ICT) for development and distribution of autologous stem cell based therapy products such as MACI, Epicel, ixmyelocel-T and Carticel in Greater China, South Korea, Singapore, and other countries in the region
Key players in the global autologous stem cell and non-stem cell based therapies market are investing heavily in their research and development activities so as to gain an upper hand in the market. Some of the major players operating in the market are Caladrius Biosciences, Vericel Corporation, Fibrocell Science, Inc., Genzyme Corporation, BrainStorm Cell Therapeutics, Regeneus Ltd., and Dendreon Corporation.
Key players operating in the market has robust pipeline of autologous stem cell and non-stem cell based therapies, which is expected to drive market over the forecast period. For instance in November 2019, Caladrius Biosciences (previously known NeoStem, Inc) announced positive results of CD34+ cell therapy CLBS16 from the ESCaPE-CMD Trial as a significant advancement in treatment of Coronary Microvascular Dysfunction (CMD), a condition that disproportionately afflicts women.
0 notes
Text
We know this condition from before the days of COVID. That's called coronary microvascular dysfunction because there are a whole subset of patients who continue to have chest pain and shortness of breath. And these patients have no blockages in their arteries. It’s just that endothelial cells are not working fine.
What is new in this study is that linking this and some patients report that initially it was linked more often to diabetes, high cholesterol, obesity, renal, kidney problems, things like that. But what we found here is that probably COVID is giving us something that is similar.
1 note
·
View note
Text
Why Women Shouldn’t Disregard Chest Pain?
Problems with the microscopic arteries within the heart muscle, which are essential for controlling the heart's blood flow, are the root cause of microvascular angina. It was formerly referred to as cardiac syndrome X.
A particularly concerning type of cardiac chest discomfort is microvascular angina, which is frequently misdiagnosed since it doesn't manifest as a blockage in the main heart arteries during testing. Doctors may overlook the underlying problem due to this condition.

Any chest pain known as angina is caused by ischemia, which is a condition where your heart muscle isn't receiving enough blood to keep up with its workload. Obstructive coronary disease, which arises when one of the heart's arteries is obstructed, is the most typical cause of angina. Insufficient blood flow to the working heart muscle may cause chest pain in people with this type of angina when they exercise or exert themselves.
The American Heart Association estimates that up to 50% of women who experience angina symptoms do not have a blocked artery. In fact, despite the possibility of other symptoms, they might not even experience chest pain.
For microvascular angina, these women should be examined. Due to spasm or cellular dysfunction, the smallest arteries in the heart may be unable to supply enough oxygen-rich blood, which may result in microvascular angina.
Microvascular angina can be challenging to identify because symptoms like nausea and indigestion might be mistaken for other conditions, and angiograms, a specialist X-ray of the heart, won't reveal constriction or blockages in these tiny arteries. A stress test is frequently used by doctors to diagnose patients by tracking the heart's performance while exercising.
Symptoms
Microvascular angina can cause chest pain that feels heavy, tight, pressurized, or squeezing.
Sweating.
Nausea and lightheadedness.
Stomach discomfort.
Breathing challenges.
Though it's extremely rare for this to be the only sign of microvascular angina, being extremely exhausted and lacking in energy.
Treatment
Surgery that can be done on larger arteries cannot be utilized to treat microvascular angina since it affects tiny arteries. Medication, on the other hand, can lessen symptoms and enhance cardiovascular health. Medications for microvascular angina include:
Nitroglycerin relaxes and dilates arteries to prevent spasms.
Beta-blockers, lower heart rate.
Statins can halt the development of fatty plaque in the arteries.
Calcium channel blockers, aid in blood vessel relaxation.
Precautions
Increased incidences of stroke, heart attack, and heart failure are observed in women with microvascular angina. Many of these risk factors can be addressed by lifestyle changes, such as a balanced diet and regular exercise, which will decrease your likelihood of developing microvascular angina. Additionally, it's critical to not be embarrassed to discuss chest discomfort or other concerns with your doctor.
Basic Life Support Louisville renders a lifesaving technique to promptly identify healthcare emergencies, and save the lives of the victims. The advanced mechanism is being duly implemented by the mentors as per the American Heart Association (AHA) guidelines. Anybody interested may visit the training site or call 502-804-6132.
0 notes
Text
Day 10 - Finding My Personal Reason for My Pilgrimage - MidDay: October 7
In March of this year, I was suddenly ill with a worsening chest pain over my heart. I was so debilitated I needed a handicap placard and was trying to figure out how to get a wheelchair to take me from my car to the cardiologist’s office, when things finally eased up a bit.
I had knew something had been going on for a while; I’d had signs of heart failure for over a year, but all normal tests.…

View On WordPress
#Angina#Belief#camino#camino-de-santiago#CMD#Coronary Microvascular Disorders#Coronary Microvascular Dysfunction#El Camino#El Camino Ingles#El Camino Trail#Grace#heart#heart disease#Heart Failure#Heart Health#Hiking#Hope#IBERIA#Pilgrim#pilgrimage#Spain#Travel#Walking#women#Women of a certain age#Women&039;s Health#women&039;s heart
0 notes
Text
Is Paxalisib Better Than Temozolomide In The First-Line Setting?

Paxalisib better than Temozolomide?
Paxalisib's mechanism of action involves directly suppressing PI3K in the PI3K/AKT kinase (also known as protein kinase B) signalling pathway, which prevents the PI3K signalling pathway from being activated. In sensitive tumour cell populations, this may prevent both cell growth and survival.
When used as first-line therapy in individuals with glioblastoma, paxalisib Phase II results from Kazia Therapeutics are comprehensive and contain interesting sensitivity analysis. In this single-arm research, paxalisib was administered as monotherapy to all 30 evaluable patients with newly diagnosed glioblastoma with unmethylated MGMT promotor status. According to the study, temozolomide had a median overall survival of 15.7 months while the median overall survival for patients who had previously received treatment was 12.7 months. Furthermore, according to the RANO criteria, the median progression-free survival (mPFS) was 8.6 months, a significant improvement above the 5.3 months linked to temozolomide. Kazia Therapeutics' profile paxalisib (safety) was quite consistent with earlier clinical investigations.
The updated data, which was presented at ASCO today, "provides a more comprehensive view of the trial and also includes some useful sensitivity assessments." - Expert Verdict.
Conclusion
With regard to newly diagnosed glioblastoma patients, paxalisib anticipates a dramatic shift in the therapeutic paradigm given the encouraging results of its Phase II data. But let's not lose sight of the fact that GBM is a very tough nut to crack given the aggressiveness of the illness and the dismal survival rate. Bevacizumab has been approved for recurrent GBM patients for years, however it has not been successful in first-line GBM treatment (except in Japan). However, paxalisib looks to provide better hope for safety and tolerability in patients with recently diagnosed glioblastoma.
The GBM market is currently experiencing some activity in the ASCO 2022, with SurVaxM (MimiVax) demonstrating promising results and paxalisib now demonstrating its promise in the first-line context. Other important GBM treatments, including Regorafenib (Bayer), Durvalumab (MEDIMMUNE/AstraZeneca), and ONC-201, are in development (Chimerix).
Newly Diagnosed Glioblastoma Companies- Aivita Biomedical, Inc., Inovio Pharmaceuticals, Denovo Biopharma, IMVAX, Merck Sharp & Dohme Corp. and Eisai, MimiVax, Bristol-Myers Squibb, Novartis Pharmaceuticals.
Get in touch with our Business Expert @ Business Consulting Services | Biotech Consulting | Technical Due Diligence Firms
Trending & Popular Market Research Reports 2022 by Delveinsight
Reactive Airway Disease Market
Reactive Airway Disease Market report delivers an in-depth understanding of the historical and forecasted epidemiology as well as the market trends in the 7MM
Coronary Microvascular Dysfunction CMD Market
DelveInsight’s Coronary Microvascular Dysfunction Market Insights, Epidemiology, & Market Forecast 2032 report delivers an in-depth understanding of the CMD epidemiology & CMD market trends
Familial Lipoprotein Lipase Deficiency Pipeline
Familial Lipoprotein Lipase Deficiency Pipeline Insights, 2022 report by DelveInsight outlays comprehensive insights of present clinical development scenario and growth prospects across.
Pediatric Growth Hormone Deficiency PGHD Market
Pediatric Growth Hormone Deficiency Market Insights, Epidemiology, and Market Forecast report delivers an understanding of the Pediatric Growth Hormone Deficiency forecasted epidemiology
Shigellosis Market
DelveInsight’s Shigellosis Market Insights, Epidemiology, and Market Forecast-2032″ report delivers an in-depth understanding of the Shigellosis, historical and forecasted epidemiology
About ASCO 2022 Conference Updates
asco 2021 highlights | asco highlights 2021 | asco medical conference | asco 2021 | asco 2021 conference | when is asco 2021 | 2021 asco | 2021 asco annual meeting | asco convention | asco conference 2021 | asco 2021 | asco 21 | asco us | asco 2022 annual meeting | asco abstract 2022
About Us
DelveInsight is a Business Consulting and Market research company, providing expert business solutions for the life science vertical and offering quintessential advisory services in the areas of R&D, Strategy Formulation, Operations, Competitive Intelligence, Competitive Landscaping, and Mergers & Acquisitions.
Contact Us
Yash
0 notes
Text
Why Vasculitis Probably can be Ameliorated with Magnesium and Antagonists of Ceramides and PlateletActivating Factor| Lupine Publishers

Journal of Surgery| Lupine Publishers
Introduction
Vasculitis is characterized as an inflammatory disease of the body’s small blood vessels, particularly in the lungs and kidneys [1-4]. Many other organ regions are usually affected which often induces morbidity and mortality [1-4]. Although the exact causes of vasculitis are not known, it appears to be an autoimmune disease even though physical, chemical injuries and infections can result in vasculitis [1-4]. It is classified as a rare disease in the USA because there are only about 200,000 cases. Although numerous treatments have been advocated, there is no known cure or preventative treatment. Vasculitis often leads to difficulties in breathing and renal shut -down. In addition, vasculitis leads to cardiac malfunctions, cardiac failure and strokes. Vasculitis is clearly more common in the aged [1-4]. Recently, we have found that a few patients that were diagnosed with vasculitis appear to have a magnesium deficiency (MgD), particularly in the serum ionized Mg2+fraction [unpublished findings].
Case Report
Unlike atherosclerosis that takes decades to develop, vasculitis of small and medium sized arterial vessels, as well as microscopic arterioles. venules and capillaries, progresses rapidly, thus producing tissue ischemia via lumen-occlusive intimal hyperplasia and inflammatory syndromes [1-4]. Whether the end result is giant cell arteritis (GCA), polyarteritis nodosa (PAN), Churg- Strauss vasculitis (CSV), Wegner granulomatosis (WG), polymyalgia rheumatica (PR), Behcets disease (BD), or other vascular diseases, invasion of the arterial and microcirculatory walls by macrophages, leukocytes and CD4 T-cells seems to be pivotal [1-4]. In addition, most of these patients appear to demonstrate coagulationopathies. The degrees of luminal stenoses vary from patient to patient. Usually, degradation of the internal elastic laminae (EL) follow suit [1-3]. It has been hypothesized that the latter stenoses are due to concentric growth of the intima which seems to be related to several angiogenic growth factors, e.g., platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) [4]. What stimulates the production of these growth factors is not completely known but is thought to involve activation of nuclearfactor-KB (NF-kB) in the macrophages and leukocytes [4]. As stated, the macrophages and leukocytes clearly play key roles in the development of vasculitis. They are activated by NF-kB to produce a host of cytokines and chemokines which are needed for tissue remodeling and granuloma formation in development of vasculitis [2,4]. What activates the macrophages and leukocytes to induce production of NF-kB is not known.
Why Magnesium Deficiency is Most Likely a Key Player in Development of Vasculitis
While we were routinely investigating the potential role of magnesium deficiency (MgD) in numerous cardiovascular- diseased patients, who presented with coronary arterial diseases, coronary vasospasm, acute myocardial infarctions (AMIs), congestive heart failure , and strokes, we noted that several of these patients had an underlying vasculitis together with significant deficits in serum ionized Mg, but not necessarily total serum Mg levels [5]. More than 50 years ago, two of us found that reduction in the concentration of extracellular free Mg ions (Mg2+ ) resulted in vasospasm of coronary, cerebral, and peripheral arterial vessels; the lower the [Mg2+ ]0, the more the intense the arterial vasospasm [6-13]. In addition, our laboratories found that microscopic blood vessels in skeletal, cutaneous, and cerebral vascular beds of intact rats, mice, rabbits, guinea- pigs, dogs, and piglets exhibited similar phenomena as dietary Mg intake was reduced over three to 12weeks [14,15]. Moreover, vascular reactivity to circulating humoral and hormonal vasoconstrictor agents (i.e., angiotensin II, norepinephrine, serotonin, numerous peptide mediators, etc.) was intensified when [Mg2+ ]0 was reduced; the lower the [Mg2+ ]0 , the greater the humoral and hormonal-induced vasoconstriction [6-10,13]. It is important to note, here, that these agents are often present at increased, circulating levels in cases of vasculitis. It is now clear that all cases of vasculitis are associated with increased levels of various cytokines and chemokines (e.g., IFN-alpha, IL-1-beta, IL-2, IL-8, IL-10, IL-4, IL-17, TNF-alpha, MCP-1, among others) which are pro-inflammatory in nature [1-4]. We have found that rats placed on MgD diets for 21days generate all of these pro-inflammatory cytokines and chemokines in the blood, cardiac tissues and arterial vessels [16,17]. Other investigators have also reported finding many of these cytokines and chemokines in MgD animals [18]. Furthermore, we have found that. These MgD animals generate growth factors similar to those found in patients presenting with various forms of vasculitis [4,5]. All of these cytokines, chemokines, and growth factors, we found in the MgD animals, were associated with microvascular wall remodeling and pathological alterations in the postcapillary venules, resulting in reduced lumen sizes, increased vascular reactivity, and adherence of leukocytes and macrophages on the endothelial cell walls [19-21], thus, in many respects, similar to what is seen in vasculitis. Last, but not least, we have found that the MgD state that we produced in the rats resulted in activation of NF-kB in cardiac, cerebral, and peripheral vascular smooth muscle cells [16-23]. In view of our findings, we believe, collectively, it is difficult to dismiss the probable role of MgD in the etiology and sustenance of a state of inflammation and vasculitis. Thus, we recommend that our hypothesis should be tested in two ways [24-26]: a) Use a Mg2+ -ion selective electrode like those we helped to pioneer [27-31], in order to carefully measure the levels of ionized free Mg; and b) Administer Mg salts, initially, intravenously, then orally, for extended periods of time.
Low Mg2+ Induces Leukocyte and Macrophage Sticking, Increased Adhesiveness to Venular Endothelial Walls, and Increased Post-Capillary Permeability in The Microcirculation
Approximately 40 years ago, Ross et al advanced the hypothesis that atherosclerosis is an inflammatory disease brought about by injury to the endothelial surfaces of the macro- and microcirculations [32]. The hypothesis stated that different forms of injury (e.g., ischemic events) will result in numerous dysfunctions in the homeostatic properties of the endothelium, e.g., increases in adhesiveness of macrophages and leukocytes and/or platelets, alteration in the procoagulant properties, formation/release of cytokines/chemokines and growth factors. Usually, inflammation is defined as a response of microcirculatory blood vessels and the tissues they perfuse to infections and damaged tissues which bring cells and host-defense factors/molecules directly from the circulation to all the diverse sites where they are required in order to eliminate/degrade the offending agents [33]. The mediators of the defense mechanisms include white blood cells, macrophages, phagocytic leukocytes, chemokines, antibodies, and complement proteins [33]. The inflammatory process brings these cells and molecules to the damaged or necrotic tissues. During the normal inflammatory process, macrophages, leukocytes, and monocytes migrate across the venous capillary walls through holes in between the endothelial cells due to increases in permeability and move to the site(s) of injury via chemotaxis. This sequence of events is thought to take place in all types of inflammatory events and in developing vasculitis [34]. The normal mediators for these processes to take place are adhesion molecules, cytokines, and chemokines, all of which we have found in patients with different forms of vasculitis and in MgD [5,23].
Probable Contributing Roles of Ceramides and Platelet Activating Factor as a Consequence of MgD to Etiology of Vasculitis
In the late 1990’s, working with proton-nuclear magnetic resonance spectroscopy (1H-NMRS), and arterial vessels exposed to low Mg2+ levels, two of us found an increased synthesis of several sphingolipids (namely, ceramides, sphingosine, and sphingosine-1- phosphate) along with an increased formation of platelet-activating factor (PAF) [35,36]. We and others have reported that many of these sphingolipids (particularly ceramides) and PAF promote vasoconstriction and vasospasm of different types of arterial blood vessels as well as arterioles and muscular venules in the living microcirculation in situ [22-24,26,37-41]. In addition, three of us found that ceramides and PAF cause increases in postcapillary permeability, leukocyte and macrophage adhesion to the endothelial linings of the postcapillary venules, and migration of these latter cell types to the extravascular tissue spaces [41]. What we found, of particular interest, is that vascular smooth muscle cells (of different types), when exposed, in primary cell culture, to low Mg2+ caused a synthesis of both ceramides and PAF, which could be selectively inhibited using specific antagonists of ceramides and PAF [42]. More than 30 years ago, Cunningham and colleagues reported that sera from rheumatoid vasculitis patients contained platelet-releasing activity [43]. Two years later, Warren and his colleagues, using a rat model of immune complex vasculitis, found that a receptor blocker of PAF inhibited an Arthus reaction [44]. Sera taken from patients in our hospitals which had an underling vasculitis (of diverse origins), and lowered serum ionized Mg, demonstrated increased levels of both ceramides and PAF [5]. We do not believe these findings are merely coincidental. It is our contention that low Mg coupled to increased cellular and serum levels of ceramides and PAF are causal agents in many types of vasculitis.
Conclusion
Although the exact cause(s) of vasculitis is not known, Mg depletion appears to be a presence in different types of vasculitis. When several of our cardiovascular- diseased patients were admitted to our hospitals, a number of them exhibited an underlying vasculitis coupled with an ionized Mg deficiency along with elevated serum levels of ceramides and PAF. Mg-deficient animals, in our labs, exhibited elevated serum and tissue levels of ceramides and PAF which could be reduced/inhibited with specific antagonists of ceramide and PAF synthesis. Elevated dietary levels of Mg also reduced the synthesis of both ceramides and PAF, at least in experimental animals. Experimental animals fed Mg deficient diets exhibited, in-vivo, inflammatory alterations in the microcirculation similar to those patients presenting with different forms of vasculitis (e.g., elevated cytokines, elevated chemokines, elevated adhesion molecules, elevated tissue levels of NF-kB, along with other substances). In view of these new findings from our laboratories, it is our belief that patients exhibiting vasculitis should be treated with oral Mg supplements along with inhibitors of ceramide and PAF synthesis in order to determine if our hypothesis is valid.
Acknowledgement
Much of our original investigations were supported, in part, by research grants from The National Heart, Lung and Blood Institute, The National Mental Health Institute, The National Institute on Drug Abuse, and The National Institute on Alcoholism and Alcohol Abuse along with unrestricted research grants from several pharmaceutical companies. Some of our studies were initiated while two of us (BMA and BTA) were on the faculty of The Albert Einstein College of Medicine. While our original studies were underway, two of our colleagues passed away, namely Professor Lawrence M. Resnick and Anthony Carella. Both of these outstanding scientists will be sorely missed.
#Lupine Publishers
#Lupine Publishers Group
#Journal of Surgery
#Case Studies journal
1 note
·
View note
Text
Cardiac syndrome X is a condition in which patients experience typical angina-like pain and have abnormal stress testing consistent with coronary artery disease. However, when these patients are further evaluated with cardiac catheterization, the result is a normal angiogram. The condition occurs more commonly in women than in men. Plausible explanations for this syndrome include cardiac microvascular dysfunction and/or an abnormal cardiac pain perception. Prognosis in these patients is generally considered good. Cardiac syndrome X should not be confused with "syndrome X," which was an older term used for metabolic syndrome.
1 note
·
View note
Text
ST Elevation and Syncope in A Young Patient with Covid-19 Disease: Brugada or Coronary Syndrome
Authored by Baris SENSOY*
Abstract
Coronavirus disease 2019 (COVID-19) has been reported to cause cardiovascular complications such as myocardial injury, thromboembolic events, arrhythmia, and heart failure. St segment elevation seen in electrocardiogram of COVID-19 patients may be associated with many diseases including acute myocardial infarction, myocarditis, Brugada syndrome and right ventricular pressure overload. Febrile states may unmask certain Brugada syndrome patients and precipitate ventricular arrhythmias. We present a case of a patient with coronavirus disease 2019 (COVID-19) and ST elevation arrived at our Emergency Department after syncope with loss of consciousness occurred during high fever.
Introduction
The COVID-19 pandemic is a major health crisis affecting several nations, with increasing cases and confirmed deaths reported to date [1]. The severe inflammatory response to COVID-19 results in a febrile illness in the vast majority of patients [2]. These patients present to the emergency department in different clinical scenarios and may require treatment modalities other than COVİD-19 disease related specific and supportive treatment. Multiple potentially overlapping mechanisms, particularly inflammation, are responsible for underlying cardiovascular complications such as myocardial injury, thromboembolic events, arrhythmias, and heart failure. The reported cardiac injury may be a result of direct viral invasion of cardiomyocytes, increased metabolic demand, immune activation, or microvascular dysfunction. Cardiac arrhythmias have also been reported with a wide range of implicated contributory factors, ranging from direct viral myocardial injury, as well as other factors, including at-risk individuals with underlying inherited arrhythmia syndromes. The presenting cardiovascular symptoms include chest pain, dyspnoea, and palpitations [3]. Atypical complaints of patients are a problem that may cause complexity in terms of differential diagnosis. Although the patients are evaluated in detail for cardiac complications at the time of admission, the differential diagnosis of cardiac complications can be confusing.
Case Presentation
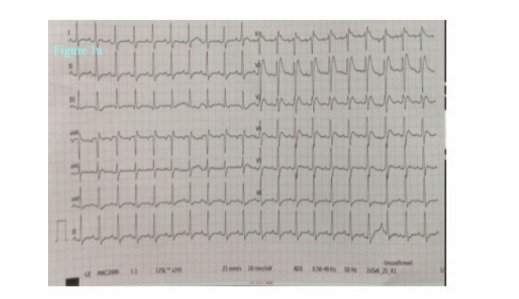
A 36-year-old man without relevant past medical history presented to the emergency department after syncope with loss of consciousness (40 seconds). He began to suffer from dyspnea, cough and fever 6 days before. His body temperature was 38.8 °C and was given antipyretic therapy 500 mg of paracetamol 2 hours before applying. The syncope occurred in the bedroom shortly after the patient got out of bed, without any prodromal symptoms and with a very brief loss of consciousness. Despite being treated with amoxicillin for four days the shortness of breath had increased. The patient also complained of chest pain radiating to the back and after his unconscious period he was making senseless gestures for 2 hours. He did not report a history of syncope before, and there was no relevant family history.In the emergency room the patient was sub febrile (37.2 °C) but noted to be tachycardic (117 beats/minute), hypotensive (80/50 mmHg), hypoxemic (SpO2 79 %) and had tachypnoea (respiratory rate 32 breaths per minute) with unaware and agitated behavior. On examination, he had increased work of breathing and diminished aeration in lung fields bilaterally. His neurological examination was normal. His initial work up revealed an elevated C‑reactive protein (468 mg/l), lactate dehydrogenase (2090 U/L), kreatinin (1.5 mg/dl), AST (328 U/L) ALT (212 U/L), kreatin kinaz (1659 U/L), D-dimer (8.8 μg/ml), ferritin (2484 ng/ml) and troponin T levels (42 pg/ml). White blood cell count was 11900 mcl and his electrolyte levels were normal. The 12‐lead electrocardiogram (ECG) showed a sinus tachycardia of 121 beats per minute and ST elevation mimicking a Brugada type 3‑like ECG pattern in lead V1 (Figure 1a). Considering a preliminary diagnosis of acute coronary syndrome with st-elevation, the patient was consulted to cardiology. Transthoracic echocardiogram was performed, which showed preserved cardiac function with dilated right chambers and an estimated systolic pulmonary artery pressure of 45 mmHg. Also, minimal (<1cm) pericardial effusion was noted. The diagnosis of acute coronary syndrome was rejected. In order to rule out pulmonary embolism and detailed examination of lung parenchyma computed tomography (CT) of the chest was planned. Chest CT demonstrated bilateral extensive consolidations (Figure 1b). The patient’s nasal/oropharyngeal swab was tested for SARS Cov-2 RT-PCR and it came out to be positive.
Due to his respiratory failure the patient was admitted to the intensive care unit of emergency room for continuous electrocardiographic monitoring and started treatment with high‐flow oxygen inhalation. Although monitorization showed no significant arrhythmic events, including premature ventricular contractions and no sustained ventricular tachycardia, within 3 hours of hospitalization, despite intubation and advanced respiratory support the patient rapidly decompensated, developing persistent hypoxemia and bradycardia, which ultimately necessitated a cardiopulmonary resuscitation with asystole. The patient died despite advanced life support.
Discussion
COVID-19 is a newly recognized infectious disease that has spread throughout the world, mainly manifested by fever, pneumonia, and respiratory failure. Recent studies reflect a wide range of cardiovascular complications and have associated this disease with an increased risk of arrhythmias and ECG abnormalities during hospitalization for COVID-19 pneumonia [3, 4]. In the literature, there are patients who applied to the emergency department with ST elevation and COVID-19 disease, needed emergency coronary angiography or a subcutaneous implantable cardioverter-defibrillator [5, 6]. Besides from direct viral invasion of cardiomyocytes, inflammation (potentially the role of IL-6), increased metabolic demand, immune activation, micro vascular dysfunction have been suggested as the underlying mechanisms for the thromboembolic events both in the venous and arterial systems related to the Covid-19 disease. Disease associated arrhythmia scenarios are tried to be explained by either direct viral myocardial damage and predisposed individuals with underlying inherited arrhythmia syndromes like Brugada syndrome [7-9].
Brugada syndrome is a sodium channelopathy characterized by the presence of ECG showing right bundle branch block and ST-segment elevation in the precordial leads. Brugada syndrome is diagnosed by the specific ECG pattern along with either family history of sudden cardiac death in a family member that is < 45 years old or type 1 ECG in relatives, dysrhythmia-related symptoms (syncope, seizure, nocturnal agonal respiration), or ventricular tachycardia or ventricular fibrillation (VF), and patients are considered to have Brugada pattern if asymptomatic. Brugada syndrome is categorized into three types. Type 1 (coved type) involves ECG showing ≥ 2-mm ST-segment elevation in one or more right precordial leads (V1 to V3) followed by an inverted T wave, occurring either spontaneously or after provocative drug test with sodium-channel blocker (flecainide, procainamide); type 2 (saddle-back type) has ≥ 0.5-mm ST-segment elevation in one or more precordial leads followed by a saddle-back morphology to the ST elevation; and type 3 can show either coved or saddleback morphologies, but with < 2-mm ST-segment elevation. Type 1 is diagnostic of Brugada syndrome, whereas Types 2 and 3 are suggestive [10].
Mediated by a cytokine-induced inflammatory cascade, COVID-19 incites high fevers, potentially instigating Brugada pattern changes. This change may be secondary to the temperature-dependent, pre-mature sodium channel inactivation found in SCN5A mutation, which is a major ionic abnormality responsible for the phenotype of Brugada pattern. Also feverinduced heart rate increase was suggested for the unmasking of the Brugada type 1 ECG pattern [11]. When Brugada syndrome is suspected, physicians should consult a cardiologist and consider ICD placement, depending on their risk of VF, though this risk stratification continues to be a controversy [10]. It was shown in a study including patients with Brugada syndrome that more than half of the study cohort had experienced syncope or cardiac arrest in the setting of a fever [12]. Different from this study, Wu and colleagues described the potential COVID-19-associated risks in known patients with Brugada syndrome. They suggested patients with Brugada syndrome to immediately attend the emergency department who develop high fever (>38.5°C) despite paracetamol treatment if: (a) they had a sodium channel disease, (b) are under 26 years old or over 70 years, (c) had a spontaneous type 1 Brugada pattern and/or cardiac syncope.
As seen in our patient’s ECG (Brugada type 3 pattern), the appearance of “saddle-back morphology” aspect in leads V1 and V2 mimicking ST elevation myocardial infarction is an additional diagnostic and therapeutic challenge in COVID‐19 patients presenting with chest pain, as recently reported [6]. We excluded acute myocardial infarction due to incompatible electrocardiographic criteria and absence of wall motion abnormalities on echocardiography. Recently, a multicentric, crosssectional, retrospective study described the electrocardiographic features of 431 hospitalized critically ill COVID-19 patients [13]. Thirty percent of the patients had ECG signs suggesting acute right ventricular pressure overload. In line with this finding, considering the lung parenchymal destruction of our patient seen in chest tomography, we think that the electrocardiographic finding is due to the pulmonary hypertension detected on the echocardiogram. Therefore, even if he had survived, in our opinion an implantable cardioverter defibrillator wouldn’t be indicated at least without provocative testing. According to our experience, in addition to assessing the severity of lung parenchymal involvement, threshold to run an ECG should be low in febrile patients with suspected COVID-19. Also, all patients with high fever and syncope especially those with known or suspected Brugada ECG patterns may warrant more aggressive antipyretic therapy and serial screening ECGs.
Conclusion
We present this case to increase awareness of the potential association between COVID-19 and the development of ST elevation pattern on ECG. Since many different pathophysiological mechanisms of Covid disease have been proposed, it is important to look at it from a broad physio pathological perspective while evaluating the clinical manifestations related to Covid 19 disease.
Read More…FullText
For more about Iris Publishers Covid-19 please click on
https://irispublishers.com/COVID-19.php
For more articles in Online Journal of Cardiology Research & Reports (OJCRR) Please click on https://irispublishers.com/ojcrr/
0 notes
Text
Juniper Publishers-Open Access Journal of Case Studies
Takotsubo Caused by Pulmonary Embolism
Authored by N Mahoungou Mackonia
Abstract
Takotsubo is a transient acute coronary myocardial infarction due to a catecholaminergic discharge accounting for 1 in 36,000 adults after intense physical or psychological stress. Most often found in women over 50 years of age. Its association with pulmonary embolism is very rare.
With this in mind, we report the case of a 76-year-old female patient with poorly followed chronic obstructive pulmonary disease (COPD). She presented to the emergency department with acute respiratory distress and lipothymia. Clinical examination revealed hypoxia with SaPO2 at 86% in free air, blood pressure at 120/80mmHg, tachycardia at 112 beats/min. The electrocardiogram showed S1Q3, hyper-right axial deviation, complete right bundle branch block with fragmented QRS, positive AVR with a tachycardia of 125 beats/min. A thoracic angioscan was performed, showing a bilateral pulmonary embolism of segmental and sub-segmental level. Ultrasensitive troponins were highly elevated at 1530ng/l with transthoracic echocardiography showing signs of acute pulmonary heart disease associated with apical ballooning, very akinetic with hyperkinesia of the bases, LVEF 26% suggestive of takotsubo confirmed by coronary angiography coupled with ventriculography giving an amphora-like appearance with a healthy coronary. The patient was initially admitted to the intensive care unit and then to the hospital for an intermediate-high risk pulmonary embolism complicated by takotsubo. The etiological work-up of the pulmonary embolism was normal. She received apixaban, Ramipril and bisoprolol. The evolution was marked by a recovery of the bi ventricular function with an LVEF of 58% in 1 month.
Takotsubo was secondary to respiratory failure caused by pulmonary embolism through catecholaminergic discharge resulting in a redistribution of beta receptors in the myocardium.
Keywords: Pulmonary embolism; takotsubo cardiomyopathy; Myocardial infarction
Abbreviations: COPD: Followed Chronic Obstructive Pulmonary Disease; APH: Acute Pulmonary Heart Disease; CICU: Cardiovascular Intensive Care Unit; LVEF: Left Ventricle; HPA: Hypothalamic-Pituitary-Adrenal; MI: Myocardial Infarction; CPA: Acute Pulmonary Heart; ARBs: Angiotensin 2 Receptor Blockers
Introduction
First described in a Japanese medical journal in 1990 about 5 cases, by the team of Hikaru Sato et al. [1-3] Takotsubo cardiomyopathy usually presents as transient left ventricular dysfunction with apical wall motion abnormalities associated with electrocardiographic changes similar to those of acute coronary syndrome in the absence of significant coronary disease [4,5]. It usually lasts about 15 days, without mortality or severity in the acute phase, and usually occurs in postmenopausal women, with 90% of cases in women aged 67-70 years [5], accounting for about 80% of cases in women over 50 years [4]. Takotsubo syndrome accounts for approximately 1-3% of all patients worldwide, or 1 case per 36,000 adults. In the USA, it accounts for 0.02% of hospital admissions and 1-2% of coronary syndromes in FRANCE [4,6]. The pathophysiological mechanism of takotsubo cardiomyopathy remains unclear, and several possible theories have been put forward, such as excess catecholamines, coronary artery spasm, microvascular dysfunction and metabolic disorders [3]. However, many of these theories focus on the central role of the sympathetic nervous system which, in response to an emotional, physical or combined trigger, releases an excess of catecholamines that cause the disturbance in myocardial kinetics. The mechanism by which catecholamines cause these contraction abnormalities is currently unclear [1], let alone its relationship to pulmonary embolism or being triggered by it. It is with this in mind that we report a case of pulmonary embolism causing takotsubo.
Case Report
We report the case of a 76-year-old female patient with poorly monitored COPD. She presented to the emergency department with acute respiratory distress and lipothymia. The clinical examination revealed hypoxia with SaPO2 at 86% in the open air and 97% under oxygen at 6litre/minute, blood pressure at 120/80mmHg, tachycardia at 112 beats/min. The electrocardiogram showed S1Q3, hyper-right axial deviation, complete right bundle branch block with fragmented QRS, positive AVR with a tachycardia of 125 beats/min. A thoracic angioscan was performed, showing bilateral segmental and sub-segmental pulmonary embolism. Ultrasensitive troponins were highly elevated at 1530ng/l with transthoracic echocardiography showing signs of acute pulmonary heart disease (APH) associated with apical ballooning, very akinetic with hyperkinesia of the bases, LVEF 26% suggestive of takotsubo confirmed by coronary angiography coupled with ventriculography giving an amphora-like appearance with a healthy coronary. The patient was initially admitted to the Cardiovascular Intensive Care Unit (CICU) and then to the hospital for an intermediate-high risk pulmonary embolism complicated by takotsubo. The etiological work-up of the pulmonary embolism was normal. She initially received oxygen therapy for 72 days, apixaban (Eliquis) 10mg x2/dr for 7 days then 5mg x2/dr for 6 months. Ramipril 5mg/dr, bisoprolol 2.5mg/dr. The evolution was marked by a recovery of the biventricular function at 1 month of the treatment with a LVEF at 45% in 2 weeks then at 58% in 1 month.
Discussion
Takotsubo cardiomyopathy is a transient stress cardiomyopathy, the symptomatology of which is highly suggestive of acute myocardial infarction [2]. It usually occurs in postmenopausal women and accounts for about 90% of women with a mean age of 67-70 years. A woman over 55 years of age is 5 times more likely to develop takotsubo than a younger woman, and 10 times more likely than a man [1,2]. Several factors are incriminated in the occurrence of takotsubo. Among them we have :
a) Contributing factors:
i. Falling blood levels of estradiol at the menopause (estradiol seems to protect the microcirculation from the vasoconstrictive effect of adrenaline);
ii. Genetic predisposition, supported by the existence of family cases;
iii. A history of psychiatric illness observed in 42% of cases (e.g. depression in 20% of cases, anxiety) or neurological illness.
b) Triggering factors:
i. Physical stress (stroke or TIA, subarachnoid haemorrhage, acute respiratory failure, accident, strenuous sports activity, cancer chemotherapy, even coronary disorders).
ii. Negative psychological stress (bereavement, divorce, anger, anxiety, financial or professional problems, floods, earthquakes, etc.), but also positive (happy surprises) [2].
Studies that have investigated the pathophysiology of Takotsubo syndrome highlight the central role of strong sympathetic stimulation and parasympathetic depression [2]. Indeed, there are two initial elements of physiology to consider. The first is the cognitive centres of the brain and the hypothalamic-pituitary-adrenal (HPA) axis, and the amount of epinephrine and norepinephrine released in response to a given stress (i.e. the "gain" of the HPA axis). The second is the response of the cardiovascular system (including the myocardium, coronary arteries and peripheral vasculature) and the sympathetic nervous system to sudden sympathetic activation and the surge in circulating catecholamines. Serum catecholamine levels at presentation are significantly higher than resting levels in the same patient or in comparable patients with acute heart failure due to acute myocardial infarction (MI), suggesting a potential for excessive HPA gain and epinephrine release. However, there is currently no proven pathophysiological mechanism to clearly explain Takotsubo syndrome. There may be a synergistic combination of more than one factor, and mechanistic studies have produced conflicting results [7]. The main manifestation of takotsubo is an acute coronary syndrome characterised by angina, repolarisation disorders on the electrocardiogram with, in particular, the pathognomonic sign of an AVR lead with positive T waves, combined with the absence of negative T waves in the V1 lead [3,4]. Elevated cardiac biomarkers and kinetic disturbances are associated with severe left ventricular dysfunction such as transient akinesia or dyskinesia of the apical segments, resulting in ballooning and base preservation [1,2,8,9]. Coronary angiography usually finds healthy coronary arteries in 70-90% of cases, with ventriculography usually showing a characteristic amphora pattern and left ventricular wall motion abnormalities [2]. Early cardiac MRI shows global kinetic disturbances in the apical and medial segments with edematous T2 hypersignal of the apex and middle part of the left ventricle without late enhancement or perfusion abnormalities suggestive of myocarditis or infarction [10].
Pulmonary embolism is a serious and fatal condition, representing the third leading cause of death worldwide after cardiovascular disease and cancer, according to the French Federation of Cardiology in 2021 [11]. Its association with takotsubo is unclear, but increased catecholamine levels during severe pain or respiratory distress associated with pulmonary perfusion defects related to pulmonary embolism appear to lead to the development of left ventricular wall motion abnormalities [12].
Our patient is a 76 year old woman, menopausal, presenting with physical stress such as respiratory distress which constitute three factors favouring takotsubo. The diagnosis in our case was oriented by an electrocardiogram which showed a positive AVR lead although we noted an S1Q3 aspect, a hyper-right axial deviation with a complete right bundle branch block associated with repolarization disorders with fragmented QRS in favour of a pulmonary embolism. Biological markers were strongly positive and rarely encountered in pulmonary embolism. On transthoracic echocardiography, apart from the signs of CPA, we noted severe dysfunction of the left ventricle involving the apical and medial segments with apical ballooning and conservation of the bases, with diagnostic confirmation on coronary angiography coupled with ventriculography as reported in the literature, which objectified healthy coronaries with an amphoric aspect of the left ventricle. Pulmonary embolism in us being at high risk, seems to be at the origin of takotsubo, given the extent of the pulmonary artery involvement and the severity, causing respiratory failure that may be at the origin of a catecholaminergic storm. This causes a redistribution of myocardial beta receptors with a predominance of Gs forms (negative inotropes) at the apex, while G1 type beta receptors (positive inotropes) remain dense at the base. This mechanism is responsible for a dysfunction of the left ventricle with an aspect of apical ballooning in systole causing a decrease in coronary perfusion by a phenomenon of microvascular spasm, responsible for direct lesions of the myocytes as well as a metabolic disorder in the myocardium [13].
The treatment of takotsubo is mainly based on the use of ACE inhibitors and angiotensin 2 receptor blockers (ARBs) as an improvement in one-year survival has been observed with a decrease in recurrence. Whereas bêta-blockers, proposed in the therapeutic strategy, do not seem to be effective in the long term with a recurrence rate of 30%. Antiplatelet agents and anticoagulants are used on a case-by-case basis in combination with treatment of the cause [1,5]. In our case, patient was treated with Ramipril, bisoprolol for takotsubo and apixaban for pulmonary embolism.
Conclusion
Pulmonary embolism associated with takotsubo cardiomyopathy is rarely described to date given its mechanism of occurrence and the severity of the two pathologies that can cause sudden death. This second entity is rarely encountered and sometimes unrecognised, and may have a poor immediate vital prognosis with rapid recovery.
To know more about Juniper Publishers please click on: https://juniperpublishers.com/manuscript-guidelines.php
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php
#JuniperPublishers#juniper publishers contact info#Emergency Medicine#Medical Genetics#Obstetrics and Gynaecology#Otolaryngology#Physiology
0 notes
Text
Autologous Stem Cell and Non-Stem Cell Based Therapies Market Development Analysis Contributing Top Vendor Landscape and Economic Growth 2028

Key players in the global autologous stem cell and non-stem cell based therapies market are investing heavily in their research and development activities so as to gain an upper hand in the market. Some of the major players operating in the market are Caladrius Biosciences, Vericel Corporation, Fibrocell Science, Inc., Genzyme Corporation, BrainStorm Cell Therapeutics, Regeneus Ltd., and Dendreon Corporation.
Key players operating in the market has robust pipeline of autologous stem cell and non-stem cell based therapies, which is expected to drive market over the forecast period. For instance in November 2019, Caladrius Biosciences (previously known NeoStem, Inc) announced positive results of CD34+ cell therapy CLBS16 from the ESCaPE-CMD Trial as a significant advancement in treatment of Coronary Microvascular Dysfunction (CMD), a condition that disproportionately afflicts women.
Moreover Major players in the industry are investing in the development of innovative and new products, in order strengthening their position in autologous stem cell and non-stem cell based therapies market. For instance in February 2018, MEDIPOST announced that the U.S. Food and Drug Administration has approved its stem cell-based Alzheimer’s disease drug, NEUROSTEM for clinical trials.
Read More: https://www.blogger.com/blog/post/preview/8474931090123965692/19288652286508012
0 notes