#U.S. Food and Drug Administration
Text

Prescription drugs accumulate in a box at the Back Cove Trail parking lot in Portland, Maine, during a take-back event. Photograph By Ben McCanna, Portland Portland Press Herald/Getty Images
How You Should Dispose of Unused or Expired Medications
Flushing or throwing away leftover drugs can contaminate waterways, threatening people and wildlife. Here are some safer solutions.
— By Priyanka Runwal | Published July 22, 2022 | Sunday August 6, 2023
If you have a stash of unwanted, unused, or expired medicines in a cabinet or drawer somewhere in your home and you don’t know the best way to get rid of them, you’re not alone.
A 2021 survey conducted on behalf of Covanta, a New Jersey-based waste management company, found that 53 percent of the 2,000 Americans polled had no idea what to do with their old medications. Pill hoarding is a common outcome, and many people eventually toss these drugs into household trash or flush them down the toilet or sink—none of which may be a good idea.
Unused or expired medicines lying around at home can get into the wrong hands, leading to accidental poisoning or drug overdose. One study found that between 2000 and 2015, U.S. Poison Control Centers received roughly 32 calls a day about children accidently ingesting opioids that had either been stored or disposed of incorrectly.
When drugs are flushed or sent to landfill, the pharmaceuticals can contaminate our groundwater, lakes, rivers, and streams, threatening human and aquatic life, although our urine and feces that contain remnants of consumed medication are bigger sources of pollution. Wastewater treatment plants aren’t designed to remove these pharmaceuticals, Tim Carroll, a spokesperson for the U.S. Environmental Protection Agency, says in an email. “EPA’s first message to everyone is do NOT put leftover, unused drugs down the drain.”
So how does one dispose of unneeded over-the-counter and prescription medications accumulating in our homes? There are a few options.
Drug Take-back Programs
In an effort to find a solution to drugs languishing in medicine cabinets or lurking in waterways, the U.S. Drug Enforcement Administration launched its first National Take-Back Day in September 2010. More than 4,000 sites across 50 states collected nearly 250,000 pounds of pharmaceuticals that people returned. Since then, DEA has hosted this single-day event biannually; in April this year, more than 5,000 sites collected about 721,000 pounds of pharmaceuticals.
People can also use mail-back envelopes or drop off their unwanted medications year-round at DEA-registered collection kiosks in police stations, pharmacies, community health centers, and hospitals. These returned drugs are then sent to medical waste incinerators.

Drug Enforcement Administration agents and Soldiers from the New Jersey National Guard's Counter Drug Task Force dumped prescription drugs to be incinerated at the Covanta Essex Resource Recovery Facility during Operation Take Back New Jersey in Newark, N.J., Oct. 31, 2017. Operation Take Back New Jersey is a DEA program that provides a safe and legal method for the citizens of New Jersey to dispose their unwanted, unused, and expired medicines. The New Jersey National Guard assisted with the collection and disposal of the medications. (U.S. Air National Guard photo by Master Sgt. Matt Hecht) Photograph By Master Sgt. Matt Hecht, AB Forces News Collection/Alamy Stock Photo
In 2021 Stericycle, the largest medical waste incinerator company in the U.S., burned 40 million pounds of unused and expired pharmaceuticals, says Jim Anderson, the company’s vice president for product management and innovation. Incineration produces an inert ash that’s sent to landfills. However, one downside of this disposal method is that transporting and burning such waste and its packaging can release greenhouse gas emissions that can be potentially greater than those generated if the drugs were dumped in landfill, according to one study estimate.
But take-back programs are preferred as they reduce the risk of drug misuse and the incineration “effectively eliminates the entrance of these pharmaceuticals into our nation’s waters,” Carroll says.
However, Steve Skerlos, a mechanical, civil, and environmental engineer at the University of Michigan argues that take-back programs could still result in medicines piling up in homes—a problem such programs were designed to address in the first place. “The question is, if I have extra, unused medication, am I going to leave my house in the next day, or week, even a month, to return that,” he says, especially in rural settings where take-back sites may not be as easily accessible. “A reasonable person may consider landfill to get it out of the house fast.”
In such cases, the DEA suggests mixing medicinal tablets and capsules with undesirable substances like coffee grounds or kitty litter and tossing the mixture into the trash inside a sealed bag or container. (Don’t crush the drugs though.)
And while the EPA advises against flushing pharmaceuticals down the drain, the U.S. Food and Drug Administration maintains a list of limited medications that have the potential to be misused or result in death if taken inappropriately and so can be flushed when safer disposal alternatives are lacking.
Pharmacies also sell drug destroyers such as DisposeRx that can work with pills, tablets, capsules, liquids, and powders. “It’s about the size of a packet of sugar,” says Thomas Menighan, former CEO of the American Pharmacists Association. “You open it, put it in a bottle of unused opioids, or any medicine, for example, you pour a little water in and shake it up, it turns into a white slurry,” which can then be tossed into the trash.
But it’s unclear if these products permanently bind or inactivate the medicinal compounds so that they don’t end up in the landfill liquid, which is released into wastewater treatment plants, and can eventually contaminate our waterways.
Recycling Pharmaceuticals
Perhaps surprisingly, not all unused medications need to be thrown away.
Every year five billion dollars’ worth of unexpired medicine ends up being discarded in the U.S. That could happen because a patient dies, their condition improves and they no longer need their prescribed medication, there’s a dose change, or they experience side effects and are put on new drugs. In such cases these unexpired medications—worth an estimated $700 million—can be recycled.
“We’re wasting a lot of medication which is already paid for,” says Anandi Law, a patient engagement specialist at the Western University of Health Sciences in California. “We could have somebody else who needs it have it.”
Millions of U.S. adults skip or delay getting their prescriptions filled due to high costs. Hence, at least 40 states have passed legislation to establish medication repository programs that allow pharmacies, drug manufactures, medical and long-term care facilities, and sometimes individuals to donate their unused drugs in original sealed containers or tamper-evident packaging.
A licensed pharmacist then inspects the donated medication to check the expiry date and look for signs of tampering, misbranding, or any indication that the drug could be compromised. Once approved, the drugs can be dispensed to uninsured or underinsured individuals via state-approved pharmacies, hospitals, charitable clinics, or community health centers.
Since its inception in 2007, Iowa’s drug donation program, SafeNetRx, has served more than 117,000 patients and redistributed nearly $54-million worth of medication and supplies. Georgia’s program formally launched in 2018, and it has already filled prescriptions worth over $50 million.
“Even though over 40 states have these drug donation laws, a lot of people don’t know that they exist,” says Kiah Williams, co-founder of SIRUM, a nonprofit organization that works with pharmacies and health facilities across the country to assist with drug donation.
Donating unused medications or using take-back programs are voluntary for households, but experts hope more people will use these options instead of disposing of their leftover drugs in the trash or down the drain, which tends to be more convenient.
“All of these efforts are still relatively new,” Carroll says. “We expect we have a long way to go until households change their habits.”
#Science#Prescription Drugs#Expired Medications#Disposing Off#Flushing | Throwing#Leftover Drugs#Contaminations of Waterways#Threat to Wildlife#Covanta | New Jersey#Pill Hoarding#Household Trash | Flush#U.S. Poison Control Centers#Wastewater Treatment Plants | Pharmaceuticals#Tim Carroll#U.S. Environmental Protection Agency#EPA#National Take-Back Day#Steve Skerlos: Mechanical Civil and Environmental Engineer#University of Michigan#U.S. Food and Drug Administration#DisposeRx#Thomas Menighan#American Pharmacists Association#Recycling Pharmaceuticals#Anandi Law#California | Western University of Health Sciences#Iowa | SafeNetRx#Kiah Williams | SIRUM#The National Geographic
0 notes
Text
Mold in marijuana? Connecticut's rules are less strict than other states
Connecticut is joined by only Florida and Maryland in setting the mold limit as high as 100,000 CFU/g. But safety standards vary widely across the nation.
Branford resident Alex London uses cannabis to help with lingering back pain and migraines that stem from a car crash.
London said he used to buy it from medical marijuana dispensaries near his home. But now, because of changes in how…

View On WordPress
#Connecticut marijuana#Legal Marijuana#marijuana laws#marijuana testing#medical marijuana#U.S. Food and Drug Administration
0 notes
Video
Grissel Trujillo de Santiago was born in Chihuahua, Mexico and is a Professor at the School of Engineering and Sciences at Tecnológico de Monterrey. She was trained as a Chemistry & Pharmacy Biologist at Universidad Autónoma de Nuevo León (summa cum laude; 2008); she obtained her MSc degree in Biotechnology (summa cum laude) from Tecnológico de Monterrey (2009); and she received her PhD degree in Biotechnology from Tecnológico de Monterrey (2014).
Mexico - 3D Ears: A Biotechnological Hope
In Mexico, the region's first transplant was performed and the world's second transplant of a custom-designed three-dimensional ear with cartilage cells from a pediatric patient born with microtia.
Despite the fact that some time later the implant had to be removed from the recipient, this biotechnological feat offers hope that, in the future, more people will receive an ear transplant.
The leader of the interdisciplinary and research project of the National Rehabilitation Institute “Luis Guillermo Ibarra Ibarra” (INR), tells us about the possibilities of this project.
The first 3D transplanted ear
Microtia is a birth deformity in which the patient has a structure with a portion of the cartilage on the outside, known as the “peanut ear,” because the entire atrial pavilion is not formed. It mainly affects the Latin American and Asian population.
It is the second congenital malformation in Mexico, after cleft lip and palate and can occur on one side of the ear or both.
After years of basic research, in vitro trials, and animal model trials, the country underwent the first transplant to a nine-year-old pediatric patient with unilateral microtia.
However, it had to withdraw some time later because the patient had an infection, but the leader of the research project believes this exercise was successful because the implant itself was not rejected by the patient’s body and gave them many lessons for future interventions.
"When we questioned the patient and family we understood that he was wearing headphones, in addition, the patient touched themselves with dirty hands."
"Children are used to not having an atrial structure, when you put one on them, they tend to touch themselves a lot," explains the project leader.
Like many investigations, this advance met with a major obstacle: The pandemic. Today, four children are waiting for transplantation, with their ear ready, but due to health restrictions it has not been possible to carry out the surgery.
Microtia and child patients
In this first stage, in the INR only the child population is chosen because they are the most vulnerable, because at school age they face ridicule and accusations from their peers.
They must be patients with unilateral microtia who do not have developed ear canal (that is, who do not hear from that side).
"In this first stage it is an aesthetic reconstruction. And we think so because we have been working since 2004 with many tests and impediments in between.”
Children in general look like they have big ears because they have an atrial structure very close to the one they will reach in adulthood.
Ears growing on 3D supports
When an otolaryngologist determines that the patient's skin is in adequate condition for a transplant, a first surgery is done to remove cartilage cells or chondrocytes that are taken to the INR laboratory, where a standardized methodology for culturing cells is performed.
Subsequently, a tomography of the patient's contralateral ear is performed to design a similar 3D structure – not the same, no person has the same ears – for the missing ear.
The support on which chondrocytes will grow is a porous material. It has already been approved by the Federal Commission for Health Hazard Protection (COFEPRIS) and the U.S. Food and Drug Administration (FDA).
"This material is in the process of patenting, it is biodegradable, as the cells synthesize the extracellular matrix, the material degrades until the body eliminates it."
Once the structure reaches the proper shape and size, a second surgical intervention is performed in which the three-dimensional mold is placed and covered with the skin of the recipient.
Protocols
In this type of advances in which everything is new, many health protocols must be carried out that demonstrate their viability.
They first demonstrated that they had good laboratory and manufacturing practices that guaranteed the sterility of the area where chondrocytes would grow.
They obtained permission to extract cartilage cells from patients who would undergo another type of surgery to send them to pathology.
Then, cartilage cultures grew in vitro with microbiological safety conditions.
They performed concept tests on the back of atypical mice—a species characterized by thin, human-like skin—in these animals the human cells are compatible and do not cause rejection. Then came the authorization to transplant into humans.
Next Steps in Ear Transplantation
One of the problems is there is a global problem of scarcity.
With funding from the Ministry of Education, Science, Technology and Innovation of Mexico City, UNAM and INR bought a bioprinting machine, and the Autonomous Metropolitan University has another and will start collaborating.
Once they have the material and bioprinters produce the first designs, researchers will need to re-perform the described viability protocols, and once approved, they may move on to a second stage of adult ear transplants.
In other stages, the biotechnologist proposes that nanomaterials could be incorporated into the pinna that conduct sounds to the inner ear so that patients can hear.
This project involves specialists from Gea González Hospital and the INR.
#🇲🇽#Grissel Trujillo de Santiago#STEM#mexico#mexican educators#Tec de Monterrey#3D ear#medicine#microtia#National Rehabilitation Institute#INR#coronavirus#covid-19#otolaryngologist#Federal Commission for Health Hazard Protection#COFEPRIS#U.S. Food and Drug Administration#FDA#mexico city#UNAM#Ministry of Education Science Technology and Innovation#Autonomous Metropolitan University#Gea González Hospital
0 notes
Text
FDA Weighs Oversight Changes After Formula, Juul Troubles
FDA Weighs Oversight Changes After Formula, Juul Troubles
The head of the Food and Drug Administration has asked for a review of the agency’s food and tobacco programs following months of criticism over their handling of the baby formula shortage and e-cigarette reviews.
Tuesday’s announcement comes as FDA Commissioner Robert Califf attempts to push past several controversies that have dominated his second stint running the agency, including the delayed…

View On WordPress
0 notes
Text
Food does not contain aborted fetuses, but the total lack of existence of such a product hasn’t stopped one Texas Republican from trying to regulate it.
Ahead of the opening of the Texas state legislature last week, Republican state Sen. Bob Hall introduced a bill to mandate that food containing “human fetal tissue” be “clearly and conspicuously labeled.” If passed, this bill would also apply to food that is “manufactured using human fetal tissue,” or “derived from research using human fetal tissue.” Medical and cosmetic products that have links to fetal tissue would also be subject to these requirements.
Fetal tissue, according to the bill, is “tissue, cells, or organs obtained from an aborted unborn child.”
To be clear, food with fetal tissue in it? Not a thing. It doesn’t exist.
“There are no conditions under which the FDA would consider human fetal tissue to be safe or legal for human or animal consumption,” an FDA spokesperson told VICE News in a statement. Eating food with fetal tissue would also likely constitute cannibalism, which is typically frowned upon.
Cannibalism has found its way into the news quite a bit lately. Prominent conspiracy theory movements like QAnon hold (falsely) that elite Democrats are running a cannibalistic, Satan-worshiping, child sex-trafficking ring. QAnon’s beliefs are linked to antisemitic and anti-LGBTQ tropes that hold that Jewish and LGBTQ people are trying to hurt children, and even drink their blood. These conspiracies, which have flourished partly through lockdown isolationism and election denialism, have radicalized a stunning number of Americans and torn families apart.
Although food would not be impacted if Hall’s bill became law, medicine and science could be, since fetal cell lines can be used to develop and test drugs. These lines can be collected from a single miscarriage or abortion, then replicated in labs, over and over again, for decades. (Cell lines derived from aborted fetal tissue can be preferable, both because it’s easier to collect and because fetal tissue derived from a miscarriage may carry whatever genetic or chromosomal problem may have caused the miscarriage in the first place.) Fetal cell lines have led to development of many major vaccines, such as the vaccines against chickenpox and Hepatitis A.
After the outbreak of the coronavirus pandemic, a split erupted in the anti-abortion community over the morality of taking COVID vaccines that may have been developed or tested using fetal cell lines. The Moderna, Pfizer, and Johnson & Johnson vaccines do not include any fetal cells, although fetal cell lines were sometimes used in the development stages.
Hall’s office did not immediately return a VICE News list of questions about the bill. However, his office told HuffPost in a statement, “Unfortunately, many Texans are unknowingly consuming products that either contain human fetal parts or were developed using human fetal parts.”
“While some may not be bothered by this, there are many Texans with religious or moral beliefs that would oppose consumption or use of these products,” the statement continued.
The U.S. Conference of Catholic Bishops has previously said that, although vaccines with links to fetal cell lines can cause “a problem of conscience for some Catholic parents,” they can take them in service of the greater good of public health. In 2020, the conference urged people to get vaccinated against COVID.
“A well-informed consumer can make whatever choice they decide on purchasing a product so long as they have all of the information in hand to make the choice,” Hall told HuffPost.
So far, Hall’s bill has not been assigned to a committee.
#us politics#news#aborted fetal tissue#texas#2023#Bob Hall#conservatives#republicans#gop#texas republicans#abortions#miscarriages#fetal tissue#food and drug administration#Cannibalism#qanon#fetal cell lines#vaccines#huffington post#U.S. Conference of Catholic Bishops#republicans be like#conservatives be like#vice news
25 notes
·
View notes
Text
If you have celiac and buy the brand Van’s for their waffles, please know that 9 days ago there was a recall because some of the packages of the gluten free waffles may contain “undeclared” wheat. And if you have celiac, you know “may” might as well mean “does”.
This recall only applies to boxes with the matching lot codes and numbers, and do not pertain to other products that Van’s has to offer. These boxes were distributed in AZ, CA, FL, GA, IL, NC, & WA. Please check your boxes immediately to ensure your own safety and save yourself the painful reactions to gluten. It’s advised the purchased packages be either thrown out (or given to someone who can eat wheat so as not to waste it) or return the product to where you’ve purchased it from.

“The U.S. Food & Drug Administration website published the recall July 3. It applies to certain packs of Van's Gluten Free Original Waffles with lot code UW40193L, expiration date Jan. 19, 2024, and UPC 0 89947 30206 4. According to the Van's recall, some of the packs of waffles may contain undeclared wheat.”
#gluten free#celiac disease#coeliac disease#gluten intolerance#gluten allergy#food allergies#chronic illness#autoimmune disorders#celiac#important
10K notes
·
View notes
Text
The Republican Taliban?
It would be a misstatement to say that Republican males hate women. They don’t hate us, but they also don’t see us as equals, either physically, intellectually, or culturally. We are, it would appear, put on this earth for their pleasure, to meet all their needs – sexually, taking care of their homes, cooking their meals, etc. Sadly, they have convinced many Republican women that this is the…

View On WordPress
#Comstock Act#Food & Drug Administration (FDA)#Joyce Vance#Judge Matthew Kacsmaryk#Mifepristone#Supreme Court&039;s Dobbs decision#U.S. Department of Justice#women&039;s rights
0 notes
Text
FDA drastically cut inspections during covid and it still hasnt been remotely restored, if you feel like youre seeing more product recalls than usual lately
#keep seeing some truly disgusting videos of meat processing plants with 0 food safety#and child laborers#:|#mine
1K notes
·
View notes
Text

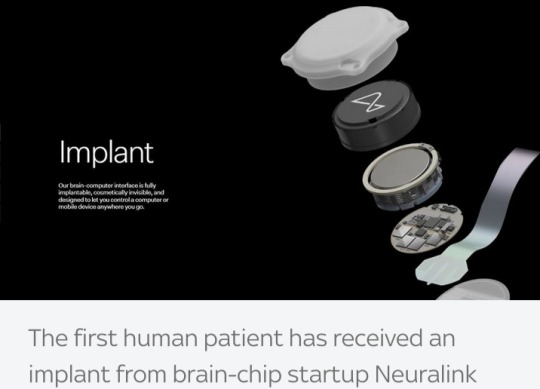




Source: Reuters
youtube
Elon Musk announces first Neuralink wireless brain chip implant in a human | BBC News
30 January 2024
Tech billionaire Elon Musk has said his company Neuralink has successfully implanted a wireless brain chip in a human for the first time.
He said initial results detected promising neuron spikes or nerve impulses and the patient is recovering well.
Posting on X, formerly known as Twitter, Mr Musk said Neuralink’s first product would be called Telepathy, and if successful, would enable "control of your phone or computer, and through them, almost any device, just by thinking."
While Mr Musk's involvement raises the profile of Neuralink, a number of rival companies have already implanted similar devices.
#Neuralink#Elon Musk#National Institute of Health#U.S. Food and Drug Administration#brain-computer interface (BCI) implant#Telepathy#PRIME Study#surgical robot#implant#neurological conditions#paralysis#brain#brain chip#technology#science#clinical trial#artificial intelligence#wireless brain chip#Youtube
1 note
·
View note
Text

Since gaining popularity online, the diabetes medication Ozempic (semaglutide) has been increasingly requested to manage weight. Now, there’s a shortage that’s affecting people who use the medication. Photograph By Imyskin, Getty Images
Ozempic is a Serious Drug with Serious Risks. Here’s What to Know.
The diabetes medication semaglutide has recently become a trendy weight loss treatment. But like every drug, there are downsides—and potentially serious side effects.
— By Allie Yang | August 1, 2023
Billionaire Elon Musk credited it for his dramatic weight loss. Celebrity sites allege that many more A-listers are using it to stay trim. And TikTok is full of influencers showing off their startling before-and-after shots showing off their weight loss after using it.
What is it? A medication called semaglutide, which is sold under different brand names, including Ozempic, approved in 2017 for treating type 2 diabetes, and Wegovy, approved just last year for weight loss.
The buzz about these drugs has created a shortage of both, according to the U.S. Food and Drug Administration, which is expected to last for several months—causing alarm among patients with diabetes who rely on Ozempic to help control their blood sugar. Experts caution that it’s important to understand these are not miracle drugs—and that there are risks to taking them outside of their intended use.
Here’s what you need to know about semaglutide, including how it works and the risks.
What’s The Science Behind The Drug?
Semaglutide helps lower blood sugar by mimicking a hormone that’s naturally secreted when food is consumed, says Ariana Chao, assistant professor at the University of Pennsylvania School of Nursing and medical director at the school’s Center for Weight and Eating Disorders. This medication, administered through injection, helps people feel full for longer, helps regulate appetite, and reduces hunger and cravings.
There is significant demand for the drug. In 2019, more than 11 percent of the population was diagnosed with diabetes, while more than four in ten adults classified as obese in 2020.
Patients with type 2 diabetes often have impairments in insulin, a hormone that helps break down food and convert it into fuel the body can use, Chao says. Semaglutide signals the pancreas to create more insulin and also lowers glucagon, which helps control blood sugar levels. This can result in weight loss but experts point out that Ozempic has not been approved for that purpose, though semaglutide at a higher dose (Wegovy) has been.
Wegovy is the first drug since 2014 to be approved for chronic weight management. The difference between the two drugs is that Wegovy is administered at a higher dose of semaglutide than Ozempic. Wegovy’s clinical trials showed more weight loss but only slightly greater improvements in glycemic control compared to Ozempic, Chao says.
The FDA sees Ozempic and Wegovy as two different medications for different uses. Chao says many insurance companies cover Ozempic for diabetes but don't cover Wegovy for obesity—a prime example of weight bias in health care. That's why some medical providers use the two doses somewhat interchangeably, as obesity and type 2 diabetes are inextricably linked–obesity is the leading risk factor for developing type 2 diabetes.
What Are The Risks?
Like every medication, there can be downsides.
The most common side effects are gastrointestinal issues, such as nausea, constipation, and diarrhea, Chao says—and more rarely, pancreatitis, gallbladder disease, and diabetic retinopathy.
Angela Godwin, nurse practitioner and clinical assistant professor at the NYU Rory Meyers College of Nursing, explains that recent reports of extreme vomiting and gastroparesis (delayed emptying of the stomach) are to be expected.
Gastroparesis “just means the food’s in your stomach longer, which then makes you feel fuller longer,” she explains.
Nausea is one of the biggest side effects of medications like Ozempic and Wegovy, and that can always lead to vomiting, Godwin says. In June, the American Society of Anesthesiologists recommended patients stop taking these medications before surgery to avoid aspiration and vomiting.
“Normally, in my experience, it's tolerable,” she says. “But then there are times when I ask [patients], ‘Well, what happened?’ And they [say] they ate too much and ate too quickly. And then yes, the body will vomit it up, because it just can't tolerate that much food anymore.”
These drugs have been extensively studied, but their relatively recent approval means researchers still don’t know what the effects of taking them long term might be.
Continuing research is helping us understand more about what happens when people stop taking these medications—which many may be forced to do amid current shortages. Research does suggest that stopping use of this medication could cause patients to regain weight, especially if they didn’t make any lifestyle changes.
“In almost all weight-loss studies, it really depends on your foundation,” says Stanford endocrinologist Sun Kim. “Your efforts at lifestyle will determine how much weight you lose. If you have your foundations like food, exercise, and sleep, you’re gonna do well.” If not, you might regain as much as 20 percent of the weight lost per year.
These medications can also be incredibly expensive, especially without insurance. Kim says an injection pen can run more than $1,000.
What Does It Mean To Use This Drug Off-label?
Using a drug off-label means using it in a way other than its intended and its FDA-approved purpose, which may not be safe or effective. Ozempic has been approved only for type 2 diabetics, and Wegovy has been approved only for patients with a BMI above 30, or 27 if they have a weight-related comorbidity like high blood pressure.
“There is no scientific evidence to show whether this medication will be effective or of benefit to those who do not fit the criteria from the FDA-approved label indications, such as people with a BMI lower than 27,” Chao says. “We also do not know the side effects or risks in these populations—there could be unknown drug reactions. These medications are not meant to be a quick fix.”
Even if you meet the criteria, experts warn against trying to obtain the medication without a prescription by traveling to countries that don't require them.
“When the medication’s not used under supervision of a health-care provider, then they can come into misuse,” Chao says. “There could be more serious adverse events that can happen.”
Godwin says recent reports of extreme vomiting and gastroparesis are a reminder that patients should schedule regular checkups with their doctor when taking these medications.
“I think it's so popular now that practitioners might be tempted to just prescribe more freely, and then maybe not monitor patients as frequently,” she says.
Patients should not increase their Ozempic dose without doctor approval—which is possible because there are multiple doses in one pen. “They could definitely have a lot of poor side effects, because they didn't titrate up to that level yet,” Godwin says. The same could be said for Wegovy, which comes in a pack of four one-dose pens.
Robert Gabbay, the American Diabetes Association’s chief scientific and medical officer, said the organization is “very much concerned” about the Ozempic shortage.
“The medication has been an important tool for people with diabetes,” he says. “Not only does it lower blood glucose and weight but it has been shown to decrease cardiovascular events—heart attacks—one of the leading causes of death for those living with diabetes.”
A Last Resort?
Still, Kim says that prescribing drugs like Ozempic and Wegovy to patients who are desperate for a new approach to weight loss can make her feel “like a superhero.” By the time patients come to her, they’ve often tried methods like Weight Watchers and following the advice of dieticians. In that case, she says, medications like Ozempic and Wegovy can be a great option.
“What I find is sometimes as they're becoming successful at losing weight, it really does feed into their lifestyle too, and then they're able to be more active,” Kim says. “It’s hard to lose weight. Seventy-five percent of the U.S. population is overweight or obese. I feel that we shouldn't be holding this back if this can help.”
Chao agrees that these medications are a good alternative for those who are unable to lose 5 percent of their body weight within about three months of making lifestyle changes. Still, she recommends trying those approaches before turning to medication.
Patients should “make sure that they're focusing on a healthy dietary pattern, reducing calories, as well as increasing physical activity,” she says. “It’s important they know that even if they are taking the medication, it's not an easy way out: They're still going to have to make lifestyle changes.”
#Science | Explainer#Ozempic (Semaglutide)#Serious Risks#Diabetes Medicatio#Allie Yang#Downsides#Potentially Serious Side Effects#Elon Musk#TikTok#Type 2 Diabetes#U.S. Food and Drug Administration#Blood Sugar#Ariana Chao#University of Pennsylvania School of Nursing#Wegovy#Risks#Gastrointestinal Issues: Nausea Constipation and Diarrhea#Pancreatitis | Gallbladder Disease | Diabetic Retinopathy.#Angela Godwin#NYU Rory Meyers College of Nursing#Stanford | Endocrinologist Sun Kim#FDA#BMI#Robert Gabbay#American Diabetes Association#The National Geographic
0 notes
Text
ISDH: List of locations for new COVID-19 boosters available
New Post has been published on https://aroundfortwayne.com/news/2022/09/13/isdh-list-of-locations-for-new-covid-19-boosters-available/
ISDH: List of locations for new COVID-19 boosters available

Today, the Indiana State Department of Health announced that it has added locations that are offering the new bivalent COVID-19 booster vaccines to its map.
#CDC Centers for Disease Control and Prevention#COVID-19 pandemic#COVID-19 vaccine#COVID-19 vaccine boosters#COVID-19 variant: Delta B.1.617.2#COVID-19 variant: Omicron B.1.1.529#FDA U.S. Food and Drug Administration#Indiana Health Commissioner Dr. Kris Box#Indianapolis Indiana#ISDH Indiana State Department of Health#Moderna COVID-19 bivalent booster#Moderna COVID-19 Vaccine#Pfizer COVID-19 bivalent booster#Pfizer-BioNTech COVID-19 vaccine
0 notes
Text
Congressional conversation underway to regulate hemp
Congressional conversation underway to regulate hemp
While efforts to decriminalize marijuana at the federal level are ongoing, there’s a simultaneous conversation underway on regulating another cannabis product: hemp.
Last Thursday, the House Agriculture Committee held a hearing on hemp, entitled, “An Examination of the USDA Hemp Production Program.”
Del. Stacey Plaskett, D-U.S. Virgin Islands, who chairs the Agriculture Subcommittee on the…

View On WordPress
0 notes
Text
Court Revives Doctors’ Lawsuit Saying FDA Overstepped Its Authority With Anti-ivermectin Campaign
NEW ORLEANS (AP) — A federal appeals court Friday revived a lawsuit by three doctors who say the Food and Drug Administration overstepped its authority in a campaign against treating COVID-19 with the anti-parasite drug ivermectin.
Ivermectin is commonly used to treat parasites in livestock. It can also be prescribed for humans and it has been championed by some conservatives as a treatment for…
View On WordPress
#5th U.S. Circuit Court of Appeal in New Orleans#Anti-ivermectin Campaign#Food and Drug Administration
0 notes
Text
The Best News of Last Week
1. ‘It was an accident’: the scientists who have turned humid air into renewable power
Greetings, readers! Welcome to our weekly dose of positivity and good vibes. In this edition, I've gathered a collection of uplifting stories that will surely bring a smile to your face. From scientific breakthroughs to environmental initiatives and heartwarming achievements, I've got it all covered.

In May, a team at the University of Massachusetts Amherst published a paper declaring they had successfully generated a small but continuous electric current from humidity in the air. They’ve come a long way since then. The result is a thin grey disc measuring 4cm across.
One of these devices can generate a relatively modest 1.5 volts and 10 milliamps. However, 20,000 of them stacked, could generate 10 kilowatt hours of energy a day – roughly the consumption of an average UK household. Even more impressive: they plan to have a prototype ready for demonstration in 2024.
2. Empty Office Buildings Are Being Turned Into Vertical Farms

Empty office buildings are being repurposed into vertical farms, such as Area 2 Farms in Arlington, Virginia. With the decline in office usage due to the Covid-19 pandemic, municipalities are seeking ways to fill vacant spaces.
Vertical farming systems like Silo and AgriPlay's modular growth systems offer efficient and adaptable solutions for converting office buildings into agricultural spaces. These initiatives not only address food insecurity but also provide economic opportunities, green jobs, and fresh produce to local communities, transforming urban centers in the process.
3. Biden-Harris Administration to Provide 804,000 Borrowers with $39 Billion in Automatic Loan Forgiveness as a Result of Fixes to Income Driven Repayment Plans

The Department of Education in the United States has announced that over 804,000 borrowers will have $39 billion in Federal student loans automatically discharged. This is part of the Biden-Harris Administration's efforts to fix historical failures in the administration of the student loan program and ensure accurate counting of monthly payments towards loan forgiveness.
The Department aims to correct the system and provide borrowers with the forgiveness they deserve, leveling the playing field in higher education. This announcement adds to the Administration's efforts, which have already approved over $116.6 billion in student loan forgiveness for more than 3.4 million borrowers.
4. F.D.A. Approves First U.S. Over-the-Counter Birth Control Pill
The move could significantly expand access to contraception. The pill is expected to be available in early 2024.
The Food and Drug Administration on Thursday approved a birth control pill to be sold without a prescription for the first time in the United States, a milestone that could significantly expand access to contraception. The medication, called Opill, will become the most effective birth control method available over the counter
5. AIDS can be ended by 2030 with investments in prevention and treatment, UN says

It is possible to end AIDS by 2030 if countries demonstrate the political will to invest in prevention and treatment and adopt non-discriminatory laws, the United Nations said on Thursday.
In 2022, an estimated 39 million people around the world were living with HIV, according to UNAIDS, the United Nations AIDS program. HIV can progress to AIDS if left untreated.
6. Conjoined twins released from Texas Children’s Hospital after successfully separated in complex surgery

Conjoined twins are finally going home after the pair was safely separated during a complex surgery at Texas Children’s Hospital in June.
Ella Grace and Eliza Faith Fuller were in the neonatal intensive care unit (NICU) for over four months after their birth on March 1. A large team of healthcare workers took six hours to complete the surgery on June 14. Seven surgeons, four anesthesiologists, four surgical nurses and two surgical technicians assisted with the procedure.
7. From villains to valued: Canadians show overwhelming support for wolves

Despite their record in popular culture, according to a recent survey, seven in 10 Canadians say they have a “very positive” view of the iconic predators.
Here's a fascinating video about how wolves changed Yellowstone nat'l park:
youtube
----
That's it for this week :)
This newsletter will always be free. If you liked this post you can support me with a small kofi donation:
Support this newsletter ❤️
Also don’t forget to reblog.
1K notes
·
View notes
Quote
I was just thinking: I have too many rights. We’ve got to cull, cull, cull! Do I really need to be voting and controlling my own body? That feels like much too much. Also, it’s spring! What better time to go through all the rights and see which ones spark joy (access to assault weapons) and which ones don’t (uncensored proximity to books, bodily autonomy). Just like they’re doing in Florida! Constitution? Please! If we were all meant to be covered by it, we would have been explicitly included!
Isn’t all this rights nonsense getting in the way of more important things, like the ability of the U.S. Court of Appeals for the 5th Circuit to consider exciting hypotheticals not borne out by science: What if a drug that has been proven safe for decades �� weren’t? Plus, millions of Americans have been given the gift of learning the name Matthew J. Kacsmaryk, most often used in the sentence, “Wait, Judge Kacsmaryk can undo the FDA approval of a drug used safely by millions for 20-plus years just … because?” It was good that the 5th Circuit did not need to think about the people most impacted by the decision to overturn Food and Drug Administration approval. After all, we’re not really people! If we were supposed to be people, we wouldn’t have uteruses.
Clearly, I have been addled by having too many rights, too much autonomy. All the voting had gone to my head. I see that now.
Alexandra Petri: Great abortion pill ruling, 5th Circuit! I felt too much like a person.
823 notes
·
View notes
Text
"Today, the U.S. Food and Drug Administration approved Opill (norgestrel) tablet for nonprescription use to prevent pregnancy— the first daily oral contraceptive approved for use in the U.S. without a prescription. Approval of this progestin-only oral contraceptive pill provides an option for consumers to purchase oral contraceptive medicine without a prescription at drug stores, convenience stores and grocery stores, as well as online.
The timeline for availability and price of this nonprescription product is determined by the manufacturer. Other approved formulations and dosages of other oral contraceptives will remain available by prescription only.
Nonprescription availability of Opill may reduce barriers to access by allowing individuals to obtain an oral contraceptive without the need to first see a health care provider. Almost half of the 6.1 million pregnancies in the U.S. each year are unintended. Unintended pregnancies have been linked to negative maternal and perinatal outcomes, including reduced likelihood of receiving early prenatal care and increased risk of preterm delivery, with associated adverse neonatal, developmental and child health outcomes. Availability of nonprescription Opill may help reduce the number of unintended pregnancies and their potential negative impacts."
308 notes
·
View notes