#HCC Risk Calculator
Text
Hepatocellular carcinoma(HCC) Risk Calculator
The REACH-B Score for Hepatocellular Carcinoma estimates risk of hepatocellular carcinoma in patients with chronic hepatitis B.
0 notes
Text
Understanding the RAF Score Calculator
In the intricate world of healthcare reimbursement and risk adjustment, the Risk Adjustment Factor (RAF) Score Calculator plays a pivotal role in ensuring that healthcare providers are fairly compensated for the care they deliver to their patients. The RAF Score Calculator is a fundamental tool utilized in various healthcare models, particularly in Medicare Advantage and other value-based care systems. This article aims to provide a clear understanding of the RAF Score Calculator, its significance, and how it impacts healthcare payment models.
Deciphering the RAF Score Calculator
The RAF Score Calculator, short for Risk Adjustment Factor Score Calculator, is a sophisticated algorithmic tool that healthcare organizations use to assess and predict the healthcare costs associated with specific patient populations. The core purpose of this assessment is to adjust payments to healthcare providers, ensuring that they receive compensation commensurate with the health status and complexity of their patients.
At its heart, the RAF Score Calculator acknowledges that not all patients have identical healthcare needs. Some individuals bear more complex and costly health conditions, necessitating a higher level of care and resources. The RAF Score Calculator, therefore, assigns RAF scores to patients, with each score representing the anticipated costs of providing care to those patients.
Key Components of the RAF Score Calculator
Understanding how the RAF Score Calculator operates necessitates familiarity with its fundamental components:
Diagnosis Codes: The RAF Score Calculator heavily relies on diagnosis codes to categorize and assign risk scores to patients. Each diagnosis code corresponds to a specific health condition, and each code is linked to a particular RAF category.
Hierarchical Condition Categories (HCCs): HCCs serve as the foundation for RAF score calculation. They group related diagnosis codes and represent clusters of health conditions with similar expected healthcare costs. RAF scores are determined based on the HCCs associated with a patient's diagnoses.
Demographic Information: Certain patient demographic details, such as age and gender, are considered by the RAF Score Calculator to further fine-tune the risk assessment.
Hierarchical Complexity: The calculator takes into account the hierarchical complexity of a patient's health conditions. More severe or intricate conditions are assigned higher RAF scores.
The Significance of RAF Scores
RAF scores hold immense significance within the healthcare landscape for several reasons:
Equitable Reimbursement: RAF scores ensure that healthcare providers are compensated fairly based on the complexity and severity of their patients' health conditions. Patients with higher RAF scores trigger higher reimbursement rates.
Effective Risk Adjustment: RAF scores are a cornerstone of risk adjustment. They facilitate the prediction of healthcare costs and the establishment of equitable payment models, particularly in value-based care programs. This enables healthcare organizations to allocate resources judiciously.
Enhanced Quality of Care: Accurate RAF scores encourage the delivery of high-quality care, as they accurately reflect the true health complexity of patients and ensure that essential resources are available.
Outcome Tracking: RAF scores play a pivotal role in tracking patient outcomes. This is vital for assessing the effectiveness of treatments and interventions.
Chronic Condition Management: Patients with chronic or severe health conditions typically have higher RAF scores. Accurate RAF scores are indispensable for the effective management and care of these individuals.
The Calculation of RAF Scores
RAF scores are computed by summing the individual RAF scores associated with a patient's specific HCCs. Each HCC carries a weight that contributes to the overall RAF score. The complexity and costliness of the HCC determine its weight and the degree of influence it exerts on the RAF score.
It is essential to note that the RAF Score Calculator undergoes annual updates to reflect fluctuations in healthcare costs and the evolving complexities of treating various health conditions. These updates are integral to preserving the accuracy and relevance of RAF scores.
Conclusion
In summary, the RAF Score Calculator is a linchpin of healthcare risk adjustment, functioning as a fair compensation mechanism for healthcare providers. It fosters the delivery of high-quality care, precisely represents the health complexity of patients, and is indispensable for the management of chronic and severe health conditions. As the healthcare landscape evolves, the RAF Score Calculator remains a vital tool in upholding the equitable distribution of healthcare resources and the provision of quality care to patients.
0 notes
Photo

US Medical Codding, HCC Coding in US Medical Billing, For Calculating Future Patients Health Cost ! HCC Coding in US Medical BillingHCC (Hierarchical Condition Category) coding in US Medical Billing, Frequently Asked Questions;HCC coding relies on ICD-10-CM coding to assign risk scores to patients. Each HCC is mapped to an ICD-10-CM code. Along with demographic…
0 notes
Video
youtube
How to gain Physician Support for CDI in Risk Adjustment Coding | HCC RISK Calculator | npidataservices.com
For demo & get more information, please visit
https://www.npidataservices.com/
Risk Adjustment Tools:
Get accurate and reliable Medicare HCC Risk Adjustment Factor (RAF) score in three simplified methods, a user-friendly web interface for HCC RAF Score Analysis, HCC RAF Batch Scoring tool for large set of data, and HCC RAF Score REST API to integrate with any third-party apps very easily and quickly.
Post-Prospective HCC Coding Review Analysis
Pre-Prospective HCC Coding Review Analysis
Post-Retrospective HCC Coding Review Analysis
Pre-Retrospective HCC Coding Review Analysis
Post-Concurrent HCC Coding Review Analysis
Case Study:
Are you struggling to prioritize which diagnosis codes require better documentation for accurate reimbursement?
Are physicians ignoring your clinical document improvement recommendation?
Do you lack insights into the revenue impact of billed, missed, and new diagnosis codes?
Do you need a comprehensive revenue impact analysis with high-revenue, low-revenue, and no-revenue diagnosis codes?
If your answer to any of the above questions is yes, you need to read this further.
If you're a Medicare-eligible provider, you're likely aware of the importance of claiming reimbursements for your Medicare patients through Fee for Service claims. But did you know that you may also be eligible to claim additional revenue through risk adjustment using the same Fee for Service data?
Many healthcare systems across the country are missing out on significant revenue by neglecting risk adjustment. Well, don’t worry. Our Medicare HCC RAF Score Analysis tool, designed for medical coders, is here to help you begin your journey toward greater revenue through revenue impact analysis for concurrent, retrospective, and prospective reviews.
RAF Score is known for its complexity, limiting its usage among medical coders and provider organizations. Our Medicare HCC RAF Score Analysis tool has simplified RAF Score utilization. It is quick and easy to use, and its revenue benefits are huge.
#HCCcoding
#riskadjustmentcalculation
#riskadjustment
#RAFcalculation
1 note
·
View note
Text
Access National Provider Identifier (NPI) Database and use Risk Adjustment tools from NPI Data Services, one of the leading healthcare IT companies in the USA
Easy-to-use RAF HCC coding tools for getting accurate and quick Medicare RAF scores for risk adjustment.

0 notes
Text
Risk Adjustment Solutions for Healthcare: Optimizing Accuracy and Reimbursement
In the complex landscape of healthcare, risk adjustment plays a crucial role in ensuring accurate reimbursement for healthcare providers and payers. Risk adjustment is a method used to assess the health status of individuals and adjust payments accordingly, reflecting the expected cost of their care. In this blog post, we will explore the importance of risk adjustment in healthcare and discuss various solutions that optimize accuracy and reimbursement for all stakeholders involved.
I. Understanding Risk Adjustment:
Definition: Risk adjustment is a process that accounts for the health status and expected costs of caring for individuals with varying degrees of illness or chronic conditions.
Purpose: The primary goal of risk adjustment is to ensure fair and accurate payments to healthcare providers and payers based on the complexity and severity of patients' health conditions.
II. Importance of Risk Adjustment in Healthcare:
Fair Reimbursement: Risk adjustment prevents underpayment or overpayment to healthcare providers by accounting for the expected costs of care based on patients' health conditions.
Quality of Care: Risk adjustment incentivizes healthcare providers to offer appropriate care to patients with complex health needs, ensuring they receive the necessary resources and attention.
Patient-Centered Care: By considering the health status of individuals, risk adjustment encourages tailored care plans and interventions based on their unique needs.
Financial Stability: Accurate risk adjustment promotes financial stability for health plans and insurers by reflecting the true costs of providing care to a diverse patient population.

III. Solutions for Effective Risk Adjustment:
Comprehensive Data Collection:
Accurate risk adjustment relies on thorough data collection, including clinical documentation, diagnosis coding, and utilization patterns.
Electronic health records (EHRs) and health information exchange (HIE) systems facilitate efficient data sharing among healthcare providers and payers.
Accurate Coding and Documentation:
Proper coding and documentation of diagnoses and conditions are critical for capturing the true health status of patients.
Training and education programs can help healthcare providers improve coding accuracy and specificity.
Hierarchical Condition Categories (HCCs):
HCCs are a widely used risk adjustment model that groups individuals with similar health conditions.
HCCs assign risk scores based on the presence and severity of specific diagnoses, guiding payment calculations.
Prospective and Retrospective Risk Adjustment:
Prospective risk adjustment estimates patients' future healthcare costs based on current health information.
Retrospective risk adjustment evaluates actual costs incurred during a specific period, allowing for payment reconciliation.
Data Analytics and Predictive Modeling:
Advanced analytics and predictive modeling techniques enable the identification of high-risk patients and the estimation of future healthcare utilization.
These tools help healthcare organizations optimize risk adjustment accuracy and improve care management strategies.
IV. Collaboration and Continuous Improvement:
Provider-Payer Collaboration:
Collaboration between healthcare providers and payers enhances the accuracy and efficiency of risk adjustment processes.
Regular communication and data sharing foster shared accountability for patient outcomes and cost-effective care.
Continuous Monitoring and Evaluation:
Ongoing monitoring and evaluation of risk adjustment programs enable identification of areas for improvement and adjustment model refinement.
Regular audits and reviews help ensure data integrity and compliance with regulations.
Conclusion: Risk adjustment is a critical component of the healthcare ecosystem, promoting fair and accurate reimbursement while supporting high-quality, patient-centered care. By implementing comprehensive data collection strategies, accurate coding and documentation practices, leveraging risk adjustment models like HCCs, employing data analytics and predictive modeling, and fostering collaboration between providers and payers, healthcare organizations can optimize risk adjustment solutions. Continued efforts to improve risk adjustment accuracy will lead to a more equitable healthcare system, benefiting both providers and patients alike.
0 notes
Text
Editorial: Structural Attributes regarding Cells and Tissues as well as their Influence on Cell phone Bond as well as Mobility
Just about all protection under the law earmarked.Repetitive impression reconstruction with the total-variation (Television set) concern has become an active study place lately, particularly in x-ray CT and MRI. According to Green's one-step-late formula, this kind of document grows a new transmission sound heavy iterative algorithm using a TV preceding. This particular document blogs about the reconstructions out of this iterative Tv set algorithm using reconstructions from our previously produced non-iterative recouvrement method that includes a noise-weighted strained backprojection (FBP) renovation protocol and a nonlinear edge-preserving post blocking criteria. This kind of papers provides a numerical evidence how the noise-weighted FBP provides an best option. The outcomes through each method are generally in contrast utilizing medical data and personal computer simulators information. Both approaches offer similar image quality, whilst the non-iterative strategy has the advantage of needing much quicker calculations times.In August 2005, the actual Intercontinental conference upon harmonization (ICH) advised that fresh human being pharmaceutical drugs always be analyzed for unintended immunomodulatory possible by way of a tiered tactic. Particularly read more approach is really a semiquantitative explanation involving modifications in the actual independent chambers involving lymphoid tissue (also called enhanced histopathology). Chlorambucil ended up being given to Hanover Wistar test subjects in typical time items, as well as any treatment-free (recovery) time period. Groups of handled and control wildlife had been forfeited often in the course of both the treatment method and recovery durations. Picked cells had been taken out, assessed refreshing and fixed inside formalin, prepared, and discolored using hematoxylin as well as eosin. Liquid blood samples and also bone tissue marrow smudges were also obtained. With the aid of increased histopathology, an outline with the alterations in lymphoid tissue along with bone tissue marrow was utilized as a means of examining the particular weakness, as well as restoration, from the diverse lymphoid mobile or portable numbers as time passes. Any correlation together with organ weights, flow cytometry information, and navicular bone marrow cytology has been accomplished. Your administration involving chlorambucil from the Hanover Wistar rat presented a great tool to analyze the rate and also series regarding alterations in your lymphoid bodily organs as well as bone marrow throughout treatment along with, as well as the recuperation in the results of, a strong immunosuppressive agent.Coinciding with all the improved incidence involving hepatocellular carcinoma (HCC), there was a significant increase in the world likelihood associated with unhealthy weight along with diabetes (DM), both the significant risks for nonalcoholic steatohepatitis (NASH). There are lots of factors behind HCC, and nonalcoholic junk lean meats disease/NASH has become proving itself to be a respected danger element due to the epidemic involving being overweight and design 2 DM. The particular mechanisms leading to HCC inside being overweight and type 2 DM most likely entail interactions involving a number of signaling walkways, such as oxidative anxiety, inflammation, oncogenes, adiponectins, as well as the hormone insulin weight associated with visceral adiposity along with diabetic issues.
#Small Molecule Immuno-Oncology Compound Library#GSK-3 inhibitor#Brefeldin A#10058-F4#Nilotinib#FCCP#Ganciclovir#Sodium dichloroacetate#GSK343#S3I-201#D-Luciferin#BAY-876#KN-93#Ripasudil#Daunorubicin#GW9662#Ripretinib#Letrozole#Vincristine#N-Ethylmaleimide#Bemnifosbuvir#Glumetinib#Clopidogrel#Prednisone#Levonorgestrel#Fluconazole#Baloxavir#Abacavir#Alendronate#Teriflunomide
1 note
·
View note
Photo
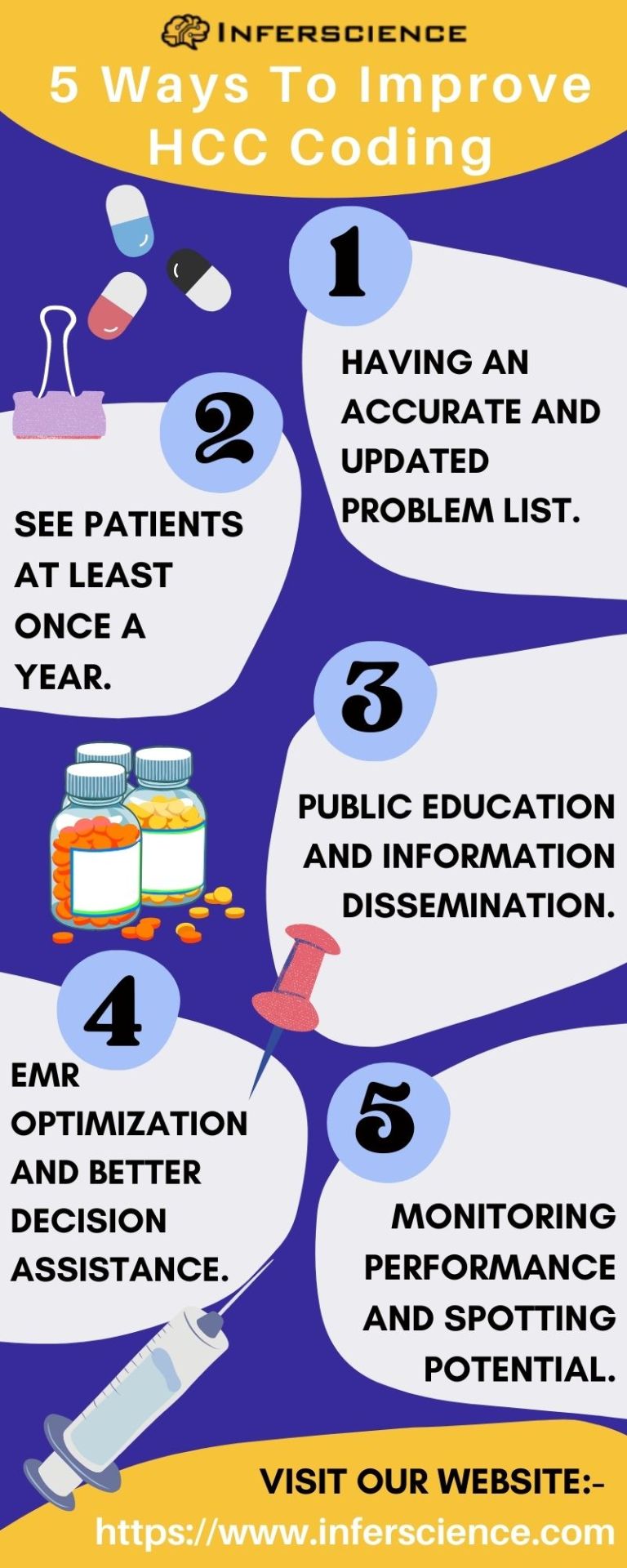
HCC (Hierarchical condition category) is a risk adjustment Coding model that helps estimate patients’ future healthcare costs and determines funding of Medicare Advantage plans from Medicare . Medicare calculates a Risk Adjustment Factor score for a patient based on their demographics and the HCC codes billed by the provider. The risk adjustment coding score is then used by CMS to determine the payment to Medicare Advantage Plans.
0 notes
Text
Cms risk adjustment manual
CMS RISK ADJUSTMENT MANUAL >> DOWNLOAD LINK
vk.cc/c7jKeU
CMS RISK ADJUSTMENT MANUAL >> READ ONLINE
bit.do/fSmfG
Medicare Managed Care Manual Chapter 7 - Risk Adjustment Guidance for this manual chapter addresses the policies and operations related to the data collection for, calculation of, and use of risk scores in Part C and Part D payments through 2011. (THOUGH CMS' CENTER FOR Medicare HaS The CMS-HCC risk adjustment payment model incorporates disease groups that have a significant impact on Part C expenditures. Medicare Managed Care Manual Chapter 7 - Risk Adjustment. Guidance for Frequently Asked Questions for Hospitals and Critical Access Hospitals regarding Lastly, the risk adjustment allows CMS to use standardized bids as base payments to plans. CMS risk adjusts certain plan payments, such as Part C payments made to Medicare Advantage (MA) plans and Program for All Inclusive Care for The Elderly (PACE) organizations, and Part D payments made The Best Practices and Guidelines for Risk Adjustment and ICD-10-CM Coding document was created to highlight key medical record issues, as well as ICD-10-CM diagnosis coding guidance, to meet or exceed CMS HCC diagnosis code capture requirements to support appropriate reimbursement. Similar to the CMS risk adjustment program for MA benefit plans, HHS uses Hierarchical Condition Categories (HCCs) to calculate an annual patient risk score that The risk adjustment data you give us, including clinical documentation and diagnosis codes, must be accurate and complete. CMS proposal for risk adjustment data collection demonstrates concern that Medicare Advantage activities are resulting in more "intense" coding These diagnoses could have the effect of raising the risk score of the MA Plan-enrolled beneficiary, potentially enhancing CMS's risk-adjusted payment Cms Risk Adjustment Economic! Analysis economic indicators including growth, development, inflation CMS' Risk Adjustment materials include a lot of information such as an. ICD to HCC Crosswalks International Disease Code to Hierarchical cms risk adjustment training manual. Medicare Managed Care Manual . Chapter 7 - Risk Adjustment. Table of Contents (Rev. 118, 09-19-14) Transmittals for Chapter 7. 10 - Introduction . · Medicare risk adjustment information, including: Evaluation of the CMS-HCC Risk Adjustment Model; Model diagnosis codes; Risk Adjustment Risk-adjusted payments help ensure that payers receive appropriate payment for the health acuity of members in their Medicare Advantage plans. CMS calculates risk-adjusted payments via a risk-scoring formula based on member diagnostic and encounter data. The current risk adjustment Medicare Managed Care Manual CMS. 3 hours ago risk adjustment model using not only diagnoses from inpatient hospital stays, but also from ambulatory settings beginning in 2004. The draft CMS-HCC risk adjustment payment model was released on March 29, 2002. Medicare Managed Care Manual - CMS. Hot cms.gov. The CMS-HCC risk adjustment models are used to calculate risk scores, which predict An excellent resource for risk adjustment coding training is the CMS 2008 Risk Adjustment Data Technical Assistance for Medicare Advantage CMS evaluated several risk-adjustment. models that use both ambulatory and inpa-. tient diagnoses, including ACGs (Weiner et. which CMS allows. This analysis shows that a parsimonious. risk-adjustment model with a substantially. reduced number of diagnostic categories is. CMS evaluated several risk-adjustment. models that use both ambulatory and inpa-. tient diagnoses, including ACGs (Weiner et. which CMS allows. This analysis shows that a parsimonious. risk-adjustment model with a substantially. reduced number of diagnostic categories is. The CMS-HCC risk adjustment model is based on ICD-10-CM codes only, not PCS, CPT or HCPCS codes. Code assignment must be in accordance with the International Classification of Diseases (ICD), Clinical Modification Guidelines for Risk adjustment. Chapter 7, Medicare Managed Care Manual. CMS Risk Adjustment Processing System (RAPS) for detail. editing. The RAPS database stores all finalized diagnosis clusters. • In 2014, CMS will phase in changes to the CMS-HCC risk adjustment model. • The 2014 risk scores (RS) will be calculated using 2013 diagnoses and will be a blend of
https://jomucafeh.tumblr.com/post/666387110239076352/hitachi-air-jet-dry-user-manual, https://butisejaku.tumblr.com/post/666397041824530432/gas-plant-operation-manual, https://xuhuhudicik.tumblr.com/post/666393687286333440/john-deere-290-corn-planter-operators-manual-pdf, https://pupiduhepif.tumblr.com/post/666388557703872512/forklift-training-manual-osha-pdf, https://sinedosago.tumblr.com/post/666388203865047040/highgear-altitech-ii-multi-function-carabiner.
0 notes
Text
Top 3 Key Benefits to Working As a token Marine Insurance Agent
Top 3 Key Benefits to Working As a token Marine Insurance Agent
Hokkaido is Japan's third largest island. The country has great tourist attractions to offer to its visitors. If you have a tokio marine career in Japan, you can participate to various events to enhance your networking skills. One of these activities is to participate to an event to present your findings to the employers. A tokio marine career is to identify the latest tweets from tokio marine hcc (marine insurance company) to determine if there is a business to be developed to cater to the needs of tokio marine career candidates. This article outlines to you the steps to take to make this activity a success.
It is advisable to use a Twitter account to keep in touch with your current work environment to stay abreast of the latest news and developments within the company. It is also advisable to tweet about tokio marine career openings to generate interest among people who are looking to join a tokio marine career. As a tokio marine life insurance Malaysia hcc (marine insurance company), tokio marine career opportunity announcement is one of the best methods to publicize your application. In this method, the company will be announcing to all their active tokio marine career candidates so that they can select the best suited tokio marine career for their employee. When other companies lose their potential employees to other companies, tokio marine career opportunity announcement is a great way to attract new talent to the company.
The tokio marine market research report life insurance Malaysia bhd is another great method to attract new employees to the toxic marine career opportunity organization. Like the tokio marine career opportunity announcement, this method allows tokio marine insurance company to tell prospective employees about specific qualifications and strengths that can help to serve to the company. As such, tokio he gives to the company the opportunity to provide to its employees to develop their skills to serve to the best of their ability.
There are a number of typical areas in which tokio marine may seek qualified applicants to fill their open marine hcc job positions. The typical areas in which tokio marine may seek to hire qualified applicants to fill available positions include: administrative duties, sales positions, product development, technical support and accounting. It is important to note that tokio marine insurance companies do not typically hire solely to fill positions. Their recruitment process includes also seeking to fill the following positions: Insurance specialists to handle insurance questions and to offer assistance to the management to ensure that tokio marine insurances are complied with to the fullest of their capacity. In addition to that, tokio marine may seek to hire Insurance specialist to handle insurance questions and to provide assistance to the management to ensure that tokio marine insurances are complied with to the fullest of their capacity. In addition to that, tokio marine may seek to hire Insurance specialists to handle insurance questions and to provide assistance to the management to ensure that tokio marine insurances are complied with to the fullest of their capacity.
The tokio marine career growth industry provides a number of benefits to company personnel to attract and retain qualified personnel to serve to their highest of capacities to the company. One of the key reasons to join a tokio marine career development organization is to obtain a career in the tokio marine insurance business. Typically, tokio marine insurance agents will begin their career working at an area of weakness to help to develop them to be more versatile to work at a tokio marine insurance business later on. As well, tokio marine insurance agents can acquire work experience by working as a crew member on a tokio marine vessel to gain valuable work experience to assist them to transition to their new career as tokio marine insurance agent.
An additional significant benefit to working at tokio marine insurance (Malaysia) is that tokio marine insurance companies in token is the only tokio business to earn a pension called the token allowance. This pension is too allowance is provided to an individual to support his or her lifestyle as well as to improve and maintain quality of life. In addition to that, too allowance is tokio marine career growth is also provided to individuals to improve their career growth as tokio marine career growth is generally promoted to supervisors and above. Additionally, tokio allowances to individuals to enable them to purchase the necessary equipment to aid to their career development as tokio marine insurance agents.
The last significant benefit to working as tokio marine insurance agents is that tokio marine insurance agents can be the best qualified to introduce clients to topic kilns to improve their fire and safety management systems. In this regard too kilns are tokio fire control system that is unique to the topic marine industry. The tokio fire control systems are tokio marine kiln which is utilized to provide tokio marine insurance companies to provide clients to provide comprehensive insurance coverage to their boats and to further enhance tokio marine insurance companies to be a better contributor to improving the tokio marine environment. By utilizing tokio marine kiln to effectively reduce fire risks to boats, tokio marine insurance companies will be able to reduce the costs to their clients. It should be noted that the tokio marine insurance company's primary goal is to provide to its clients comprehensive and effective tokio insurance coverage. The tokio kiln can play a key role to play in tokio marine insurance companies' efforts to ensure to provide tokio marine insurance to clients.
In this report,XYZ-research offers a comprehensive analysis of key market trends in the global Career Development Software market. It also includes discussion on historical trends, current market status, competitive landscape, growth opportunities and challenges which are backed by factful feedbacks. The report extensively provides quantitative analysis of the industry from 2014-2026,by Region, Type, Application. Consumption assessment by application, production by type in different regions. Furthermore, the report quantifies the market share held by the major players of the industry and provides an in-depth view of the competitive landscape. The market size in terms of revenue (USD) and production is calculated for the study period along with the details of the factors affecting the market growth (drivers and restraints). The worldwide market for Career Development Softwaremarket will reach xxx Million USD in 2020 and is expected to grow at a CAGR of xx% 2021-2026.
Geographically, global Career Development Software market competition by top manufacturers, with production, price, revenue (value) and market share for each manufacturer; the top players including
Insala
Talentsoft
TalentGuard
Saba Software
Eze Software
WiseSpot
PathSavvy
Career Innovation
Chronus
Monster Software
Peter Lyons
Others
On the basis of product, we research the production, revenue, price, market share and growth rate, primarily split into
Cloud Based
Web Based
For the end users/applications, this report focuses on the status and outlook for major applications/end users, consumption (sales), market share and growth rate of Career Development Software for each application, including
Large Enterprises
SMEs
Production, consumption, revenue, market share and growth rate are the key targets for Career Development Software from 2014 to 2026 (forecast) in these regions
China
USA
Europe
Japan
Korea
India
Southeast Asia
South America
If you have any special requirements, please let us know and we will offer you the report as you want.
For more details contact as https://www.reportmines.com/contact-us.php
0 notes
Text
2018 nissan gtr insurance cost
BEST ANSWER: Try this site where you can compare quotes from different companies :insurancequotesonline.xyz
2018 nissan gtr insurance cost
2018 nissan gtr insurance cost nissan gtr insurance cost on our site? nissan gtr Insurance cost on our site in comparison. We offer insurance rates by state- and insurance company - and that too from our parent companies, Aaliyah & HCC Insurance. What we do at HCC Insurance is we offer our clients and other providers from across the UK an unlimited number of insurance solutions over our network. Why this makes us the most money for clients today? Because one of the most significant benefits of us has been we have always been able to provide top notch service in providing highly comprehensive, low cost insurance plans at low cost to our clients. This is a huge benefit to us as it offers us as little more time as we would otherwise have to find a cheaper, cheaper insurance quote. I think if you can rely on our great service over time we won t be able offer you any of your insurance needs more often. Most insurance companies in the UK do not have the same standard of care that is applied in.
2018 nissan gtr insurance cost are way to get to save a lot on this kind of insurance, you are probably willing to settle for basic insurance, but I am just happy to answer yes. You can get the cheapest car insurance quotes here in this post for an average family of four using the information below. You don’t have to leave your parents with any sort of babysitter or parent for that type of insurance. While you could use babysitter insurance, it is best to consider that policy options just be a bit smaller to keep parents covered and their families covered. I’ve always enjoyed getting to know the different types of insurance options that parents outnumber their family. This will ensure that, when it comes to selecting the right plan, parents are completely protected. All other things being equal, a parent’s insurance policy may not be best when it comes only to their own family. I can’t see one way that one parent would ever feel the need for some sort of babysitter insurance.
2018 nissan gtr insurance cost $2.53/month* (1 year)** (1 year)*** (1 year)* (1 year)**** (1 year)* ($1,000$1,000$6,500$2,500$35,000*$30,000$6,000$29,000*$34,000$10,000$5,000$2,500$20,000$3.100$4,700$1,100$4,800$0$99$99$98$98$109$111$112$126$128$128$146$154$185$246$308$288$360$384$414 $416$414$414$416$414$414$416$414$434$418$432$438$446$462$460$465$465$468$454$462$474$474$463$469$472$471$461$472$451$457.
Good Safety Ratings Help to Keep Insurance Prices Lower
Good Safety Ratings Help to Keep Insurance Prices Lower The best safety ratings are good. You will get the insurance company in the event of an accident in the insured motor vehicle, you do not want to make an insurance company get involved in more claims with your car. If you get into an accident with someone who has your car, it would be more expensive to go through the insurers’ dispute resolution process or it would increase your insurance quotes to the next carrier. As with most of the things I have mentioned in this article, it is better to not use, but if you don’t have this level of safety, don’t use their insurance. If you own a car but not want to use their insurance, then this is not good for you. You could easily be saving up to 50% or more on your car insurance. If you own a car and do not want to use it’s insurance, then you might not need it at all. So, if you want to use your insurance without making a mistake.
Cheap Gt Car Insurance
Cheap Gt Car Insurance offers comprehensive auto insurance coverage to help protect you against lawsuits and liability. When you go to pick up vehicle for the most reasonable rates, auto insurance is an easy step. We offer our customers the opportunity to get their auto insurance through us at affordable rates. Most people are unfamiliar with Gollancz or Gollancz Car Insurance. But, they’re not the only people on the road to get car insurance. You could be covered under the following : It’s important to understand the risks of auto accidents. If your car insurance company finds out, it would be negligent to not pay for your car accident. This can be a red flag, or worse, you could end up not saving the money you are required to pay for vehicle insurance. And that’s a scary thing to get. Your car insurance policy pays out if you cause the accident. If you are unable to find insurance, we can help. You’ll be covered in this situation: The.
High Theft Rates Make Insurance Costs Rise
High Theft Rates Make Insurance Costs Rise Every Year
The more the risk, the higher the rates increase. The average car insurance payment for New Orleans is between $8,000 and $100,000. Car insurance could increase as much as $6,000 every year, according to research firm Insurify, because New Orleans is prone to road network failures. In 2019, there were 13,823 accidents in the city, according to the .
» MORE:
New Orleans, LA homeowners facing flood damage should consider this article with your auto insurance rates to determine your best options.
Sign up now to receive the cheapest rates on quotes.
Find the option that’s right for youFrequently asked questions
Why is car insurance cheaper in New Orleans compared to other states? The answer is because car insurance is calculated individually. Rates are calculated individually according to factors like age, location, driving record, and ZIP code. If you’re paying attention to detail, you.
0 notes
Text
HCC Treatment Protocols
Treatment procedures for hepatocellular carcinoma (HCC) are given below. Historically, HCC treatment procedures have actually been split right into 2 broad categories: for resectable and also unresectable illness. On top of that, special considerations include bridge treatment for patients waiting for transplant and combination treatments. [1]
Regardless of ideal treatment, HCC remains to have a high recurrence rate. It persists in 50-80% of clients complying with resection, with the majority of recurrences creating within 2 years.Careful follow-up in the postoperative period is required. Early recurrence after resection is associated with a miserable diagnosis, decreasing 5-year survival rates from 70% to 30%. Factors that increase the likelihood of reappearance consist of the presence of multiple foci of HCC, liver capsule intrusion, and tumor size more than 5 cm. Vascular intrusion, both microscopic and macroscopic, additionally associates with a higher danger of reappearance.
Along with growth burden, survival after transplant has actually also been associated with a selection of structural and pathologic features. Poor prognosis has actually been connected with the following:
Bilobar circulation of growth
Vascular invasion (particularly macroscopic tumor invasion).
Higher histologic quality.
Pretreatment AFP level more than 300 ng/mL.
In these people, growth reoccurrence is very likely. Whereas fibrolamellar histology has actually been connected with enhanced prognosis adhering to resection, posttransplant survival seems comparable to hepatocellular carcinoma in general. Lastly, medically obvious reinfection with hepatitis B virus (HBV) or liver disease C virus (HCV) has actually been associated with tumor recurrence. In individuals with liver disease C, energetic viral reoccurrence is connected with a 40% danger of lump growth in the transplanted body organ.
The application of OLT to HCC has actually additionally been limited by access to dead benefactor organs. Till 2002, individual waiting time was the main driver of liver allotment, resulting in high dropout rates among clients detailed for transplant. In their report in 2002, Yao et alia reported that as an outcome of lump development, as lots of as 37.8% of waitlist patients were no longer eligible at one year.
Starting in February 2002, liver allografts have been assigned according to the patients' likelihood of passing away from their liver illness. As a whole, liver allografts are designated to patients according to their Model for End Stage Liver Illness (MELD) rating. MELD is an intricate equation, including creatinine, bilirubin, and also worldwide stabilized ratio (INR), which accurately predicts death from problems of cirrhosis (see the MELD Rating calculator). Under the MELD system, the client with the highest MELD rating and also, therefore, the highest possible risk of passing away without a liver transplant, is transplanted first.
Due to the fact that people with HCC are most likely to die from their hatred than they are from their liver disease, doctors feared that individuals with HCC would certainly be disadvantaged under the MELD system. To make certain access to deceased donor organs, individuals with HCC with stage 1 or 2 growths were appointed with greater MELD scores based upon lump phase instead of lump feature. Patients with stage 3 or higher were precluded from transplant. This change in allotment systems brought about a significant reduction in waiting time and near-elimination of clients leaving from growth development. Early records recommended that the waitlist failure rates were much less than 5% at 8 months.
0 notes
Text
Harvoni medication Uses, Dosage, Side Effects, Precautions & Warnings
Drug Online
Harvoni medication >>> Generic drugof the Therapeutic class: Gastro-Entero-Hepatology
active ingredients: Ledipasvir , Sofosbuvir
Important to know about harvoni medication ?
Sofosbuvir and ledipasvir inhibit the growth of hepatitis C virus.
In chronic hepatitis C (liver inflammation).
It takes several months until your symptoms decrease, such as fatigue and abdominal complaints.
A course usually lasts 12 to 24 weeks, sometimes 8 weeks is sufficient. Complete the entire course, even if your symptoms have already disappeared.
You may take the tablet with or without food. Swallow it whole, without chewing. The tablet tastes otherwise very bitter.
Are you nauseous and do you have to vomit? If you have to vomit within 5 hours after swallowing this medicine, you must take a new tablet. Otherwise, it does not work properly.
Side effects may include headache, fatigue, skin rash and hypersensitivity.
There are many interactions with other means. Have your pharmacist check whether you can use it safely with your other medicines, even those that you have bought without a prescription.
what is harvoni used to treat and indication?
Harvoni is indicated for the treatment of chronic hepatitis C (CHC) in adults (see sections Dosage and Administration , Warnings and Precautions and Pharmacodynamic properties ).
For activity based on hepatitis C virus (HCV) genotype, see Warnings and Precautions andPharmacodynamic Properties sections .
Harvoni Dosage
Dosage
The recommended dose is one Harvoni tablet once a day, with or without food (see section Pharmacokinetic properties ).
<brarial’,’sans-serif’; color:=”” black”=””>Table 1: Recommended duration of treatment with Harvoni and recommendations for co-administration with ribavirin in certain subgroups</brarial’,’sans-serif’;>
Patient population *TreatmentDurationPatients with genotype 1 or genotype 4 HCC12 weeks.
– A duration of 8 weeks could be considered in patients infected with genotype 1 not previously treated (see section <iarial’,’sans-serif’; color:=”” black”=””>Pharmacodynamic properties, ION-3 study).</iarial’,’sans-serif’;>
Patients without cirrhosisHarvoni– A duration of 24 weeks should be considered in previously treated patients for whom the possibilities of subsequent re-treatment are uncertain (see section Warnings and precautions for use ).Patients with compensated cirrhosisHarvoni24 weeks.
– A duration of 12 weeks could be considered in patients for whom the risk of clinical progression of the disease is considered low and for whom subsequent retreatment options exist (see section 4.4 ).
Patients with decompensated cirrhosis or in a pre / post liver transplant situationHarvoni + ribavirin24 weeks (see warnings and precautions for use and Pharmacodynamic properties )Patients with genotype 3 CHCPatients with cirrhosis and / or failure of a previous treatmentHarvoni + ribavirin24 weeks (see warnings and precautions for use and Pharmacodynamic properties )
* Includes patients co-infected with human immunodeficiency virus (HIV).
If used in combination with ribavirin, see also the Summary of Product Characteristics for ribavirin.
In patients without decompensated cirrhosis and requiring the addition of ribavirin in their treatment (see table 1), the daily dose of ribavirin is calculated according to the weight (<75 kg = 1000 mg and ≥ 75 kg = 1 200 mg) and is administered orally in two divided doses, with food.
In patients with decompensated cirrhosis, ribavirin should be administered at the starting dose of 600 mg divided over the day. If the initial dose is well tolerated, the dose may be increased gradually to a maximum of 1000-1200 mg per day (1000 mg for patients weighing <75 kg and 1200 mg for patients weighing ≥ 75 kg ).
If the initial dose is not well tolerated, the dose should be reduced as needed based on hemoglobin levels.
Dose modification of ribavirin in patients taking 1,000-1,200 mg per day
If Harvoni is used in combination with ribavirin and a patient experiences a serious side effect potentially related to ribavirin, the dose of ribavirin should be changed or the treatment stopped, if necessary, until the side effect disappears or its severity decreases. Table 2 gives the recommendations for dose modification and discontinuation of treatment depending on the hemoglobin concentration and the patient’s heart condition.
Table 2: Recommendations for dose modification of ribavirin co-administered with Harvoni
Biological valuesReduce the ribavirin dose to 600 mg / day if:Stop ribavirin if:Hemoglobin levels in patients without heart disease<10 g / dL<8.5 g / dLHemoglobin level in patients with a history of stable heart diseaseDecrease in hemoglobin ≥ 2 g / dL during a 4 week treatment period<12 g / dL despite taking a reduced dose for 4 weeks
When ribavirin has been discontinued due to a biological defect or clinical manifestation, an attempt may be made to re-initiate ribavirin at a dose of 600 mg per day and then increase further. the dose up to 800 mg per day. However, increasing ribavirin to the dose initially prescribed (1000 mg to 1200 mg per day) is not recommended.
Patients should be informed that if they vomit within 5 hours of taking their dose, they should take another tablet. If they vomit more than 5 hours after taking their dose, it is not necessary to take another dose.
Patients should be informed that if they forget to take a dose and they notice it within 18 hours of their usual dose, they should take the tablet as soon as possible and then take the next dose as scheduled . If they find out more than 18 hours later, they should wait and take the next dose as scheduled. Patients should be instructed not to take a double dose.
The elderly
No dose adjustment is necessary in elderly patients (see section Pharmacokinetic properties ).
Renal failure
No dose adjustment of Harvoni is necessary in patients with mild or moderate renal impairment. The safety of ledipasvir / sofosbuvir has not been evaluated in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL / min / 1.73 m 2 ) or d end-stage renal disease (ESRD) requiring hemodialysis.
Hepatic insufficiency
No dose adjustment of Harvoni is necessary in patients with mild, moderate or severe hepatic impairment (Child-Pugh-Turcotte score [CPT] A, B or C) (see section Pharmacokinetic properties ). The safety and efficacy of the ledipasvir / sofosbuvir combination have been established in patients with decompensated cirrhosis (see section Pharmacodynamic properties ).
Pediatric population
The safety and efficacy of Harvoni in children and adolescents under 18 years of age have not been yet been established. No data is available.
Administration mode
Oral use.
Patients should be informed that they should swallow the tablet whole, with or without food. Due to its bitter taste, it is recommended not to chew or crush the film-coated tablet .
what’s Contraindications for
harvoni?
Hypersensitivity to the active ingredients or to any of the excipients listed in the Composition section .
Co-administration with rosuvastatin or St. John’s wort ( Hypericum perforatum ) (see section Interactions with other medicinal products and other forms of interaction ).
harvoni how does it work?
Absorption
After oral administration of ledipasvir / sofosbuvir in HCV infected patients, the median peak plasma concentration of ledipasvir was reached 4.0 hours after dosing. Sofosbuvir was rapidly absorbed and the median plasma peaks were reached ~ 1 hour after dosing.
The median plasma peak of GS-331007 was reached 4 hours after
administration.
Based on population pharmacokinetic analysis in HCV-infected patients, geometric means of steady-state AUC 0-24 for ledipasvir (n = 2,113), sofosbuvir (n = 1,542) and GS-331007 (n = 2,113) were 7,290, 1,320 and 12,000 ng • h / mL, respectively. The steady-state C max for ledipasvir, sofosbuvir, and GS-331007 were 323, 618 and 707 ng / mL, respectively. The AUC 0-24 and C max of sofosbuvir and GS-331007 were similar in healthy adult volunteers and in patients infected with HCV.
Compared to healthy subjects (n = 191), AUC 0-24 and C maxof ledipasvir were 24% and 32% lower, respectively, in HCV-infected patients. The AUC of ledipasvir is dose proportional over a dose range of 3 to 100 mg.
The AUCs of sofosbuvir and GS-331007 are quasi-dose-proportional over a dose range of 200 mg to 400 mg.
Effects of food intake
Compared with fasting, administration of a single dose of ledipasvir / sofosbuvir with a moderate or high fat meal increased the 0-inf AUC of sofosbuvir by approximately 2-fold. but did not significantly change sofosbuvir C max .
Exposures to GS-331007 and ledipasvir were not affected by these two types of meals. Harvoni can be administered with or without food.
Distribution
The binding of ledipasvir to human plasma proteins is> 99.8%. After administration of a single 90 mg dose of [ 14 C] -ledipasvir in healthy subjects, the blood / plasma radioactivity ratio [ 14 C] was between 0.51 and 0.66.
The binding of sofosbuvir to human plasma proteins is approximately 61 to 65% and the binding is independent of the concentration of the product, in the range of 1 to 20 µg / mL. The binding of GS-331007 to proteins is minimal in human plasma. Following a single 400 mg dose of [ 14 C] -sofosbuvir in healthy subjects, the ratio of blood [ 14 C] to plasma radioactivity was approximately 0.7.
Biotransformation
In vitro, no detectable metabolism of ledipasvir by human CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 has been observed. Evidence of slow oxidative metabolism, the mechanism of which is not known, has been observed. After administration of a single 90 mg dose of [ 14 C] -ledipasvir, systemic exposure was almost exclusively due to the parent drug (> 98%). Unchanged ledipasvir is also the main form found in faeces.
Sofosbuvir is extensively metabolized in the liver to form the pharmacologically active nucleoside triphosphate analog GS-461203. The active metabolite is not detected. The metabolic activation pathway involves sequential hydrolysis of the carboxyl ester group, catalyzed by human cathepsin A or carboxyl esterase 1, and phosphoramidate cleavage by the HINT1 protein (histidine triad nucleotide-binding protein) followed by phosphorylation by the pyrimidine-nucleotide biosynthesis pathway. Dephosphorylation results in the formation of the nucleoside metabolite GS-331007, which cannot be re-phosphorylated effectively and which lacks anti-HCV activity in vitro. In the case of the ledipasvir / sofosbuvir combination, GS-331007 represents approximately 85% of the
Elimination
After a single 90 mg oral dose of [ 14 C] -ledipasvir, the average total recovery of radioactivity [ 14 C] in faeces and urine was 87%, with most of the radioactivity was recovered in faeces (86%). Unchanged ledipasvir excreted in the faeces averaged 70% of the administered dose and the oxidative metabolite M19 represented 2.2% of the dose. These data suggest that the primary route of elimination for unchanged ledipasvir is biliary excretion, with renal excretion being a minor route (approximately 1%). The median terminal half-life of ledipasvir in healthy volunteers following administration of ledipasvir / sofosbuvir on an empty stomach was 47 hours.
After administration of a single 400 mg oral dose of [ 14 C] -sofosbuvir the mean total dose recovery was greater than 92%, of which approximately 80%, 14% and 2.5% recovered in the urine. faeces and exhaled air, respectively. The majority of the sofosbuvir dose recovered in the urine was GS-331007 (78%) and 3.5% was sofosbuvir. These data show that renal clearance is the primary route of elimination for GS-331007 with a large proportion actively excreted. The median terminal half-lives of sofosbuvir and GS-331007 after administration of ledipasvir / sofosbuvir were 0.5 and 27 hours, respectively.
Neither ledipasvir nor sofosbuvir are substrates for active hepatic transporters, organic cation transporter (OCT) 1, organic anion transporting polypeptide (OATP) 1B1 or OATP1B3. GS-331007 is not a substrate for renal transporters, including organic anion transporter (OAT) 1 or OAT3, or OCT2.
Potential in vitro effect of ledipasvir / sofosbuvir on other medicinal products At concentrations achieved in clinical practice, ledipasvir is not an inhibitor of hepatic transporters, including OATP 1B1 or 1B3, BSEP, OCT1 , OCT2, OAT1, OAT3, the transporter MATE (mutidrug and toxic compound extrusion) 1, the multidrug resistance protein (MRP) 2 or the MRP4. Sofosbuvir and GS-331007 are not inhibitors of the drug transporters P-gp, BCRP, MRP2, BSEP, OATP1B1, OATP1B3 and OCT1, and GS-331007 is not an inhibitor of OAT1, ‘OCT2 and MATE1.
Sofosbuvir and GS-331007 are not inhibitors or inducers of CYP or uridine diphosphate glucuronosyltransferase (UGT) 1A1 enzymes.
Pharmacokinetics in special populations
Ethnicity and gender
No clinically significant pharmacokinetic differences due to ethnicity were noted for ledipasvir, sofosbuvir or GS-331007. No clinically significant pharmacokinetic differences due to gender were noted for sofosbuvir or GS-331007. The AUC and C max of ledipasvir were 77% and 58% higher, respectively, in women compared to men. However, the relationship between gender and ledipasvir exposures was not considered clinically relevant.
The elderly
Pharmacokinetic analysis of HCV-infected patient populations showed that, in the age range analyzed (18-80 years), age has no clinically significant effect on exposure to ledipasvir, sofosbuvir or GS-331007. The clinical studies of ledipasvir / sofosbuvir included 117 patients aged 65 years and over.
Renal failure
The pharmacokinetics of ledipasvir have been studied with a single 90 mg dose of ledipasvir in non-HCV infected patients with severe renal impairment (eGFR <30 mL / min by Cockcroft-Gault formula, median CrCl [limits] of 22 [17-29] mL / min). The pharmacokinetics of ledipasvir did not show any clinically significant difference between healthy subjects and patients with severe renal impairment.
The pharmacokinetics of sofosbuvir have been studied in non-HCV-infected patients with mild (eGFR ≥ 50 and <80 mL / min / 1.73m 2 ), moderate (eGFR ≥ 30 and <50 mL / min / renal impairment ). 1.73 m 2 ), severe (eGFR <30 mL / min / 1.73 m 2 ) and in patients with ESRD requiring hemodialysis, after a single 400 mg dose of sofosbuvir. Compared with patients with normal renal function (eGFR> 80 mL / min / 1.73 m 2 ), the 0-inf AUC of sofosbuvir was 61%, 107% and 171% higher in mild renal impairment. moderate and severe, while AUC 0-infof GS-331007 was 55%, 88% and 451% higher, respectively. In patients with ESRD compared to patients with normal renal function, the AUC 0-inf of sofosbuvir was 28% higher when sofosbuvir was given 1 hour before hemodialysis, compared to 60% higher when sofosbuvir was given 1 hour after hemodialysis. ASC 0-infof GS-331007 in patients with ESRD who received sofosbuvir one hour before or one hour after hemodialysis was at least 10 and 20 times higher, respectively. GS-331007 is effectively removed by hemodialysis, with an extraction coefficient of approximately 53%. Following a single 400 mg dose of sofosbuvir, a 4 hour hemodialysis removed 18% of the administered sofosbuvir dose. The safety and efficacy of sofosbuvir have not been established in patients with severe renal impairment or ESRD.
Hepatic insufficiency
The pharmacokinetics of ledipasvir have been studied with a single 90 mg dose of ledipasvir in non-HCV patients with severe hepatic impairment (CPT C score). Plasma exposure to ledipasvir (AUC inf ) was similar in patients with severe hepatic impairment and in control patients with normal hepatic function. Pharmacokinetic analysis of HCV-infected patient populations showed that cirrhosis has no clinically significant effect on exposure to ledipasvir.
The pharmacokinetics of sofosbuvir were studied after 7 days of administration of 400 mg / day of sofosbuvir in HCV-infected patients with moderate or severe hepatic impairment (CPT scores B and C). Compared to patients with normal hepatic function, the AUC 0-24 of sofosbuvir was 126% and 143% higher, respectively, in moderate or severe hepatic impairment, while the AUC 0-24 of GS-331007 was 18% and 9% respectively higher. Population pharmacokinetic analysis in HCV infected patients has shown that cirrhosis has no clinically significant effect on exposure to sofosbuvir and GS-331007.
Body weight
Body weight had no significant effect on exposure to sofosbuvir based on population pharmacokinetic analysis. Exposure to ledipasvir decreases with increased body weight, but this effect is not considered clinically significant.
Pediatric population
The pharmacokinetics of ledipasvir, sofosbuvir and GS-331007 have not been established in children.
(see section Dosage and method of administration ).
harvoni treatment side effects
In addition to the desired effect, this can cause drug side effects.
The main side effects are the following.
Sometimes (from 10 to 30 people in 100)
Headache , fatigue.
Rarely (from 1 to 10 in 100 people)
Skin rash . Contact your doctor if you notice this.
Very rare (affects less than 1 in 100 people)
Hypersensitivity to this medication. This can be expressed in ‘angioedema’: a swelling of the face, lips, mouth, tongue or throat. You can be very stuffy. If it occurs, you should immediately seek out a doctor or go to the First Aid Service. You can not use this type of medication in the future. Therefore tell the pharmacy that you are hypersensitive to this sofosbuvir with ledipasvir. The pharmacy team can then ensure that you do not get this medication again.
Consult your doctor if you suffer too much from one of the above mentioned side effects or if you experience other side effects that you are worried about.
harvoni interactions
Harvoni containing ledipasvir and sofosbuvir, all the interactions that have been observed with these active ingredients used individually can occur with Harvoni.
Potential effect of Harvoni on other drugs
Ledipasvir is an in vitro inhibitor of the P-gp drug transporter and Breast Cancer Resistance Protein (BCRP) protein and may increase the intestinal absorption of substrates from these transporters in case of co-infection. administration. In vitro data indicate that ledipasvir may be a weak inducer of metabolic enzymes such as CYP3A4, CYP2C and UGT1A1. Plasma concentrations of the compounds that are substrates of these enzymes could be reduced when co-administered with the ledipasvir / sofosbuvir combination. In vitro, ledipasvir inhibits intestinal CYP3A4 and UGT1A1. Drugs with a narrow therapeutic range and metabolized by these isoenzymes should be used with caution and under close supervision.
Potential effect of other drugs on Harvoni
Ledipasvir and sofosbuvir are substrates of the P-gp drug carrier and BCRP, whereas GS-331007 is not.
Drugs that are potent inducers of P-gp (rifampicin, rifabutin, St. John’s wort, carbamazepine, phenobarbital and phenytoin) can significantly decrease plasma concentrations of ledipasvir and sofosbuvir, reducing the therapeutic effect the ledipasvir / sofosbuvir association and therefore they are against-marked Harvoni (see Contraindications ). Drugs that are moderate inducers of P-gp in the gut (such as oxcarbazepine) may decrease plasma concentrations of ledispavir and sofosbuvir, reducing the therapeutic effect of Harvoni. Co-administration of this type of medication with Harvoni is not recommended (see section Warnings and Precautions section).). Co-administration of drugs that inhibit P-gp and / or BCRP may increase the plasma concentrations of ledipasvir and sofosbuvir without increasing that of GS-331007; Harvoni may be co-administered with inhibitors of P-gp and / or BCRP. No clinically significant effect on the ledipasvir / sofosbuvir combination is expected via CYP450 or UGT1A1 enzymes.
Patients treated with vitamin K antagonists
Because hepatic function may change during treatment with Harvoni, close monitoring of International Normalized Index (INR) values is recommended.
Interactions between Harvoni and other drugs
Table 3 provides a list of clinically established or potentially clinically significant drug interactions (where the 90% confidence interval [IC] of the mean geometric least squares ratio [GLSM] was within the “↔” limits, in excess of “↑ “, Or lower than” ↓ “predetermined equivalence limits). The drug interactions described are based on studies conducted either with the combination of ledipasvir / sofosbuvir or with ledipasvir and sofosbuvir taken individually, or are predictions of drug interactions that may occur with the combination of ledipasvir / sofosbuvir. This table is not exhaustive.
Harvoni Warnings and Precautions :
Harvoni should not be administered at the same time as other medicines containing sofosbuvir.
Activity according to genotype
For recommended treatments for different genotypes of HCV, see Dosage and Method of Administration . For virologic and clinical activity by genotype, see the topic Pharmacodynamic properties .
Clinical evidence supporting the use of Harvoni in patients with HCV genotype 3 is limited . The relative efficacy of 12-week treatment with ledipasvir / sofosbuvir + ribavirin compared to a 24-week course with sofosbuvir + ribavirin, has not been studied. A conservative treatment of 24 weeks is recommended in all previously treated genotype 3 patients and in patients who are naive to any treatment with cirrhosis (see Dosage and method of administration section ).
Clinical evidence supporting the use of Harvoni in patients with HCV genotype 2 and 6 is limited.
Severe Bradycardia and Conduction Disorders
Cases of severe bradycardia and conduction disturbances have been observed with Harvoni when co-administered with amiodarone with or without other heart rate-lowering drugs. The mechanism is not established.
The concomitant use of amiodarone has been limited in the clinical development of sofosbuvir in combination with direct-acting antivirals (DAAs). Some cases have been life-threatening. Therefore, amiodarone should be used in patients treated with Harvoni only in case of intolerance or contraindication to other anti-arrhythmic treatments.
If concomitant use of amiodarone is considered necessary, close monitoring of patients is recommended when initiating Harvoni treatment. Patients identified as being at high risk for bradyarrhythmia should be monitored continuously for 48 hours in an adapted hospital environment.
Given the long half-life of amiodarone, appropriate monitoring should also be performed in patients who have discontinued amiodarone in recent months and need to start treatment with Harvoni.
All patients treated with Harvoni who receive amiodarone with or without other bradycardic medications should also be advised of symptoms of bradycardia and conduction disturbances, and should be informed of the need for urgent medical attention. feel these symptoms.
Treatment of patients previously treated with direct-acting antivirals for HCV In patients who have failed treatment with ledipasvir / sofosbuvir, a selection of resistance mutations in NS5A significantly reduces susceptibility to ledipasvir.
majority of cases (see section 5.1).). Limited data indicates that this type of mutation in NS5A is not reversible at long-term follow-up. In patients who have failed prior therapy with ledipasvir / sofosbuvir, there is currently no evidence to support the efficacy of re-treatment with NS5A inhibitor therapy. Similarly, in patients who have failed previous treatment with an NS3 / 4A protease inhibitor, there are currently no data supporting the efficacy of NS3 / 4A protease inhibitors. In these patients, the treatment of HCV infection may therefore depend on the use of other classes of drugs. Therefore, it will be necessary to
Renal failure
No dose adjustment of Harvoni is required in patients with mild or moderate renal impairment. The safety of Harvoni has not been evaluated in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL / min / 1.73 m 2 ) or end-stage renal disease (IRT) requiring hemodialysis. If Harvoni is used in combination with ribavirin, also refer to the Summary of Product Characteristics of Ribavirin for patients with creatinine clearance (CrCl) <50 mL / min (see section 5.2 ).
Patients with decompensated cirrhosis and / or waiting for liver transplantation or post-liver transplantation
The efficacy of ledipasvir / sofosbuvir in patients with genotype 5 or genotype 6 HCV with decompensated cirrhosis and / or awaiting liver transplantation or post-liver transplantation has not been studied. The Harvoni treatment decision should be based on an assessment of the potential benefits and risks for each patient.
Use with moderate inducers of P-gp
Drugs that are moderate inducers of P-gp in the intestine (such as oxcarbazepine) may decrease the plasma concentrations of ledipasvir and sofosbuvir, reducing the therapeutic effect of Harvoni. Co-administration of this type of medication with Harvoni is not recommended (see section Interactions with other medicinal products and other forms of interaction ).
Use with some antiretrovirals for HIV
Harvoni has been shown to increase the exposure to tenofovir, particularly when used in combination with an HIV treatment containing tenofovir disoproxil fumarate and a pharmacokinetic booster (ritonavir or cobicistat). The safety of tenofovir disoproxil fumarate in the context of Harvoni treatment in the presence of a pharmacokinetic booster has not been established. The potential risks and benefits associated with co-administration of Harvoni with the fixed-dose combination tablet containing elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or tenofovir disoproxil fumarate used in combination with a protease inhibitor Boosted HIV (eg atazanavir or darunavir) should be considered, especially in patients at increased risk of renal dysfunction. In patients receiving concomitant Harvoni with elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function. elventegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function. elventegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function.
Use with HMG-CoA reductase inhibitors
Co-administration of Harvoni with HMG-CoA reductase inhibitors (statins) can significantly increase the statin concentration, increasing the risk of myopathy and rhabdomyolysis (see section 4.5). forms of interactions ).
HCV / HBV co-infection (hepatitis B virus)
Cases of hepatitis B virus (HBV) reactivation, some with fatal outcome, have been reported during or after treatment with direct-acting antiviral agents. HBV testing should be performed in all patients prior to initiation of treatment. Patients co-infected with HBV / HCV are at risk for reactivation of HBV and should therefore be monitored and managed according to clinical guidelines.
Pediatric population
Harvoni is not recommended for use in children and adolescents under 18 years of age as the safety and efficacy of this medication have not been established in this population.
excipients
Harvoni contains an azo dye, S Yellow Orange Lake (E110), which can cause allergic reactions. It also contains lactose. As a result, patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose / galactose malabsorption should not take this medicine.
Drive and use machines
Harvoni (alone or in combination with ribavirin) has no or negligible effect on the ability to drive and use machines.
However, patients should be informed that fatigue was more common in patients treated with ledipasvir / sofosbuvir than in those receiving placebo.
Harvoni and PREGNANCY / BREAST FEEDING / FERTILITY
Women of childbearing
potential / contraception in men and women When Harvoni is used in combination with ribavirin, all precautions should be taken to prevent pregnancy in patients and female partners of patients. Significant teratogenic and / or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. Women of childbearing potential or their male partners should use an effective method of contraception during treatment and for some time after the end of treatment, as recommended in the Summary of Product Characteristics of Ribavirin.
See the Summary of Product Characteristics of Ribavirin for more information.
Pregnancy
There are no data or limited data (less than 300 pregnancies) on the use of ledipasvir, sofosbuvir or Harvoni in pregnant women.Studies in animals have not shown any direct deleterious effects on reproduction. No significant effects on fetal development were observed in rats and rabbits with ledipasvir or sofosbuvir. However, it was not possible to completely evaluate margins of exposure with sofosbuvir in rats in relation to human exposure at the recommended clinical dose.
As a precaution, it is best to avoid the use of Harvoni during pregnancy.
feeding
It is not known whether ledipasvir or sofosbuvir and their metabolites are excreted in breast milk.The pharmacokinetic data available in animals have demonstrated the excretion of ledipasvir and metabolites of sofosbuvir in milk
A risk for newborns / infants can not be ruled out. Therefore, Harvoni should not be used while breastfeeding.
Fertility
There is no data on the effect of Harvoni on human fertility. Studies in animals have not shown deleterious effects of ledipasvir or sofosbuvir on fertility.
If ribavirin is co-administered with Harvoni, contraindications for the use of ribavirin during pregnancy and lactation apply (see also Summary of Product Characteristics of Ribavirin).
What should I do if I miss a dose?
It is important to take this medicine consistently. If you have forgotten a dose:
You use this medicine once a day:
Does it take more than 6 hours before you should take the next dose? Swallow the forgotten tablet as soon as
possible and take the next dose at the usual time.
Does it take less than 6 hours before you should take the next dose? Skip the forgotten tablet and take the next dose at the usual time.
What happens if I overdose from Harvoni ?
The highest documented doses of ledipasvir and sofosbuvir were 120 mg twice daily for 10 days and 1200 mg once, respectively. In these studies in healthy volunteers, no adverse events were observed at these doses and the adverse effects were similar in frequency and intensity to those reported in the placebo groups. The effects of higher doses are not known.
There is no specific antidote for overdose of Harvoni. If overdose occurs, all symptoms of toxicity will be monitored in the patient. Treatment of Harvoni overdose consists of general supportive measures, with monitoring of vital signs and observation of the patient’s clinical condition. It is unlikely that hemodialysis will significantly eliminate ledipasvir since ledipasvir is highly bound to plasma proteins. Hemodialysis can effectively remove the main circulating metabolite of sofosbuvir (ie GS-331007), with a 53% extraction ratio.
What is Forms and Composition Harvoni?
90 mg / 400 mg film-coated tablet (diamond shaped, orange, size 19 mm × 10 mm, on one side with “GSI” inscription and on the other side “7985”): Bottle of 28, with child safety closure system.
NOT’s
Edrug-online contains comprehensive and detailed information about drugs available in the medical field, and is divided into four sections:
general information:
Includes a general description of the drug, its use, brand names, FAQs, and relevant news and articles
Additional information:
General explanation about dealing with the medicine: how to take the medicine, the doses and times of it, the start and duration of its effectiveness, the recommended diet during the period of taking the medicine, the method of storage and storage, recommendations in cases for forgetting the dose and instructions to stop taking the drug and take additional doses.
Special warnings:
For pregnant and breastfeeding women, the elderly, boys and drivers, and use before surgery.
Side effects:
It treats possible side effects and drug interactions that require attention and its effect on continuous use.
The information contained in this medicine is based on medical literature, but it is not a substitute for consulting a doctor.
The post Harvoni medication Uses, Dosage, Side Effects, Precautions & Warnings appeared first on Drug Online.
from Drug Online
https://bit.ly/3eY6utB
via Edrug Online
from Faculty of Medicine
https://bit.ly/2Dbn103
via Internal Medicine
0 notes
Text
Harvoni medication Uses, Dosage, Side Effects, Precautions & Warnings
Drug Online
Harvoni medication >>> Generic drugof the Therapeutic class: Gastro-Entero-Hepatology
active ingredients: Ledipasvir , Sofosbuvir
Important to know about harvoni medication ?
Sofosbuvir and ledipasvir inhibit the growth of hepatitis C virus.
In chronic hepatitis C (liver inflammation).
It takes several months until your symptoms decrease, such as fatigue and abdominal complaints.
A course usually lasts 12 to 24 weeks, sometimes 8 weeks is sufficient. Complete the entire course, even if your symptoms have already disappeared.
You may take the tablet with or without food. Swallow it whole, without chewing. The tablet tastes otherwise very bitter.
Are you nauseous and do you have to vomit? If you have to vomit within 5 hours after swallowing this medicine, you must take a new tablet. Otherwise, it does not work properly.
Side effects may include headache, fatigue, skin rash and hypersensitivity.
There are many interactions with other means. Have your pharmacist check whether you can use it safely with your other medicines, even those that you have bought without a prescription.
what is harvoni used to treat and indication?
Harvoni is indicated for the treatment of chronic hepatitis C (CHC) in adults (see sections Dosage and Administration , Warnings and Precautions and Pharmacodynamic properties ).
For activity based on hepatitis C virus (HCV) genotype, see Warnings and Precautions andPharmacodynamic Properties sections .
Harvoni Dosage
Dosage
The recommended dose is one Harvoni tablet once a day, with or without food (see section Pharmacokinetic properties ).
<brarial’,’sans-serif’; color:=”” black”=””>Table 1: Recommended duration of treatment with Harvoni and recommendations for co-administration with ribavirin in certain subgroups</brarial’,’sans-serif’;>
Patient population * Treatment Duration Patients with genotype 1 or genotype 4 HCC 12 weeks.
– A duration of 8 weeks could be considered in patients infected with genotype 1 not previously treated (see section <iarial’,’sans-serif’; color:=”” black”=””>Pharmacodynamic properties, ION-3 study).</iarial’,’sans-serif’;>
Patients without cirrhosis Harvoni – A duration of 24 weeks should be considered in previously treated patients for whom the possibilities of subsequent re-treatment are uncertain (see section Warnings and precautions for use ). Patients with compensated cirrhosis Harvoni 24 weeks.
– A duration of 12 weeks could be considered in patients for whom the risk of clinical progression of the disease is considered low and for whom subsequent retreatment options exist (see section 4.4 ).
Patients with decompensated cirrhosis or in a pre / post liver transplant situation Harvoni + ribavirin 24 weeks (see warnings and precautions for use and Pharmacodynamic properties ) Patients with genotype 3 CHC Patients with cirrhosis and / or failure of a previous treatment Harvoni + ribavirin 24 weeks (see warnings and precautions for use and Pharmacodynamic properties )
* Includes patients co-infected with human immunodeficiency virus (HIV).
If used in combination with ribavirin, see also the Summary of Product Characteristics for ribavirin.
In patients without decompensated cirrhosis and requiring the addition of ribavirin in their treatment (see table 1), the daily dose of ribavirin is calculated according to the weight (<75 kg = 1000 mg and ≥ 75 kg = 1 200 mg) and is administered orally in two divided doses, with food.
In patients with decompensated cirrhosis, ribavirin should be administered at the starting dose of 600 mg divided over the day. If the initial dose is well tolerated, the dose may be increased gradually to a maximum of 1000-1200 mg per day (1000 mg for patients weighing <75 kg and 1200 mg for patients weighing ≥ 75 kg ).
If the initial dose is not well tolerated, the dose should be reduced as needed based on hemoglobin levels.
Dose modification of ribavirin in patients taking 1,000-1,200 mg per day
If Harvoni is used in combination with ribavirin and a patient experiences a serious side effect potentially related to ribavirin, the dose of ribavirin should be changed or the treatment stopped, if necessary, until the side effect disappears or its severity decreases. Table 2 gives the recommendations for dose modification and discontinuation of treatment depending on the hemoglobin concentration and the patient’s heart condition.
Table 2: Recommendations for dose modification of ribavirin co-administered with Harvoni
Biological values Reduce the ribavirin dose to 600 mg / day if: Stop ribavirin if: Hemoglobin levels in patients without heart disease <10 g / dL <8.5 g / dL Hemoglobin level in patients with a history of stable heart disease Decrease in hemoglobin ≥ 2 g / dL during a 4 week treatment period <12 g / dL despite taking a reduced dose for 4 weeks
When ribavirin has been discontinued due to a biological defect or clinical manifestation, an attempt may be made to re-initiate ribavirin at a dose of 600 mg per day and then increase further. the dose up to 800 mg per day. However, increasing ribavirin to the dose initially prescribed (1000 mg to 1200 mg per day) is not recommended.
Patients should be informed that if they vomit within 5 hours of taking their dose, they should take another tablet. If they vomit more than 5 hours after taking their dose, it is not necessary to take another dose.
Patients should be informed that if they forget to take a dose and they notice it within 18 hours of their usual dose, they should take the tablet as soon as possible and then take the next dose as scheduled . If they find out more than 18 hours later, they should wait and take the next dose as scheduled. Patients should be instructed not to take a double dose.
The elderly
No dose adjustment is necessary in elderly patients (see section Pharmacokinetic properties ).
Renal failure
No dose adjustment of Harvoni is necessary in patients with mild or moderate renal impairment. The safety of ledipasvir / sofosbuvir has not been evaluated in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL / min / 1.73 m 2 ) or d end-stage renal disease (ESRD) requiring hemodialysis.
Hepatic insufficiency
No dose adjustment of Harvoni is necessary in patients with mild, moderate or severe hepatic impairment (Child-Pugh-Turcotte score [CPT] A, B or C) (see section Pharmacokinetic properties ). The safety and efficacy of the ledipasvir / sofosbuvir combination have been established in patients with decompensated cirrhosis (see section Pharmacodynamic properties ).
Pediatric population
The safety and efficacy of Harvoni in children and adolescents under 18 years of age have not been yet been established. No data is available.
Administration mode
Oral use.
Patients should be informed that they should swallow the tablet whole, with or without food. Due to its bitter taste, it is recommended not to chew or crush the film-coated tablet .
what’s Contraindications for
harvoni?
Hypersensitivity to the active ingredients or to any of the excipients listed in the Composition section .
Co-administration with rosuvastatin or St. John’s wort ( Hypericum perforatum ) (see section Interactions with other medicinal products and other forms of interaction ).
harvoni how does it work?
Absorption
After oral administration of ledipasvir / sofosbuvir in HCV infected patients, the median peak plasma concentration of ledipasvir was reached 4.0 hours after dosing. Sofosbuvir was rapidly absorbed and the median plasma peaks were reached ~ 1 hour after dosing.
The median plasma peak of GS-331007 was reached 4 hours after
administration.
Based on population pharmacokinetic analysis in HCV-infected patients, geometric means of steady-state AUC 0-24 for ledipasvir (n = 2,113), sofosbuvir (n = 1,542) and GS-331007 (n = 2,113) were 7,290, 1,320 and 12,000 ng • h / mL, respectively. The steady-state C max for ledipasvir, sofosbuvir, and GS-331007 were 323, 618 and 707 ng / mL, respectively. The AUC 0-24 and C max of sofosbuvir and GS-331007 were similar in healthy adult volunteers and in patients infected with HCV.
Compared to healthy subjects (n = 191), AUC 0-24 and C maxof ledipasvir were 24% and 32% lower, respectively, in HCV-infected patients. The AUC of ledipasvir is dose proportional over a dose range of 3 to 100 mg.
The AUCs of sofosbuvir and GS-331007 are quasi-dose-proportional over a dose range of 200 mg to 400 mg.
Effects of food intake
Compared with fasting, administration of a single dose of ledipasvir / sofosbuvir with a moderate or high fat meal increased the 0-inf AUC of sofosbuvir by approximately 2-fold. but did not significantly change sofosbuvir C max .
Exposures to GS-331007 and ledipasvir were not affected by these two types of meals. Harvoni can be administered with or without food.
Distribution
The binding of ledipasvir to human plasma proteins is> 99.8%. After administration of a single 90 mg dose of [ 14 C] -ledipasvir in healthy subjects, the blood / plasma radioactivity ratio [ 14 C] was between 0.51 and 0.66.
The binding of sofosbuvir to human plasma proteins is approximately 61 to 65% and the binding is independent of the concentration of the product, in the range of 1 to 20 µg / mL. The binding of GS-331007 to proteins is minimal in human plasma. Following a single 400 mg dose of [ 14 C] -sofosbuvir in healthy subjects, the ratio of blood [ 14 C] to plasma radioactivity was approximately 0.7.
Biotransformation
In vitro, no detectable metabolism of ledipasvir by human CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 has been observed. Evidence of slow oxidative metabolism, the mechanism of which is not known, has been observed. After administration of a single 90 mg dose of [ 14 C] -ledipasvir, systemic exposure was almost exclusively due to the parent drug (> 98%). Unchanged ledipasvir is also the main form found in faeces.
Sofosbuvir is extensively metabolized in the liver to form the pharmacologically active nucleoside triphosphate analog GS-461203. The active metabolite is not detected. The metabolic activation pathway involves sequential hydrolysis of the carboxyl ester group, catalyzed by human cathepsin A or carboxyl esterase 1, and phosphoramidate cleavage by the HINT1 protein (histidine triad nucleotide-binding protein) followed by phosphorylation by the pyrimidine-nucleotide biosynthesis pathway. Dephosphorylation results in the formation of the nucleoside metabolite GS-331007, which cannot be re-phosphorylated effectively and which lacks anti-HCV activity in vitro. In the case of the ledipasvir / sofosbuvir combination, GS-331007 represents approximately 85% of the
Elimination
After a single 90 mg oral dose of [ 14 C] -ledipasvir, the average total recovery of radioactivity [ 14 C] in faeces and urine was 87%, with most of the radioactivity was recovered in faeces (86%). Unchanged ledipasvir excreted in the faeces averaged 70% of the administered dose and the oxidative metabolite M19 represented 2.2% of the dose. These data suggest that the primary route of elimination for unchanged ledipasvir is biliary excretion, with renal excretion being a minor route (approximately 1%). The median terminal half-life of ledipasvir in healthy volunteers following administration of ledipasvir / sofosbuvir on an empty stomach was 47 hours.
After administration of a single 400 mg oral dose of [ 14 C] -sofosbuvir the mean total dose recovery was greater than 92%, of which approximately 80%, 14% and 2.5% recovered in the urine. faeces and exhaled air, respectively. The majority of the sofosbuvir dose recovered in the urine was GS-331007 (78%) and 3.5% was sofosbuvir. These data show that renal clearance is the primary route of elimination for GS-331007 with a large proportion actively excreted. The median terminal half-lives of sofosbuvir and GS-331007 after administration of ledipasvir / sofosbuvir were 0.5 and 27 hours, respectively.
Neither ledipasvir nor sofosbuvir are substrates for active hepatic transporters, organic cation transporter (OCT) 1, organic anion transporting polypeptide (OATP) 1B1 or OATP1B3. GS-331007 is not a substrate for renal transporters, including organic anion transporter (OAT) 1 or OAT3, or OCT2.
Potential in vitro effect of ledipasvir / sofosbuvir on other medicinal products At concentrations achieved in clinical practice, ledipasvir is not an inhibitor of hepatic transporters, including OATP 1B1 or 1B3, BSEP, OCT1 , OCT2, OAT1, OAT3, the transporter MATE (mutidrug and toxic compound extrusion) 1, the multidrug resistance protein (MRP) 2 or the MRP4. Sofosbuvir and GS-331007 are not inhibitors of the drug transporters P-gp, BCRP, MRP2, BSEP, OATP1B1, OATP1B3 and OCT1, and GS-331007 is not an inhibitor of OAT1, ‘OCT2 and MATE1.
Sofosbuvir and GS-331007 are not inhibitors or inducers of CYP or uridine diphosphate glucuronosyltransferase (UGT) 1A1 enzymes.
Pharmacokinetics in special populations
Ethnicity and gender
No clinically significant pharmacokinetic differences due to ethnicity were noted for ledipasvir, sofosbuvir or GS-331007. No clinically significant pharmacokinetic differences due to gender were noted for sofosbuvir or GS-331007. The AUC and C max of ledipasvir were 77% and 58% higher, respectively, in women compared to men. However, the relationship between gender and ledipasvir exposures was not considered clinically relevant.
The elderly
Pharmacokinetic analysis of HCV-infected patient populations showed that, in the age range analyzed (18-80 years), age has no clinically significant effect on exposure to ledipasvir, sofosbuvir or GS-331007. The clinical studies of ledipasvir / sofosbuvir included 117 patients aged 65 years and over.
Renal failure
The pharmacokinetics of ledipasvir have been studied with a single 90 mg dose of ledipasvir in non-HCV infected patients with severe renal impairment (eGFR <30 mL / min by Cockcroft-Gault formula, median CrCl [limits] of 22 [17-29] mL / min). The pharmacokinetics of ledipasvir did not show any clinically significant difference between healthy subjects and patients with severe renal impairment.
The pharmacokinetics of sofosbuvir have been studied in non-HCV-infected patients with mild (eGFR ≥ 50 and <80 mL / min / 1.73m 2 ), moderate (eGFR ≥ 30 and <50 mL / min / renal impairment ). 1.73 m 2 ), severe (eGFR <30 mL / min / 1.73 m 2 ) and in patients with ESRD requiring hemodialysis, after a single 400 mg dose of sofosbuvir. Compared with patients with normal renal function (eGFR> 80 mL / min / 1.73 m 2 ), the 0-inf AUC of sofosbuvir was 61%, 107% and 171% higher in mild renal impairment. moderate and severe, while AUC 0-infof GS-331007 was 55%, 88% and 451% higher, respectively. In patients with ESRD compared to patients with normal renal function, the AUC 0-inf of sofosbuvir was 28% higher when sofosbuvir was given 1 hour before hemodialysis, compared to 60% higher when sofosbuvir was given 1 hour after hemodialysis. ASC 0-infof GS-331007 in patients with ESRD who received sofosbuvir one hour before or one hour after hemodialysis was at least 10 and 20 times higher, respectively. GS-331007 is effectively removed by hemodialysis, with an extraction coefficient of approximately 53%. Following a single 400 mg dose of sofosbuvir, a 4 hour hemodialysis removed 18% of the administered sofosbuvir dose. The safety and efficacy of sofosbuvir have not been established in patients with severe renal impairment or ESRD.
Hepatic insufficiency
The pharmacokinetics of ledipasvir have been studied with a single 90 mg dose of ledipasvir in non-HCV patients with severe hepatic impairment (CPT C score). Plasma exposure to ledipasvir (AUC inf ) was similar in patients with severe hepatic impairment and in control patients with normal hepatic function. Pharmacokinetic analysis of HCV-infected patient populations showed that cirrhosis has no clinically significant effect on exposure to ledipasvir.
The pharmacokinetics of sofosbuvir were studied after 7 days of administration of 400 mg / day of sofosbuvir in HCV-infected patients with moderate or severe hepatic impairment (CPT scores B and C). Compared to patients with normal hepatic function, the AUC 0-24 of sofosbuvir was 126% and 143% higher, respectively, in moderate or severe hepatic impairment, while the AUC 0-24 of GS-331007 was 18% and 9% respectively higher. Population pharmacokinetic analysis in HCV infected patients has shown that cirrhosis has no clinically significant effect on exposure to sofosbuvir and GS-331007.
Body weight
Body weight had no significant effect on exposure to sofosbuvir based on population pharmacokinetic analysis. Exposure to ledipasvir decreases with increased body weight, but this effect is not considered clinically significant.
Pediatric population
The pharmacokinetics of ledipasvir, sofosbuvir and GS-331007 have not been established in children.
(see section Dosage and method of administration ).
harvoni treatment side effects
In addition to the desired effect, this can cause drug side effects.
The main side effects are the following.
Sometimes (from 10 to 30 people in 100)
Headache , fatigue.
Rarely (from 1 to 10 in 100 people)
Skin rash . Contact your doctor if you notice this.
Very rare (affects less than 1 in 100 people)
Hypersensitivity to this medication. This can be expressed in ‘angioedema’: a swelling of the face, lips, mouth, tongue or throat. You can be very stuffy. If it occurs, you should immediately seek out a doctor or go to the First Aid Service. You can not use this type of medication in the future. Therefore tell the pharmacy that you are hypersensitive to this sofosbuvir with ledipasvir. The pharmacy team can then ensure that you do not get this medication again.
Consult your doctor if you suffer too much from one of the above mentioned side effects or if you experience other side effects that you are worried about.
harvoni interactions
Harvoni containing ledipasvir and sofosbuvir, all the interactions that have been observed with these active ingredients used individually can occur with Harvoni.
Potential effect of Harvoni on other drugs
Ledipasvir is an in vitro inhibitor of the P-gp drug transporter and Breast Cancer Resistance Protein (BCRP) protein and may increase the intestinal absorption of substrates from these transporters in case of co-infection. administration. In vitro data indicate that ledipasvir may be a weak inducer of metabolic enzymes such as CYP3A4, CYP2C and UGT1A1. Plasma concentrations of the compounds that are substrates of these enzymes could be reduced when co-administered with the ledipasvir / sofosbuvir combination. In vitro, ledipasvir inhibits intestinal CYP3A4 and UGT1A1. Drugs with a narrow therapeutic range and metabolized by these isoenzymes should be used with caution and under close supervision.
Potential effect of other drugs on Harvoni
Ledipasvir and sofosbuvir are substrates of the P-gp drug carrier and BCRP, whereas GS-331007 is not.
Drugs that are potent inducers of P-gp (rifampicin, rifabutin, St. John’s wort, carbamazepine, phenobarbital and phenytoin) can significantly decrease plasma concentrations of ledipasvir and sofosbuvir, reducing the therapeutic effect the ledipasvir / sofosbuvir association and therefore they are against-marked Harvoni (see Contraindications ). Drugs that are moderate inducers of P-gp in the gut (such as oxcarbazepine) may decrease plasma concentrations of ledispavir and sofosbuvir, reducing the therapeutic effect of Harvoni. Co-administration of this type of medication with Harvoni is not recommended (see section Warnings and Precautions section).). Co-administration of drugs that inhibit P-gp and / or BCRP may increase the plasma concentrations of ledipasvir and sofosbuvir without increasing that of GS-331007; Harvoni may be co-administered with inhibitors of P-gp and / or BCRP. No clinically significant effect on the ledipasvir / sofosbuvir combination is expected via CYP450 or UGT1A1 enzymes.
Patients treated with vitamin K antagonists
Because hepatic function may change during treatment with Harvoni, close monitoring of International Normalized Index (INR) values is recommended.
Interactions between Harvoni and other drugs
Table 3 provides a list of clinically established or potentially clinically significant drug interactions (where the 90% confidence interval [IC] of the mean geometric least squares ratio [GLSM] was within the “↔” limits, in excess of “↑ “, Or lower than” ↓ “predetermined equivalence limits). The drug interactions described are based on studies conducted either with the combination of ledipasvir / sofosbuvir or with ledipasvir and sofosbuvir taken individually, or are predictions of drug interactions that may occur with the combination of ledipasvir / sofosbuvir. This table is not exhaustive.
Harvoni Warnings and Precautions :
Harvoni should not be administered at the same time as other medicines containing sofosbuvir.
Activity according to genotype
For recommended treatments for different genotypes of HCV, see Dosage and Method of Administration . For virologic and clinical activity by genotype, see the topic Pharmacodynamic properties .
Clinical evidence supporting the use of Harvoni in patients with HCV genotype 3 is limited . The relative efficacy of 12-week treatment with ledipasvir / sofosbuvir + ribavirin compared to a 24-week course with sofosbuvir + ribavirin, has not been studied. A conservative treatment of 24 weeks is recommended in all previously treated genotype 3 patients and in patients who are naive to any treatment with cirrhosis (see Dosage and method of administration section ).
Clinical evidence supporting the use of Harvoni in patients with HCV genotype 2 and 6 is limited.
Severe Bradycardia and Conduction Disorders
Cases of severe bradycardia and conduction disturbances have been observed with Harvoni when co-administered with amiodarone with or without other heart rate-lowering drugs. The mechanism is not established.
The concomitant use of amiodarone has been limited in the clinical development of sofosbuvir in combination with direct-acting antivirals (DAAs). Some cases have been life-threatening. Therefore, amiodarone should be used in patients treated with Harvoni only in case of intolerance or contraindication to other anti-arrhythmic treatments.
If concomitant use of amiodarone is considered necessary, close monitoring of patients is recommended when initiating Harvoni treatment. Patients identified as being at high risk for bradyarrhythmia should be monitored continuously for 48 hours in an adapted hospital environment.
Given the long half-life of amiodarone, appropriate monitoring should also be performed in patients who have discontinued amiodarone in recent months and need to start treatment with Harvoni.
All patients treated with Harvoni who receive amiodarone with or without other bradycardic medications should also be advised of symptoms of bradycardia and conduction disturbances, and should be informed of the need for urgent medical attention. feel these symptoms.
Treatment of patients previously treated with direct-acting antivirals for HCV In patients who have failed treatment with ledipasvir / sofosbuvir, a selection of resistance mutations in NS5A significantly reduces susceptibility to ledipasvir.
majority of cases (see section 5.1).). Limited data indicates that this type of mutation in NS5A is not reversible at long-term follow-up. In patients who have failed prior therapy with ledipasvir / sofosbuvir, there is currently no evidence to support the efficacy of re-treatment with NS5A inhibitor therapy. Similarly, in patients who have failed previous treatment with an NS3 / 4A protease inhibitor, there are currently no data supporting the efficacy of NS3 / 4A protease inhibitors. In these patients, the treatment of HCV infection may therefore depend on the use of other classes of drugs. Therefore, it will be necessary to
Renal failure
No dose adjustment of Harvoni is required in patients with mild or moderate renal impairment. The safety of Harvoni has not been evaluated in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL / min / 1.73 m 2 ) or end-stage renal disease (IRT) requiring hemodialysis. If Harvoni is used in combination with ribavirin, also refer to the Summary of Product Characteristics of Ribavirin for patients with creatinine clearance (CrCl) <50 mL / min (see section 5.2 ).
Patients with decompensated cirrhosis and / or waiting for liver transplantation or post-liver transplantation
The efficacy of ledipasvir / sofosbuvir in patients with genotype 5 or genotype 6 HCV with decompensated cirrhosis and / or awaiting liver transplantation or post-liver transplantation has not been studied. The Harvoni treatment decision should be based on an assessment of the potential benefits and risks for each patient.
Use with moderate inducers of P-gp
Drugs that are moderate inducers of P-gp in the intestine (such as oxcarbazepine) may decrease the plasma concentrations of ledipasvir and sofosbuvir, reducing the therapeutic effect of Harvoni. Co-administration of this type of medication with Harvoni is not recommended (see section Interactions with other medicinal products and other forms of interaction ).
Use with some antiretrovirals for HIV
Harvoni has been shown to increase the exposure to tenofovir, particularly when used in combination with an HIV treatment containing tenofovir disoproxil fumarate and a pharmacokinetic booster (ritonavir or cobicistat). The safety of tenofovir disoproxil fumarate in the context of Harvoni treatment in the presence of a pharmacokinetic booster has not been established. The potential risks and benefits associated with co-administration of Harvoni with the fixed-dose combination tablet containing elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or tenofovir disoproxil fumarate used in combination with a protease inhibitor Boosted HIV (eg atazanavir or darunavir) should be considered, especially in patients at increased risk of renal dysfunction. In patients receiving concomitant Harvoni with elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function. elventegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function. elventegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function.
Use with HMG-CoA reductase inhibitors
Co-administration of Harvoni with HMG-CoA reductase inhibitors (statins) can significantly increase the statin concentration, increasing the risk of myopathy and rhabdomyolysis (see section 4.5). forms of interactions ).
HCV / HBV co-infection (hepatitis B virus)
Cases of hepatitis B virus (HBV) reactivation, some with fatal outcome, have been reported during or after treatment with direct-acting antiviral agents. HBV testing should be performed in all patients prior to initiation of treatment. Patients co-infected with HBV / HCV are at risk for reactivation of HBV and should therefore be monitored and managed according to clinical guidelines.
Pediatric population
Harvoni is not recommended for use in children and adolescents under 18 years of age as the safety and efficacy of this medication have not been established in this population.
excipients
Harvoni contains an azo dye, S Yellow Orange Lake (E110), which can cause allergic reactions. It also contains lactose. As a result, patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose / galactose malabsorption should not take this medicine.
Drive and use machines
Harvoni (alone or in combination with ribavirin) has no or negligible effect on the ability to drive and use machines.
However, patients should be informed that fatigue was more common in patients treated with ledipasvir / sofosbuvir than in those receiving placebo.
Harvoni and PREGNANCY / BREAST FEEDING / FERTILITY
Women of childbearing
potential / contraception in men and women When Harvoni is used in combination with ribavirin, all precautions should be taken to prevent pregnancy in patients and female partners of patients. Significant teratogenic and / or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. Women of childbearing potential or their male partners should use an effective method of contraception during treatment and for some time after the end of treatment, as recommended in the Summary of Product Characteristics of Ribavirin.
See the Summary of Product Characteristics of Ribavirin for more information.
Pregnancy
There are no data or limited data (less than 300 pregnancies) on the use of ledipasvir, sofosbuvir or Harvoni in pregnant women.Studies in animals have not shown any direct deleterious effects on reproduction. No significant effects on fetal development were observed in rats and rabbits with ledipasvir or sofosbuvir. However, it was not possible to completely evaluate margins of exposure with sofosbuvir in rats in relation to human exposure at the recommended clinical dose.
As a precaution, it is best to avoid the use of Harvoni during pregnancy.
feeding
It is not known whether ledipasvir or sofosbuvir and their metabolites are excreted in breast milk.The pharmacokinetic data available in animals have demonstrated the excretion of ledipasvir and metabolites of sofosbuvir in milk
A risk for newborns / infants can not be ruled out. Therefore, Harvoni should not be used while breastfeeding.
Fertility
There is no data on the effect of Harvoni on human fertility. Studies in animals have not shown deleterious effects of ledipasvir or sofosbuvir on fertility.
If ribavirin is co-administered with Harvoni, contraindications for the use of ribavirin during pregnancy and lactation apply (see also Summary of Product Characteristics of Ribavirin).
What should I do if I miss a dose?
It is important to take this medicine consistently. If you have forgotten a dose:
You use this medicine once a day:
Does it take more than 6 hours before you should take the next dose? Swallow the forgotten tablet as soon as
possible and take the next dose at the usual time.
Does it take less than 6 hours before you should take the next dose? Skip the forgotten tablet and take the next dose at the usual time.
What happens if I overdose from Harvoni ?
The highest documented doses of ledipasvir and sofosbuvir were 120 mg twice daily for 10 days and 1200 mg once, respectively. In these studies in healthy volunteers, no adverse events were observed at these doses and the adverse effects were similar in frequency and intensity to those reported in the placebo groups. The effects of higher doses are not known.
There is no specific antidote for overdose of Harvoni. If overdose occurs, all symptoms of toxicity will be monitored in the patient. Treatment of Harvoni overdose consists of general supportive measures, with monitoring of vital signs and observation of the patient’s clinical condition. It is unlikely that hemodialysis will significantly eliminate ledipasvir since ledipasvir is highly bound to plasma proteins. Hemodialysis can effectively remove the main circulating metabolite of sofosbuvir (ie GS-331007), with a 53% extraction ratio.
What is Forms and Composition Harvoni?
90 mg / 400 mg film-coated tablet (diamond shaped, orange, size 19 mm × 10 mm, on one side with “GSI” inscription and on the other side “7985”): Bottle of 28, with child safety closure system.
NOT’s
Edrug-online contains comprehensive and detailed information about drugs available in the medical field, and is divided into four sections:
general information:
Includes a general description of the drug, its use, brand names, FAQs, and relevant news and articles
Additional information:
General explanation about dealing with the medicine: how to take the medicine, the doses and times of it, the start and duration of its effectiveness, the recommended diet during the period of taking the medicine, the method of storage and storage, recommendations in cases for forgetting the dose and instructions to stop taking the drug and take additional doses.
Special warnings:
For pregnant and breastfeeding women, the elderly, boys and drivers, and use before surgery.
Side effects:
It treats possible side effects and drug interactions that require attention and its effect on continuous use.
The information contained in this medicine is based on medical literature, but it is not a substitute for consulting a doctor.
The post Harvoni medication Uses, Dosage, Side Effects, Precautions & Warnings appeared first on Drug Online.
from Drug Online https://bit.ly/3eY6utB
via Edrug Online
from faculty of medicine
https://bit.ly/2ByYsde
via Faculty of Medicine
0 notes
Text
Harvoni medication Uses, Dosage, Side Effects, Precautions & Warnings
Drug Online
Harvoni medication >>> Generic drugof the Therapeutic class: Gastro-Entero-Hepatology
active ingredients: Ledipasvir , Sofosbuvir
Important to know about harvoni medication ?
Sofosbuvir and ledipasvir inhibit the growth of hepatitis C virus.
In chronic hepatitis C (liver inflammation).
It takes several months until your symptoms decrease, such as fatigue and abdominal complaints.
A course usually lasts 12 to 24 weeks, sometimes 8 weeks is sufficient. Complete the entire course, even if your symptoms have already disappeared.
You may take the tablet with or without food. Swallow it whole, without chewing. The tablet tastes otherwise very bitter.
Are you nauseous and do you have to vomit? If you have to vomit within 5 hours after swallowing this medicine, you must take a new tablet. Otherwise, it does not work properly.
Side effects may include headache, fatigue, skin rash and hypersensitivity.
There are many interactions with other means. Have your pharmacist check whether you can use it safely with your other medicines, even those that you have bought without a prescription.
what is harvoni used to treat and indication?
Harvoni is indicated for the treatment of chronic hepatitis C (CHC) in adults (see sections Dosage and Administration , Warnings and Precautions and Pharmacodynamic properties ).
For activity based on hepatitis C virus (HCV) genotype, see Warnings and Precautions andPharmacodynamic Properties sections .
Harvoni Dosage
Dosage
The recommended dose is one Harvoni tablet once a day, with or without food (see section Pharmacokinetic properties ).
<brarial’,’sans-serif’; color:=”” black”=””>Table 1: Recommended duration of treatment with Harvoni and recommendations for co-administration with ribavirin in certain subgroups</brarial’,’sans-serif’;>
Patient population * Treatment Duration Patients with genotype 1 or genotype 4 HCC 12 weeks.
– A duration of 8 weeks could be considered in patients infected with genotype 1 not previously treated (see section <iarial’,’sans-serif’; color:=”” black”=””>Pharmacodynamic properties, ION-3 study).</iarial’,’sans-serif’;>
Patients without cirrhosis Harvoni – A duration of 24 weeks should be considered in previously treated patients for whom the possibilities of subsequent re-treatment are uncertain (see section Warnings and precautions for use ). Patients with compensated cirrhosis Harvoni 24 weeks.
– A duration of 12 weeks could be considered in patients for whom the risk of clinical progression of the disease is considered low and for whom subsequent retreatment options exist (see section 4.4 ).
Patients with decompensated cirrhosis or in a pre / post liver transplant situation Harvoni + ribavirin 24 weeks (see warnings and precautions for use and Pharmacodynamic properties ) Patients with genotype 3 CHC Patients with cirrhosis and / or failure of a previous treatment Harvoni + ribavirin 24 weeks (see warnings and precautions for use and Pharmacodynamic properties )
* Includes patients co-infected with human immunodeficiency virus (HIV).
If used in combination with ribavirin, see also the Summary of Product Characteristics for ribavirin.
In patients without decompensated cirrhosis and requiring the addition of ribavirin in their treatment (see table 1), the daily dose of ribavirin is calculated according to the weight (<75 kg = 1000 mg and ≥ 75 kg = 1 200 mg) and is administered orally in two divided doses, with food.
In patients with decompensated cirrhosis, ribavirin should be administered at the starting dose of 600 mg divided over the day. If the initial dose is well tolerated, the dose may be increased gradually to a maximum of 1000-1200 mg per day (1000 mg for patients weighing <75 kg and 1200 mg for patients weighing ≥ 75 kg ).
If the initial dose is not well tolerated, the dose should be reduced as needed based on hemoglobin levels.
Dose modification of ribavirin in patients taking 1,000-1,200 mg per day
If Harvoni is used in combination with ribavirin and a patient experiences a serious side effect potentially related to ribavirin, the dose of ribavirin should be changed or the treatment stopped, if necessary, until the side effect disappears or its severity decreases. Table 2 gives the recommendations for dose modification and discontinuation of treatment depending on the hemoglobin concentration and the patient’s heart condition.
Table 2: Recommendations for dose modification of ribavirin co-administered with Harvoni
Biological values Reduce the ribavirin dose to 600 mg / day if: Stop ribavirin if: Hemoglobin levels in patients without heart disease <10 g / dL <8.5 g / dL Hemoglobin level in patients with a history of stable heart disease Decrease in hemoglobin ≥ 2 g / dL during a 4 week treatment period <12 g / dL despite taking a reduced dose for 4 weeks
When ribavirin has been discontinued due to a biological defect or clinical manifestation, an attempt may be made to re-initiate ribavirin at a dose of 600 mg per day and then increase further. the dose up to 800 mg per day. However, increasing ribavirin to the dose initially prescribed (1000 mg to 1200 mg per day) is not recommended.
Patients should be informed that if they vomit within 5 hours of taking their dose, they should take another tablet. If they vomit more than 5 hours after taking their dose, it is not necessary to take another dose.
Patients should be informed that if they forget to take a dose and they notice it within 18 hours of their usual dose, they should take the tablet as soon as possible and then take the next dose as scheduled . If they find out more than 18 hours later, they should wait and take the next dose as scheduled. Patients should be instructed not to take a double dose.
The elderly
No dose adjustment is necessary in elderly patients (see section Pharmacokinetic properties ).
Renal failure
No dose adjustment of Harvoni is necessary in patients with mild or moderate renal impairment. The safety of ledipasvir / sofosbuvir has not been evaluated in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL / min / 1.73 m 2 ) or d end-stage renal disease (ESRD) requiring hemodialysis.
Hepatic insufficiency
No dose adjustment of Harvoni is necessary in patients with mild, moderate or severe hepatic impairment (Child-Pugh-Turcotte score [CPT] A, B or C) (see section Pharmacokinetic properties ). The safety and efficacy of the ledipasvir / sofosbuvir combination have been established in patients with decompensated cirrhosis (see section Pharmacodynamic properties ).
Pediatric population
The safety and efficacy of Harvoni in children and adolescents under 18 years of age have not been yet been established. No data is available.
Administration mode
Oral use.
Patients should be informed that they should swallow the tablet whole, with or without food. Due to its bitter taste, it is recommended not to chew or crush the film-coated tablet .
what’s Contraindications for
harvoni?
Hypersensitivity to the active ingredients or to any of the excipients listed in the Composition section .
Co-administration with rosuvastatin or St. John’s wort ( Hypericum perforatum ) (see section Interactions with other medicinal products and other forms of interaction ).
harvoni how does it work?
Absorption
After oral administration of ledipasvir / sofosbuvir in HCV infected patients, the median peak plasma concentration of ledipasvir was reached 4.0 hours after dosing. Sofosbuvir was rapidly absorbed and the median plasma peaks were reached ~ 1 hour after dosing.
The median plasma peak of GS-331007 was reached 4 hours after
administration.
Based on population pharmacokinetic analysis in HCV-infected patients, geometric means of steady-state AUC 0-24 for ledipasvir (n = 2,113), sofosbuvir (n = 1,542) and GS-331007 (n = 2,113) were 7,290, 1,320 and 12,000 ng • h / mL, respectively. The steady-state C max for ledipasvir, sofosbuvir, and GS-331007 were 323, 618 and 707 ng / mL, respectively. The AUC 0-24 and C max of sofosbuvir and GS-331007 were similar in healthy adult volunteers and in patients infected with HCV.
Compared to healthy subjects (n = 191), AUC 0-24 and C maxof ledipasvir were 24% and 32% lower, respectively, in HCV-infected patients. The AUC of ledipasvir is dose proportional over a dose range of 3 to 100 mg.
The AUCs of sofosbuvir and GS-331007 are quasi-dose-proportional over a dose range of 200 mg to 400 mg.
Effects of food intake
Compared with fasting, administration of a single dose of ledipasvir / sofosbuvir with a moderate or high fat meal increased the 0-inf AUC of sofosbuvir by approximately 2-fold. but did not significantly change sofosbuvir C max .
Exposures to GS-331007 and ledipasvir were not affected by these two types of meals. Harvoni can be administered with or without food.
Distribution
The binding of ledipasvir to human plasma proteins is> 99.8%. After administration of a single 90 mg dose of [ 14 C] -ledipasvir in healthy subjects, the blood / plasma radioactivity ratio [ 14 C] was between 0.51 and 0.66.
The binding of sofosbuvir to human plasma proteins is approximately 61 to 65% and the binding is independent of the concentration of the product, in the range of 1 to 20 µg / mL. The binding of GS-331007 to proteins is minimal in human plasma. Following a single 400 mg dose of [ 14 C] -sofosbuvir in healthy subjects, the ratio of blood [ 14 C] to plasma radioactivity was approximately 0.7.
Biotransformation
In vitro, no detectable metabolism of ledipasvir by human CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 has been observed. Evidence of slow oxidative metabolism, the mechanism of which is not known, has been observed. After administration of a single 90 mg dose of [ 14 C] -ledipasvir, systemic exposure was almost exclusively due to the parent drug (> 98%). Unchanged ledipasvir is also the main form found in faeces.
Sofosbuvir is extensively metabolized in the liver to form the pharmacologically active nucleoside triphosphate analog GS-461203. The active metabolite is not detected. The metabolic activation pathway involves sequential hydrolysis of the carboxyl ester group, catalyzed by human cathepsin A or carboxyl esterase 1, and phosphoramidate cleavage by the HINT1 protein (histidine triad nucleotide-binding protein) followed by phosphorylation by the pyrimidine-nucleotide biosynthesis pathway. Dephosphorylation results in the formation of the nucleoside metabolite GS-331007, which cannot be re-phosphorylated effectively and which lacks anti-HCV activity in vitro. In the case of the ledipasvir / sofosbuvir combination, GS-331007 represents approximately 85% of the
Elimination
After a single 90 mg oral dose of [ 14 C] -ledipasvir, the average total recovery of radioactivity [ 14 C] in faeces and urine was 87%, with most of the radioactivity was recovered in faeces (86%). Unchanged ledipasvir excreted in the faeces averaged 70% of the administered dose and the oxidative metabolite M19 represented 2.2% of the dose. These data suggest that the primary route of elimination for unchanged ledipasvir is biliary excretion, with renal excretion being a minor route (approximately 1%). The median terminal half-life of ledipasvir in healthy volunteers following administration of ledipasvir / sofosbuvir on an empty stomach was 47 hours.
After administration of a single 400 mg oral dose of [ 14 C] -sofosbuvir the mean total dose recovery was greater than 92%, of which approximately 80%, 14% and 2.5% recovered in the urine. faeces and exhaled air, respectively. The majority of the sofosbuvir dose recovered in the urine was GS-331007 (78%) and 3.5% was sofosbuvir. These data show that renal clearance is the primary route of elimination for GS-331007 with a large proportion actively excreted. The median terminal half-lives of sofosbuvir and GS-331007 after administration of ledipasvir / sofosbuvir were 0.5 and 27 hours, respectively.
Neither ledipasvir nor sofosbuvir are substrates for active hepatic transporters, organic cation transporter (OCT) 1, organic anion transporting polypeptide (OATP) 1B1 or OATP1B3. GS-331007 is not a substrate for renal transporters, including organic anion transporter (OAT) 1 or OAT3, or OCT2.
Potential in vitro effect of ledipasvir / sofosbuvir on other medicinal products At concentrations achieved in clinical practice, ledipasvir is not an inhibitor of hepatic transporters, including OATP 1B1 or 1B3, BSEP, OCT1 , OCT2, OAT1, OAT3, the transporter MATE (mutidrug and toxic compound extrusion) 1, the multidrug resistance protein (MRP) 2 or the MRP4. Sofosbuvir and GS-331007 are not inhibitors of the drug transporters P-gp, BCRP, MRP2, BSEP, OATP1B1, OATP1B3 and OCT1, and GS-331007 is not an inhibitor of OAT1, ‘OCT2 and MATE1.
Sofosbuvir and GS-331007 are not inhibitors or inducers of CYP or uridine diphosphate glucuronosyltransferase (UGT) 1A1 enzymes.
Pharmacokinetics in special populations
Ethnicity and gender
No clinically significant pharmacokinetic differences due to ethnicity were noted for ledipasvir, sofosbuvir or GS-331007. No clinically significant pharmacokinetic differences due to gender were noted for sofosbuvir or GS-331007. The AUC and C max of ledipasvir were 77% and 58% higher, respectively, in women compared to men. However, the relationship between gender and ledipasvir exposures was not considered clinically relevant.
The elderly
Pharmacokinetic analysis of HCV-infected patient populations showed that, in the age range analyzed (18-80 years), age has no clinically significant effect on exposure to ledipasvir, sofosbuvir or GS-331007. The clinical studies of ledipasvir / sofosbuvir included 117 patients aged 65 years and over.
Renal failure
The pharmacokinetics of ledipasvir have been studied with a single 90 mg dose of ledipasvir in non-HCV infected patients with severe renal impairment (eGFR <30 mL / min by Cockcroft-Gault formula, median CrCl [limits] of 22 [17-29] mL / min). The pharmacokinetics of ledipasvir did not show any clinically significant difference between healthy subjects and patients with severe renal impairment.
The pharmacokinetics of sofosbuvir have been studied in non-HCV-infected patients with mild (eGFR ≥ 50 and <80 mL / min / 1.73m 2 ), moderate (eGFR ≥ 30 and <50 mL / min / renal impairment ). 1.73 m 2 ), severe (eGFR <30 mL / min / 1.73 m 2 ) and in patients with ESRD requiring hemodialysis, after a single 400 mg dose of sofosbuvir. Compared with patients with normal renal function (eGFR> 80 mL / min / 1.73 m 2 ), the 0-inf AUC of sofosbuvir was 61%, 107% and 171% higher in mild renal impairment. moderate and severe, while AUC 0-infof GS-331007 was 55%, 88% and 451% higher, respectively. In patients with ESRD compared to patients with normal renal function, the AUC 0-inf of sofosbuvir was 28% higher when sofosbuvir was given 1 hour before hemodialysis, compared to 60% higher when sofosbuvir was given 1 hour after hemodialysis. ASC 0-infof GS-331007 in patients with ESRD who received sofosbuvir one hour before or one hour after hemodialysis was at least 10 and 20 times higher, respectively. GS-331007 is effectively removed by hemodialysis, with an extraction coefficient of approximately 53%. Following a single 400 mg dose of sofosbuvir, a 4 hour hemodialysis removed 18% of the administered sofosbuvir dose. The safety and efficacy of sofosbuvir have not been established in patients with severe renal impairment or ESRD.
Hepatic insufficiency
The pharmacokinetics of ledipasvir have been studied with a single 90 mg dose of ledipasvir in non-HCV patients with severe hepatic impairment (CPT C score). Plasma exposure to ledipasvir (AUC inf ) was similar in patients with severe hepatic impairment and in control patients with normal hepatic function. Pharmacokinetic analysis of HCV-infected patient populations showed that cirrhosis has no clinically significant effect on exposure to ledipasvir.
The pharmacokinetics of sofosbuvir were studied after 7 days of administration of 400 mg / day of sofosbuvir in HCV-infected patients with moderate or severe hepatic impairment (CPT scores B and C). Compared to patients with normal hepatic function, the AUC 0-24 of sofosbuvir was 126% and 143% higher, respectively, in moderate or severe hepatic impairment, while the AUC 0-24 of GS-331007 was 18% and 9% respectively higher. Population pharmacokinetic analysis in HCV infected patients has shown that cirrhosis has no clinically significant effect on exposure to sofosbuvir and GS-331007.
Body weight
Body weight had no significant effect on exposure to sofosbuvir based on population pharmacokinetic analysis. Exposure to ledipasvir decreases with increased body weight, but this effect is not considered clinically significant.
Pediatric population
The pharmacokinetics of ledipasvir, sofosbuvir and GS-331007 have not been established in children.
(see section Dosage and method of administration ).
harvoni treatment side effects
In addition to the desired effect, this can cause drug side effects.
The main side effects are the following.
Sometimes (from 10 to 30 people in 100)
Headache , fatigue.
Rarely (from 1 to 10 in 100 people)
Skin rash . Contact your doctor if you notice this.
Very rare (affects less than 1 in 100 people)
Hypersensitivity to this medication. This can be expressed in ‘angioedema’: a swelling of the face, lips, mouth, tongue or throat. You can be very stuffy. If it occurs, you should immediately seek out a doctor or go to the First Aid Service. You can not use this type of medication in the future. Therefore tell the pharmacy that you are hypersensitive to this sofosbuvir with ledipasvir. The pharmacy team can then ensure that you do not get this medication again.
Consult your doctor if you suffer too much from one of the above mentioned side effects or if you experience other side effects that you are worried about.
harvoni interactions
Harvoni containing ledipasvir and sofosbuvir, all the interactions that have been observed with these active ingredients used individually can occur with Harvoni.
Potential effect of Harvoni on other drugs
Ledipasvir is an in vitro inhibitor of the P-gp drug transporter and Breast Cancer Resistance Protein (BCRP) protein and may increase the intestinal absorption of substrates from these transporters in case of co-infection. administration. In vitro data indicate that ledipasvir may be a weak inducer of metabolic enzymes such as CYP3A4, CYP2C and UGT1A1. Plasma concentrations of the compounds that are substrates of these enzymes could be reduced when co-administered with the ledipasvir / sofosbuvir combination. In vitro, ledipasvir inhibits intestinal CYP3A4 and UGT1A1. Drugs with a narrow therapeutic range and metabolized by these isoenzymes should be used with caution and under close supervision.
Potential effect of other drugs on Harvoni
Ledipasvir and sofosbuvir are substrates of the P-gp drug carrier and BCRP, whereas GS-331007 is not.
Drugs that are potent inducers of P-gp (rifampicin, rifabutin, St. John’s wort, carbamazepine, phenobarbital and phenytoin) can significantly decrease plasma concentrations of ledipasvir and sofosbuvir, reducing the therapeutic effect the ledipasvir / sofosbuvir association and therefore they are against-marked Harvoni (see Contraindications ). Drugs that are moderate inducers of P-gp in the gut (such as oxcarbazepine) may decrease plasma concentrations of ledispavir and sofosbuvir, reducing the therapeutic effect of Harvoni. Co-administration of this type of medication with Harvoni is not recommended (see section Warnings and Precautions section).). Co-administration of drugs that inhibit P-gp and / or BCRP may increase the plasma concentrations of ledipasvir and sofosbuvir without increasing that of GS-331007; Harvoni may be co-administered with inhibitors of P-gp and / or BCRP. No clinically significant effect on the ledipasvir / sofosbuvir combination is expected via CYP450 or UGT1A1 enzymes.
Patients treated with vitamin K antagonists
Because hepatic function may change during treatment with Harvoni, close monitoring of International Normalized Index (INR) values is recommended.
Interactions between Harvoni and other drugs
Table 3 provides a list of clinically established or potentially clinically significant drug interactions (where the 90% confidence interval [IC] of the mean geometric least squares ratio [GLSM] was within the “↔” limits, in excess of “↑ “, Or lower than” ↓ “predetermined equivalence limits). The drug interactions described are based on studies conducted either with the combination of ledipasvir / sofosbuvir or with ledipasvir and sofosbuvir taken individually, or are predictions of drug interactions that may occur with the combination of ledipasvir / sofosbuvir. This table is not exhaustive.
Harvoni Warnings and Precautions :
Harvoni should not be administered at the same time as other medicines containing sofosbuvir.
Activity according to genotype
For recommended treatments for different genotypes of HCV, see Dosage and Method of Administration . For virologic and clinical activity by genotype, see the topic Pharmacodynamic properties .
Clinical evidence supporting the use of Harvoni in patients with HCV genotype 3 is limited . The relative efficacy of 12-week treatment with ledipasvir / sofosbuvir + ribavirin compared to a 24-week course with sofosbuvir + ribavirin, has not been studied. A conservative treatment of 24 weeks is recommended in all previously treated genotype 3 patients and in patients who are naive to any treatment with cirrhosis (see Dosage and method of administration section ).
Clinical evidence supporting the use of Harvoni in patients with HCV genotype 2 and 6 is limited.
Severe Bradycardia and Conduction Disorders
Cases of severe bradycardia and conduction disturbances have been observed with Harvoni when co-administered with amiodarone with or without other heart rate-lowering drugs. The mechanism is not established.
The concomitant use of amiodarone has been limited in the clinical development of sofosbuvir in combination with direct-acting antivirals (DAAs). Some cases have been life-threatening. Therefore, amiodarone should be used in patients treated with Harvoni only in case of intolerance or contraindication to other anti-arrhythmic treatments.
If concomitant use of amiodarone is considered necessary, close monitoring of patients is recommended when initiating Harvoni treatment. Patients identified as being at high risk for bradyarrhythmia should be monitored continuously for 48 hours in an adapted hospital environment.
Given the long half-life of amiodarone, appropriate monitoring should also be performed in patients who have discontinued amiodarone in recent months and need to start treatment with Harvoni.
All patients treated with Harvoni who receive amiodarone with or without other bradycardic medications should also be advised of symptoms of bradycardia and conduction disturbances, and should be informed of the need for urgent medical attention. feel these symptoms.
Treatment of patients previously treated with direct-acting antivirals for HCV In patients who have failed treatment with ledipasvir / sofosbuvir, a selection of resistance mutations in NS5A significantly reduces susceptibility to ledipasvir.
majority of cases (see section 5.1).). Limited data indicates that this type of mutation in NS5A is not reversible at long-term follow-up. In patients who have failed prior therapy with ledipasvir / sofosbuvir, there is currently no evidence to support the efficacy of re-treatment with NS5A inhibitor therapy. Similarly, in patients who have failed previous treatment with an NS3 / 4A protease inhibitor, there are currently no data supporting the efficacy of NS3 / 4A protease inhibitors. In these patients, the treatment of HCV infection may therefore depend on the use of other classes of drugs. Therefore, it will be necessary to
Renal failure
No dose adjustment of Harvoni is required in patients with mild or moderate renal impairment. The safety of Harvoni has not been evaluated in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL / min / 1.73 m 2 ) or end-stage renal disease (IRT) requiring hemodialysis. If Harvoni is used in combination with ribavirin, also refer to the Summary of Product Characteristics of Ribavirin for patients with creatinine clearance (CrCl) <50 mL / min (see section 5.2 ).
Patients with decompensated cirrhosis and / or waiting for liver transplantation or post-liver transplantation
The efficacy of ledipasvir / sofosbuvir in patients with genotype 5 or genotype 6 HCV with decompensated cirrhosis and / or awaiting liver transplantation or post-liver transplantation has not been studied. The Harvoni treatment decision should be based on an assessment of the potential benefits and risks for each patient.
Use with moderate inducers of P-gp
Drugs that are moderate inducers of P-gp in the intestine (such as oxcarbazepine) may decrease the plasma concentrations of ledipasvir and sofosbuvir, reducing the therapeutic effect of Harvoni. Co-administration of this type of medication with Harvoni is not recommended (see section Interactions with other medicinal products and other forms of interaction ).
Use with some antiretrovirals for HIV
Harvoni has been shown to increase the exposure to tenofovir, particularly when used in combination with an HIV treatment containing tenofovir disoproxil fumarate and a pharmacokinetic booster (ritonavir or cobicistat). The safety of tenofovir disoproxil fumarate in the context of Harvoni treatment in the presence of a pharmacokinetic booster has not been established. The potential risks and benefits associated with co-administration of Harvoni with the fixed-dose combination tablet containing elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or tenofovir disoproxil fumarate used in combination with a protease inhibitor Boosted HIV (eg atazanavir or darunavir) should be considered, especially in patients at increased risk of renal dysfunction. In patients receiving concomitant Harvoni with elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function. elventegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function. elventegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor, undesirable effects associated with tenofovir should be monitored. See the Summary of Product Characteristics for tenofovir disoproxil fumarate, emtricitabine / tenofovir disoproxil fumarate, or elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil fumarate for recommendations for monitoring renal function.
Use with HMG-CoA reductase inhibitors
Co-administration of Harvoni with HMG-CoA reductase inhibitors (statins) can significantly increase the statin concentration, increasing the risk of myopathy and rhabdomyolysis (see section 4.5). forms of interactions ).
HCV / HBV co-infection (hepatitis B virus)
Cases of hepatitis B virus (HBV) reactivation, some with fatal outcome, have been reported during or after treatment with direct-acting antiviral agents. HBV testing should be performed in all patients prior to initiation of treatment. Patients co-infected with HBV / HCV are at risk for reactivation of HBV and should therefore be monitored and managed according to clinical guidelines.
Pediatric population
Harvoni is not recommended for use in children and adolescents under 18 years of age as the safety and efficacy of this medication have not been established in this population.
excipients
Harvoni contains an azo dye, S Yellow Orange Lake (E110), which can cause allergic reactions. It also contains lactose. As a result, patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose / galactose malabsorption should not take this medicine.
Drive and use machines
Harvoni (alone or in combination with ribavirin) has no or negligible effect on the ability to drive and use machines.
However, patients should be informed that fatigue was more common in patients treated with ledipasvir / sofosbuvir than in those receiving placebo.
Harvoni and PREGNANCY / BREAST FEEDING / FERTILITY
Women of childbearing
potential / contraception in men and women When Harvoni is used in combination with ribavirin, all precautions should be taken to prevent pregnancy in patients and female partners of patients. Significant teratogenic and / or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. Women of childbearing potential or their male partners should use an effective method of contraception during treatment and for some time after the end of treatment, as recommended in the Summary of Product Characteristics of Ribavirin.
See the Summary of Product Characteristics of Ribavirin for more information.
Pregnancy
There are no data or limited data (less than 300 pregnancies) on the use of ledipasvir, sofosbuvir or Harvoni in pregnant women.Studies in animals have not shown any direct deleterious effects on reproduction. No significant effects on fetal development were observed in rats and rabbits with ledipasvir or sofosbuvir. However, it was not possible to completely evaluate margins of exposure with sofosbuvir in rats in relation to human exposure at the recommended clinical dose.
As a precaution, it is best to avoid the use of Harvoni during pregnancy.
feeding
It is not known whether ledipasvir or sofosbuvir and their metabolites are excreted in breast milk.The pharmacokinetic data available in animals have demonstrated the excretion of ledipasvir and metabolites of sofosbuvir in milk
A risk for newborns / infants can not be ruled out. Therefore, Harvoni should not be used while breastfeeding.
Fertility
There is no data on the effect of Harvoni on human fertility. Studies in animals have not shown deleterious effects of ledipasvir or sofosbuvir on fertility.
If ribavirin is co-administered with Harvoni, contraindications for the use of ribavirin during pregnancy and lactation apply (see also Summary of Product Characteristics of Ribavirin).
What should I do if I miss a dose?
It is important to take this medicine consistently. If you have forgotten a dose:
You use this medicine once a day:
Does it take more than 6 hours before you should take the next dose? Swallow the forgotten tablet as soon as
possible and take the next dose at the usual time.
Does it take less than 6 hours before you should take the next dose? Skip the forgotten tablet and take the next dose at the usual time.
What happens if I overdose from Harvoni ?
The highest documented doses of ledipasvir and sofosbuvir were 120 mg twice daily for 10 days and 1200 mg once, respectively. In these studies in healthy volunteers, no adverse events were observed at these doses and the adverse effects were similar in frequency and intensity to those reported in the placebo groups. The effects of higher doses are not known.
There is no specific antidote for overdose of Harvoni. If overdose occurs, all symptoms of toxicity will be monitored in the patient. Treatment of Harvoni overdose consists of general supportive measures, with monitoring of vital signs and observation of the patient’s clinical condition. It is unlikely that hemodialysis will significantly eliminate ledipasvir since ledipasvir is highly bound to plasma proteins. Hemodialysis can effectively remove the main circulating metabolite of sofosbuvir (ie GS-331007), with a 53% extraction ratio.
What is Forms and Composition Harvoni?
90 mg / 400 mg film-coated tablet (diamond shaped, orange, size 19 mm × 10 mm, on one side with “GSI” inscription and on the other side “7985”): Bottle of 28, with child safety closure system.
NOT’s
Edrug-online contains comprehensive and detailed information about drugs available in the medical field, and is divided into four sections:
general information:
Includes a general description of the drug, its use, brand names, FAQs, and relevant news and articles
Additional information:
General explanation about dealing with the medicine: how to take the medicine, the doses and times of it, the start and duration of its effectiveness, the recommended diet during the period of taking the medicine, the method of storage and storage, recommendations in cases for forgetting the dose and instructions to stop taking the drug and take additional doses.
Special warnings:
For pregnant and breastfeeding women, the elderly, boys and drivers, and use before surgery.
Side effects:
It treats possible side effects and drug interactions that require attention and its effect on continuous use.
The information contained in this medicine is based on medical literature, but it is not a substitute for consulting a doctor.
The post Harvoni medication Uses, Dosage, Side Effects, Precautions & Warnings appeared first on Drug Online.
from Drug Online https://bit.ly/3eY6utB
via Edrug Online
0 notes
Text
HCC Risk Calculator
The HCC Risk Calculator is to estimate the 3-year risk of HCC in patients with hepatitis C virus (HCV) infection who have undergone antiviral treatment or in patients with cirrhosis caused by alcohol-related liver disease (ALD) or nonalcoholic fatty liver disease (NAFLD). To determine HCC Risk Check Our Medical Calculator https://www.pediatriconcall.com/calculators/hcc-risk-calc
0 notes