#animals injected with deadly mRNA
Text
🧬💉⚰️
#Covid#premature death#inoculation#animals injected with deadly mRNA#food#health#dna#crimes against humanity#these people are evil#health care workers#warning#danger#food hazards#crippling the natural immune system#diseases#speaktruth#corruption#fight for justice#standup#speak up#truth#please share#wwg1wga
349 notes
·
View notes
Text
mRNA Vaccines for Livestock? - Questions For Corbett #097 - The Corbett Report
家畜用のmRNAワクチンは開発されているのでしょうか?もちろんです。では、これは何を意味するのでしょうか?いつものように、それは誰に聞くかによるのです。今週の「Questions For Corbett」で、第3世代ワクチンの悪いところ、悪いところ、腐敗したところ、そして食の未来について調べてみましょう。
SHOW NOTES
Bill Gates Vows To Pump mRNA Into Food Supply To ‘Force-Jab’ the Unvaccinated
Original video: Bill Gates and Penny Mordaunt launch the Global Academy of Agriculture and Food Security
Instagram post: someone's friend's neighbour's cows died from mRNA vaccines
Report: mRNA vaccines may be injected into livestock
Healthfeedback funded by Meta/TikTok/Google
Healthfeedback.org: Misrepresented 2018 clip of Bill Gates trigger inaccurate claims that mRNA COVID-19 vaccines for livestock could transfer to people through diet
Gates/Omidyar/US State Department-funding of International Fact-Checking Network
USA Today Fact check is an IFCN partner
USA Today Fact check: False claim about mandatory mRNA vaccines, deaths in Australian cattle
About AFP Fact Check
AFP Fact Check: Australian farmers not 'forced to inject livestock with deadly mRNA vaccines'
AFP Fact Check: mRNA vaccine cannot transfer through meat consumption
NSW fast-tracks mRNA FMD and Lumpy Skin Disease vaccines
Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines
NOVEL MRNA VACCINE TECHNOLOGY FOR PREVENTION OF BOVINE RESPIRATORY SYNCYTIAL VIRUS
The Future of Livestock Vaccines
Big Pharma pushes to get farm animals off antibiotics and on vaccines
Bayer Partners with BioNTech to Develop mRNA Vaccines, Drugs for Animal Health
mRNA Vaccines in Livestock and Companion Animals are here now.
SEQUIVITY
DNA vaccines in veterinary use
Veterinary biologics licensed in Canada
Paul Offitt: Can mRNA vaccines alter a person's DNA?
The Future of Vaccines
mRNA Vaccines: Disruptive Innovation in Vaccination
ビル・ゲイツ、ワクチン未接種者を「強制ジャブ」するためにmRNAを食品に注入することを誓う
オリジナル動画です。ビル・ゲイツとペニー・モーダントが「農業と食料安全保障のグローバル・アカデミー」を設立
Instagramの投稿:誰かの友達の近所の牛がmRNAワクチンで死亡した。
報告:mRNAワクチンを家畜に注射する可能性
ヘルスフィードバックはMeta/TikTok/Googleから出資を受けています。
Healthfeedback.org:家畜用mRNA COVID-19ワクチンが食事を通じて人に移行する可能性があるという不正確な主張を誘発するビル・ゲイツの2018年のクリップを誤報した。
ゲイツ/オミダイア/米国務省、国際ファクトチェック・ネットワークに資金提供
USA TodayのファクトチェックはIFCNのパートナーです。
USA Today ファクトチェック。mRNAワクチン義務化に関する虚偽の主張、オーストラリア牛の死亡例
AFPファクトチェックについて
AFPファクトチェック。オーストラリアの農家は「致命的なmRNAワクチンを家畜に注射することを強制された」わけではない
AFPファクトチェック:mRNAワクチンは肉食で移行できない
NSW州、FMDおよびLumpy Skin DiseaseのmRNAワクチンを早期開発
獣医学部における新規ワクチン技術。ヒト医療用ワクチンへのヘラルド
牛呼吸器シンシチアルウイルス予防のための新規MRNAワクチン技術
家畜用ワクチンの未来
大手製薬会社は、家畜に抗生物質を与えず、ワクチンを投与するよう働きかけています。
バイエル、バイオエヌテック社と提携し、動物用mRNAワクチンおよび薬剤の開発を開始
家畜とコンパニオンアニマルにおけるmRNAワクチン「公式」はコチラから
シークイビティ
動物用DNAワクチン
カナダで認可された動物用生物製剤
Paul Offitt: mRNAワクチンは人のDNAを変えることができるのか?
ワクチンの未来
mRNA ワクチン。ワクチン接種における破壊的イノベーション
3 notes
·
View notes
Text
NIH and CDC Directors Lie To Congress again.
♦ National Institutes of Health (NIH) Director Lawrence A. Tabak officially took office on December 20, 2021. Previously Tabak served as the Principal Deputy Director and the Deputy Ethics Counselor of NIH since August 2010. Today he was asked about the origin of SARS-CoV-2, commonly known as Covid-19. Mr. Tabak claims he has no idea where the virus came from.
– Non pretending version: Tabak knows exactly where it came from and is protecting Anthony Fauci.
♦ Rochelle P. Walensky, is the Director of the Centers for Disease Control and Prevention (CDC) and the Administrator of the Agency for Toxic Substances and Disease Registry. Today Ms. Walensky was asked if her prior claims of vaccinated people not being able to spread or transmit the COVID-19 virus were accurate. Walensky clams the statements were accurate at the time they were made (March ’21).
– Non pretending version: the entire predicate of the govt vaccination mandate rests on the vaccine stopping the spread of COVID-19. The vaccine stopping transmission was a lie when Walensky said it in March ’21, and it is a lie she must maintain in 2023 because the OSHA mandate could not have existed without it.
Top 7 reasons mRNA COVID JABS are much more DEADLY than any other “vaccine” ever created
Never before have so many humans suffered from post-traumatic vaccine disorder. PTVD is sweeping the globe as the spike protein prions clog and clot the vascular system of those “vaccinated” with the gene mutation injections. In fact, mRNA “technology” is so deadly that the FDA never got approval to use it for humans, and even the “emergency use authorization” was not valid because there were several other alternative treatments for Covid that proved effective, including hydroxychloroquine, ivermectin, vitamin D and zinc.
Even though many vaccines contain known neurotoxins and carcinogens, like mercury, aluminum, peanut oil, dairy, GMOs, latex, MSG, and human albumin, they still are not as dangerous and deadly as mRNA Fauci Flu jabs. In fact, the Wuhan Virus injections have killed and maimed more humans that ALL other vaccines.
#1. mRNA jabs were never even approved by the FDA (even the “emergency use authorization” was faked because there were other alternatives to fighting Covid available).
#2. They clog your vascular system with spike proteins, causing mass inflammation (MIS).
#3. There is no guarantee of when mRNA stops producing toxic spike prions.
#4. They trick your cells and alter their natural function.
#5. Each jab and booster causes worsened auto-immune dysfunction and less immunity against future infections.
#6. mRNA is an experiment and has never been proven safe or effective.
#7. The jabs kill animals tested and human babies in the womb, and cause infertility.
vaccines.news/2023-04-18-7-reasons-mrna-covid-jabs-more-deadly.html
Captain Convey JAB Note
One of the worst things to do for your health is get the covid-19 "vaccine" and booster jabs.
These JABS are deadly ticking mRNA gene therapy ticking time bombs.
The biden regime/big pharma/big business etc are making big bucks from this FAKE KILLER JAB!
The best source to know the truth about vaccines and the covid-19 FAKE "vaccine" is vaccines.news
0 notes
Text
Enzalutamide enhances kidney damage induced simply by Iodixanol within person suffering from diabetes test subjects by simply deactivating the particular mTOR/p70S6K signaling process
Specifically, RelE exhibits a desire for the cease codons UAG along with UGA and feeling codons CAG and also UCG throughout vitro. In this examine #Link# , all of us employed an extensive paint primer extension way of #Link# chart the frequency and also codon specificity associated with RelE bosom activity in vivo. We all discovered extensive cleavage at the beginning of the code location of five records, ompA, lpp, ompF, rpsA, as well as tufA. We then planned RelE cleavage web sites across 1 quick records (lpp) and a couple long records (ompF and ompA). RelE minimize most of these transcripts regularly as well as successfully within the very first comparable to A hundred codons, only occasionally reduce outside of this time, and barely reduce with internet sites in vicinity for the 3' stop. Amid 196 RelE web sites in these #Link# five records, there wasn't any choice with regard to CAG or perhaps UCG perception codons. In fact, bioinformatic research RelE cleavage websites didn't recognize virtually any series personal preferences. These kind of benefits advise a model of RelE purpose dissimilar to individuals proposed earlier, because RelE aimed repeated codon-independent mRNA cleavage coincident using the beginning associated with translation elongation.Cardiac arrest patients may experience short-term brain hypoperfusion bringing about late death associated with hippocampal CA1 neurons along with mental incapacity. Many of us avoided this specific throughout grownup subjects through conquering the actual term associated with transient receptor prospective melastatin Several (TRPM7), a short-term receptor potential route that is certainly essential for embryonic development, is critical regarding mobile or portable tactical and trace ion homeostasis within vitro, as well as whose world-wide deletion throughout these animals is deadly. TRPM7 has been suppressed throughout CA1 neurons by simply intrahippocampal injection therapy associated with well-liked vectors bearing shRNA certain regarding TRPM7. This did not have any not well influence on animal survival, neuronal and dendritic morphology, neuronal excitability, or perhaps synaptic plasticity, because exemplified simply by robust long-term potentiation (LTP). Even so, TRPM7 reductions made neurons proof against ischemic demise right after human brain ischemia and conserved neuronal morphology overall performance. Additionally, the idea prevented ischemia-induced loss throughout LTP along with maintained efficiency within fear-associated and also spatial-navigational recollection jobs. Hence, local suppression of TRPM7 is possible, effectively tolerated and also suppresses late neuronal loss of life inside vivo.This specific cardstock offers real-time MRI-based charge of a ferromagnetic microcapsule for endovascular routing. The concept had been examined with regard to upcoming growth and development of microdevices built to conduct noninvasive treatments in remote control websites obtainable with the human being cardiovascular system. A system application architecture is shown illustrating different application segments to permit 3-D routing of a microdevice within arteries, particularly: (my partner and i) vessel course coordinator, (2) magnet incline guiding, (three) monitoring and also (4) closed-loop routing handle. First, the position identification from the microrobot into the circulatory is actually taken out making use of Frangi vesselness filter through the pre-operation images (3-D MRI photo). And then, some minimum trajectories is actually predetermined, making use of path-planning algorithms, to guide the actual microrobot through the shot point to the actual growth place over the anarchic vessel circle.
0 notes
Photo

How Science Beat the Virus DEC. 14,2020
And what it lost in the process
“As of this writing, the biomedical library PubMed lists more than 74,000 COVID-related scientific papers—more than twice as many as there are about polio, measles, cholera, dengue, or other diseases that have plagued humanity for centuries.
Michael D. L. Johnson at the University of Arizona normally studies copper’s toxic effects on bacteria. But when he learned that SARS‑CoV‑2 persists for less time on copper surfaces than on other materials, he partially pivoted to see how the virus might be vulnerable to the metal. No other disease has been scrutinized so intensely, by so much combined intellect, in so brief a time.
Scientists who’d already been studying other emerging diseases were even quicker off the mark. Lauren Gardner, an engineering professor at Johns Hopkins University who has studied dengue and Zika, knew that new epidemics are accompanied by a dearth of real-time data. So she and one of her students created an online global dashboard to map and tally all publicly reported COVID‑19 cases and deaths. After one night of work, they released it, on January 22. The dashboard has since been accessed daily by governments, public-health agencies, news organizations, and anxious citizens.
Studying deadly viruses is challenging at the best of times, and was especially so this past year. To handle SARS‑CoV‑2, scientists must work in “biosafety level 3” labs, fitted with special airflow systems and other extreme measures; although the actual number is not known, an estimated 200 such facilities exist in the U.S. Researchers often test new drugs and vaccines on monkeys before proceeding to human trials, but the U.S. is facing a monkey shortage after China stopped exporting the animals, possibly because it needed them for research.
Most vaccines comprise dead, weakened, or fragmented pathogens, and must be made from scratch whenever a new threat emerges. But over the past decade, the U.S. and other countries have moved away from this slow “one bug, one drug” approach. Instead, they’ve invested in so-called platform technologies, in which a standard chassis can be easily customized with different payloads that target new viruses. For example, the Pfizer/BioNTech and Moderna vaccines both consist of nanoparticles that contain pieces of SARS‑CoV‑2’s genetic material—its mRNA. When volunteers are injected with these particles, their cells use the mRNA to reconstruct a noninfectious fragment of the virus, allowing their immune system to prepare antibodies that neutralize it. No company has ever brought an mRNA vaccine to market before, but because the basic platform had already been refined, researchers could quickly repurpose it with SARS‑CoV‑2’s mRNA. Moderna got its vaccine into Phase 1 clinical trials on March 16, just 66 days after the new virus’s genome was first uploaded—far faster than any pre-COVID vaccine.
Respiratory viruses, though extremely common, are often neglected. Respiratory syncytial virus, parainfluenza viruses, rhinoviruses, adenoviruses, bocaviruses, a quartet of other human coronaviruses—they mostly cause mild coldlike illnesses, but those can be severe. How often? Why? It’s hard to say, because, influenza aside, such viruses attract little funding or interest. “There’s a perception that they’re just colds and there’s nothing much to learn,” says Emily Martin of the University of Michigan, who has long struggled to get funding to study them. Such reasoning is shortsighted folly. Respiratory viruses are the pathogens most likely to cause pandemics, and those outbreaks could potentially be far worse than COVID‑19’s.
The incentives to trespass are substantial. Academia is a pyramid scheme: Each biomedical professor trains an average of six doctoral students across her career, but only 16 percent of the students get tenure-track positions. Competition is ferocious, and success hinges on getting published—a feat made easier by dramatic results.
In March, when the U.S. started shutting down, one of the biggest questions on the mind of Whitney Robinson of UNC at Chapel Hill was: Are our kids going to be out of school for two years? While biomedical scientists tend to focus on sickness and recovery, social epidemiologists like her “think about critical periods that can affect the trajectory of your life,” she told me. Disrupting a child’s schooling at the wrong time can affect their entire career, so scientists should have prioritized research to figure out whether and how schools could reopen safely. But most studies on the spread of COVID‑19 in schools were neither large in scope nor well-designed enough to be conclusive. No federal agency funded a large, nationwide study, even though the federal government had months to do so. The NIH received billions for COVID‑19 research, but the National Institute of Child Health and Human Development—one of its 27 constituent institutes and centers—got nothing. “
* The print version of this article stated that the Moderna and Pfizer/BioNTech vaccines were reported to be 95 percent effective at preventing COVID-19 infections. In fact, the vaccines prevent disease, not infection.
READ MORE https://www.theatlantic.com/magazine/archive/2021/01/science-covid-19-manhattan-project/617262/?utm_source=pocket-newtab
0 notes
Text
Search speeds up for vaccine against the new coronavirus
A mystery illness emerged in China late last December. As word of its spread got out, researchers at Inovio Pharmaceuticals paid close attention. This was even before anyone knew what was making people sick — a new coronavirus.
Inovio, based in San Diego, Calif., is no stranger to such viruses. A different novel coronavirus had emerged in 2012. It, too, caused potentially deadly infections. This disease would come to be known as MERS. (That’s short for Middle East respiratory syndrome.) Inovio became one of the first companies to develop a vaccine against MERS. (That vaccine is still experimental.)
Explainer: What is a coronavirus?
Early in the latest outbreak, Chinese researchers posted details of the genetic makeup of the virus that was making so many people sick. The disease, which causes fevers, pneumonia and other serious symptoms, is now called COVID-19. The virus responsible has just been named SARS-CoV-2. Based on Inovio’s work on the MERS vaccine, its scientists sprang into action. They thought they might be able to roll out a version of the MERS drug to tackle COVID-19.
Kate Broderick is Inovio’s senior vice president for research and development. “We’d all hoped that there would be enough overlap that our previously developed MERS vaccine would be helpful in this case,” she recalls. Like MERS and another severe coronavirus — one that causes the disease SARS — the new virus uses RNA as its genetic material.
But in the end, Inovio’s researchers found, SARS-CoV-2 was too different for its vaccine against MERS to take down this virus. So the scientists set to work on creating a new vaccine.
Its design relies on a relatively new approach. Vaccines usually are made from weakened or killed forms of viruses or parts of viruses. Those viral parts may include some proteins that serve as building blocks of the germ. When injected into somebody new, their immune system recognizes these viral bits as an invader. It now makes antibodies. These should help to fight off future invasions by the whole, live virus. But to make enough vaccine for millions of people, it can take months or even years to grow enough disabled virus or to purify enough viral proteins.
So for their SARS-CoV-2 vaccine, Inovio scientists took a different approach. They converted the virus’s RNA into DNA. They also selected pieces of the virus that computer models suggested would prod the immune system into making antibodies. Then they inserted selected bits of the DNA into bacteria. Those bacteria now used instructions in the bits of DNA to make large quantities of the viral protein. And it’s those proteins that will be used in the vaccine.
Explainer: What is a vaccine?
This approach drastically shortens how long it takes to make a vaccine, Broderick says. Normally, it might take two to three years. For Inovio’s product, it took three hours to design. Then it took roughly a month to make, Broderick reports.
The company started testing the vaccine in animals at the beginning of this month. It hopes to begin safety tests in people by early summer.
Even so, Inovio’s vaccine is still at least a year away from widespread use. As the number of cases of COVID-19 continues to rise, several other research teams are also racing to develop vaccines and treatments. They, too, are using unusual ways to fight the new virus.
Novel vaccines for a novel coronavirus
Messenger RNAs are copies of protein-making instructions found in the DNA of genes. Cells “read” these instructions to build proteins. Researchers are now developing a messenger RNA — or mRNA — vaccine. Its goal would be to stimulate the body to produce vaccine components.
Part of the research team behind this project works for Moderna. It’s a Cambridge, Mass.–based biotechnology company. The other scientists work for the National Institute of Allergy and Infectious Diseases, or NIAID. It’s one of the National Institutes of Health.
Kizzmekia Corbett is an immunologist at NIAID’s Vaccine Research Center. It’s in Bethesda, Md. She’s also the scientific leader on the center’s effort to develop a COVID-19 vaccine. Scientists on this project have selected portions of the SARS-CoV-2 virus that may spark a vigorous immune reaction. This mRNA vaccine, she explains, would then tell human cells which viral proteins to make.
“We’re literally giving the cells a genetic code,” Corbett says. It’s being delivered as RNA. And it will tell cells, ‘Hey, make this protein.’”
Explainer: What are proteins?
Those proteins — Corbett wouldn’t say which ones — will then prod the immune system to make antibodies against the virus. Here, the body does all of the protein-making work. That means researchers can skip the time-consuming and costly step of making those proteins in some lab.
This approach could be used in making vaccines against future new coronaviruses or other new infectious diseases, Corbett says. “What we feel we have developed,” she adds, “is [a new way] to quickly deploy a vaccine if another novel coronavirus should pop up.”
Other mRNA vaccines for other infections are still undergoing tests.
On February 24, Moderna announced that the new mRNA vaccine is ready for testing in people. It will be tested first in 45 adults to see if it is safe. If it passes that test, researchers will do more testing to see if it also protects against the virus.
But even if the vaccine works, there is another problem. The researchers don’t yet have a company willing or able to produce the huge amounts of mRNA doses. And that would be needed to make enough vaccine to treat a large share of the public.
The long road to a new vaccine
Inovio’s work on its MERS vaccine shows just how long it can take to make sure a vaccine is safe and works as it should. The company ran safety tests of its MERS vaccine from February 2016 to May 2017. They recruited 75 healthy adults to take part in this phase I clinical trial. And there were no serious side effects, the researchers reported last September 1 in Lancet Infectious Diseases.
Explainer: What is a clinical trial?
In August 2018, the vaccine moved into a Phase II trial. This tests a drug’s safety in a far more people. It also tests whether the vaccine spurs the immune system to make protective antibodies, as it was designed to do. That trial is expected to wrap up later this year.
Even if everything goes well, the MERS vaccine must still pass yet another clinical trial. This Phase III type investigates the drug’s safety and how well it works. Data from this type of trial must be submitted to the U.S. Food and Drug Administration, or FDA, before a company can release a new vaccine. All new vaccines and prescription drugs must pass such tests.
Inovio and the NIAID-Moderna team have both received funding from an organization in Oslo, Norway. It’s called CEPI. That’s short for Coalition for Epidemic Preparedness Innovations. CEPI also is funding work on yet another type of novel vaccine. CEPI and researchers at the University of Queensland in Brisbane, Australia, have found a way to keep a coronavirus from infecting cells. They refer to it as a molecular clamp.
The Queensland group had already been working with CEPI on such a “clamp” against other viruses for about a year, says Trent Munro. He’s a biotechnologist on the project. A molecular clamp, he explains is a protein stitched onto a second protein. In this case, it’s stitched onto the spike protein of a coronavirus.
With SARS and MERS, spike proteins work a bit like shape-shifting lock picks. They can change shape to interact with a protein on the surface of human cells. This allows them to enter those cells. On February 19, researchers described the 3-D structure of the spike protein of the new coronavirus in Science. This confirmed that new virus’s spike protein is also a shape-shifter. What’s more, it clings to its target on human cells 10 to 20 times as tightly as the SARS spike protein does to the same target. Such a tight grip may help the COVID-19 virus spread more easily from person to person, researchers say.
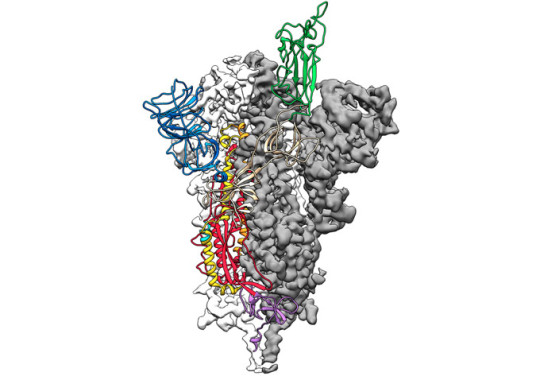
Scientists have determined the 3-D structure of the spike protein (illustrated here) in the virus that causes COVID-19. That protein helps the virus enter cells. The new work reveals that this protein binds more tightly to proteins on the surface of human cells than does the version of this protein in the virus that causes SARS. Tighter binding may explain why the new virus appears more infectious. Jason McLellan/Univ. of Texas at Austin
The molecular clamp that the Queensland team devised keeps the spike protein from shape-shifting. It locks it into a shape that triggers antibody production. This makes it a potent vaccine, Munro says.
When the Queensland group began working with CEPI to develop a molecular clamp vaccine, “we thought it would take three years as a test case,” Munro says. But the emergence of the new coronavirus forced the researchers to speed up their work.
The team is using mammal cells to make this vaccine. And a specialized machine figures out which cells are making the clamped protein. With this machine, Munro says, in just days researchers can “do things that would have taken weeks before.” Lab testing of the COVID-19 vaccine has already started. Testing in animals may begin soon. A safety test of it in people might be just months away. Still, Munro notes, it will be much longer than that — at least a year — before the vaccine is ready to roll out to the public.
“I know the timeline feels long,” Munro says. “I imagine it feels just unacceptable to those folks who are in areas of serious outbreak.” But he wants the public to know that they’re “pushing things forward as fast as possible.”
CEPI has plans to work with other groups on new vaccines. On January 31, it said it was partnering with a company in Tübingen, Germany. It’s for another mRNA vaccine to protect against the COVID-19 virus.
Beating vaccines to the punch
People who get over infections will retain antibodies to the germ that made them sick. It may stick around in their blood for years — even decades. Those antibodies can give them some protection when that person later encounters a similar germ. But those antibodies also might protect others.
How? Give people a shot of somebody else’s protective antibodies. This might prevent infections in healthy people. It might even treat infections in people who are already sick. And these injections could work faster than vaccines.
Vaccines can take weeks or months to prod the immune system into making enough antibodies to stave off an infection, says Christos Kyratsous. He works at at Regeneron Pharmaceuticals, based in Tarrytown, N.Y. There he is the vice president of infectious-disease research and technologies for fighting viruses. (Regeneron is a major financial supporter of Society for Science & the Public, which publishes Science News for Students.)
Ebola vaccines take at least a week to stimulate antibody production. In contrast, shots of “antibodies offer immediate protection,” Kyratsous says.
In studies by other research teams, blood serum taken from people who had recovered from Ebola helped infected people recover from the disease. And it did so because that serum was loaded with protective antibodies. Doctors and scientists in China have already begun using blood plasma from people who have recovered from COVID-19 to treat people who are sick with the disease.
One problem: Giving people antibodies from survivors doesn’t always work. Regeneron and other companies have developed antibodies that can offer more reliable protection. The company is already testing antibodies against Ebola and the MERS virus. Human trials and laboratory work with the company’s MERS antibodies suggest that these treatments can help protect against infection. They also can treat infections in people who are already sick, Kyratsous says.
Regeneron is now developing antibodies against the COVID-19 virus. “We have learned a lot of things from the MERS project that we can now apply to the novel coronavirus,” Kyratsous says.

A researcher at Regeneron’s infectious-disease labs in Tarrytown, N.Y., is working to develop antibodies to combat the new coronavirus. Rani Levy/Regeneron
For instance, the team has learned more about which parts of the virus make the best antibody targets. The proteins on the surface of a virus that are needed to infect cells — such as the spike protein — generally are best, he says.
Regeneron researchers have made SARS-CoV-2 proteins in the lab. They have injected them into mice that have human versions of antibody-producing genes. These “humanized” mice make human antibodies, Kyratsous says. Such mice could provide a ready supply of antibodies to treat people.
As soon as those antibodies are available, the company hopes to run lab tests on how well they work against the virus. If they work well, safety tests in animals and people might be able to start soon.
The team also hopes to get some blood from people who recovered from COVID-19 and retrieve some of their antibody-producing cells. But, Kyratsous cautions, mining antibodies from people’s blood won’t easily yield enough to treat masses of people.
As these programs all show, getting a treatment for a new virus is not something that can be done overnight. It can take months or years.
So in the midst of a new outbreak, “You’re not going to just pull a vaccine out of your pocket,” notes Anthony Fauci. He directs NIAID in Bethesda, Md. If the current outbreak proves to be “really bad,” the FDA might allow emergency use of promising vaccines that haven’t completed their full safety and efficacy testing. But researchers won’t know for at least six months whether any of the vaccines in development will help against COVID-19 virus.
Other strategies to fight the new virus, including using existing drugs designed to fight other diseases (such as AIDS and hepatitis C), also are underway. But no one knows which ones are winners. So for now, people exposed to the new virus must rely on their own immune systems and care from doctors and nurses to fight off COVID-19.
Search speeds up for vaccine against the new coronavirus published first on https://triviaqaweb.tumblr.com/
0 notes