#glia
Text



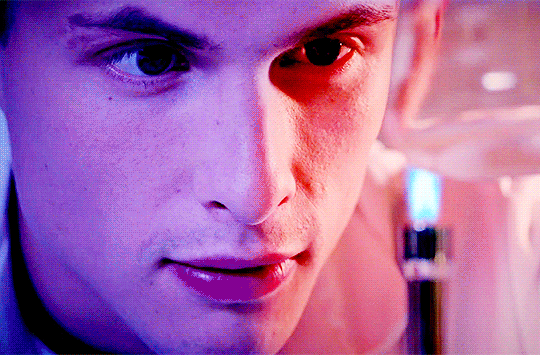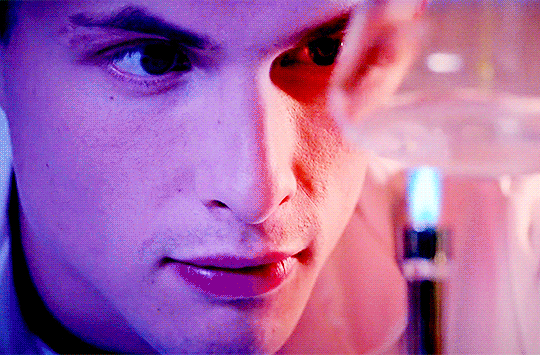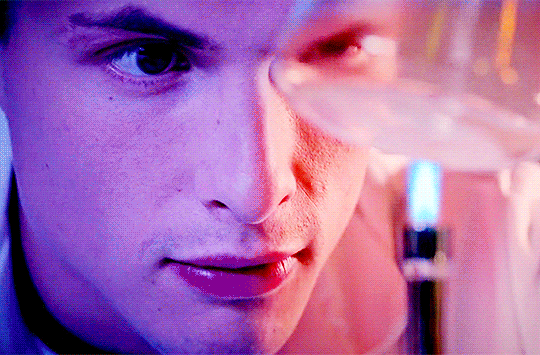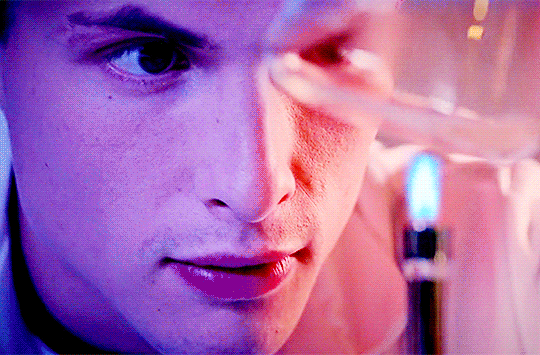
Arnas Fedaravicius // Glia
Taglist: @sihtricfedaraaahvicius @whumpappreciation @siimonesvensson @gloriouslyalivetoday @melissarose234 @crusader1997 @sivulele @gemini-mama @bathedinheat @vashole @losstboi @fox-bright @whumpybromance @umfood @elwegencyn @keenbagelsharkbanana @the-irish-girl @tinumiel @hb8301 @miss-sparkel-mr-hitch @simpforfictionalaisela25 @alexagirlie @uunotheangel
60 notes
·
View notes
Text

Glial Diversity Atlas
Study in fruit flies of the relationship between the shapes (morphologies) of distinct glial cells – non-neuronal cells of the nervous system – and their gene activity (transcription) reveals several new morphologies but no clear correlation with the transcription signatures
Read the published research article here
Image from work by Inês Lago-Baldaia and colleagues
Department of Cell and Developmental Biology, University College London, London, UK
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in PLOS Biology, October 2023
You can also follow BPoD on Instagram, Twitter and Facebook
7 notes
·
View notes
Text
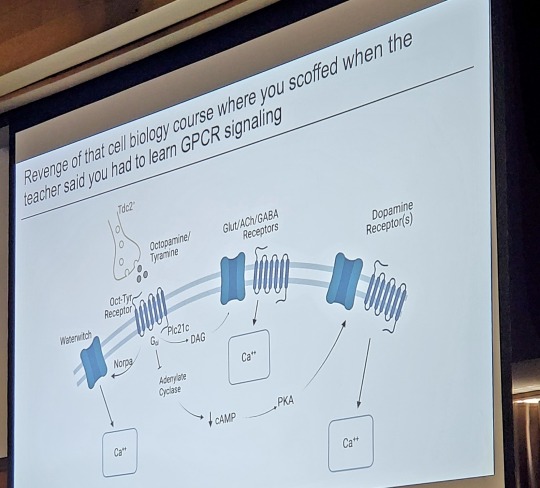
I love it when science talks are funny
#science#science talk#neuroscience#biology#glia#GPCR#laboratory#research#scientist#grad student#phd#phd student#gradblr#public speaking#presentations#ppt#hawtchocolate
80 notes
·
View notes
Text
#glia #shoegaze
10 notes
·
View notes
Audio
Listen/purchase: On The Take by Glia
album
#bandcamp#music#alternative#dream pop#shoegaze#glia#on the take#happens all the time#swirl#fuzz#fuzzy
3 notes
·
View notes
Text
youtube
glia set on lines, 2/24/2024
0 notes
Text
I’d learned by this point that comparing brains is a difficult business in general. In explaining how clever humans are, we often point out the extraordinarily large size of our thinking organs. Their bulk is the bane of childbirth and consumes 90 percent of the glucose in our blood. But size itself is not a clear guide for comparing animal intelligences, as some bigger animals with larger brains seem to lack the cognitive abilities of smaller ones. Size, as the saying goes, isn’t everything. Relative brain-to-body size, how wrinkled and complex brains are, the thickness of their layers, the structures within them, and the types of neurons these are made of are all helpful—though our human brains are, naturally, the yardstick that other brains are measured against. And yet it is impossible to look at a whale brain and not be surprised by its size. When Hof first saw one, despite knowing they were big, its mass still shocked him. The human brain is about 1,350 grams, three times larger than our big-brained relative, the chimpanzee. A sperm whale or killer whale brain can be 10 kilograms. These are the biggest brains on Earth and possibly the biggest brains ever, anywhere. It’s perhaps not a fair comparison: in relation to the size of our bodies, our brains are bigger than those of whales. Ours are similar in proportion to our body mass, as are the brains of some rodents; mice and men both invest a lot of themselves in their thinking organs. But we both lag far behind small birds and ants, which have much bigger brains compared to their body size than any big animals.
The outer layer of a mammal’s brain is called the cerebral cortex. In cross section, it looks a little like a wraparound bicycle helmet sitting on top of the other parts of the brain. This is the most recently evolved part of our brains, and it was by using their own cerebral cortexes that brain scientists have learned that this area is responsible for rational, conscious thought.
It handles tasks like perceiving senses, thinking, movement, figuring out how you relate to the space around you, and language. You are using yours now to read and think about this sentence. Many biologists define “intelligence” as something along the lines of the mental and behavioral flexibility of an organism to solve problems and come up with novel solutions. In humans, the cerebral cortex, acting with other bits of the brain (the basal ganglia, basal forebrain, and dorsal thalamus), appears to be the seat of this form of “intelligence.” The more cortex you have and the more wrinkled it is, the more surface area available for making connections—and voila! More thinking.
Humans have a really large neocortex surface area, but it’s still just over half that of a common dolphin, and miles behind the sperm whale. Even if you divide the cortex area by the total weight of the brain to remove the cetacean size advantage, humans still lag behind dolphins and killer whales. But there are other measurements in the cortex that seem to be associated with intelligence, and here, dolphins and whales lag behind humans.
The more neurons are packed in, how closely and effectively they are wired, and how fast they transmit impulses are also extremely important in brain function. Just as the composition and layout of the chipset in your tiny, cheap cellphone allows it to pack more computing power than a five-tonne room-sized 1970s supercomputer. Both cetaceans and elephants, the biggest mammals on sea and land, seem to have large distances between their neurons and slower conduction speeds. In raw numbers of neurons, humans here, too, have the edge, with a human cortex containing an estimated 15 billion neurons. Given the larger size of cetacean brains, you’d think they’d have more, but in fact their cerebral cortex is thinner, and the neurons are fatter, taking up more room.
Nevertheless, some cetaceans such as the false killer whale are close behind human levels with 10.5 billion cerebral neurons, about the same as an elephant. Chimps have 6.2 billion and gorillas 4.3 billion. Further complicating comparisons, whales have huge numbers of other kinds of cells, called glia, packing their cortexes. Until recently, we believed these glial cells to be an unthinking filler, but we’ve now discovered that they actually seem important for cognition, too. I don’t know about you, but all this cortex measurement and comparison makes my own feeble organ hurt.
— In the Mind of a Whale
#tom mustill#in the mind of a whale#science#zoology#biology#human biology#marine biology#neuroscience#brain#cerebral cortex#basal ganglia#basal forebrain#thalamus#neocortex#neurons#glia#patrick hof#whales#intelligence
1 note
·
View note
Photo

Gleeful Look
Glial cells are like an unsung network of city support workers – regulating traffic, sweeping streets, keeping things orderly and hygienic in our brain and nervous system. Their shape is key to how they interact with brain cells, but current techniques to assess this morphology are limited. A new imaging tool, GliaMorph, combines different modules to in turn quantify texture, segmentation, and other features at the individual cell and global scale. Researchers turned GliaMorph’s focus to zebrafish Müller glia – cells important to eye health. They were able to measure Müller glia development, observing the growing complexity and rearrangement (pictured, at 72 - top - and 120 - bottom - hours post fertilisation) – with the original image on the left followed left to right by segmentation, structural edges, distance between elements, and the cellular skeleton highlighted. They then measured changing protein positioning in a mouse model of glaucoma, showing the approach’s direct potential for revealing the precise morphology of human disease.
Written by Anthony Lewis
Image from work by Elisabeth Kugler and colleagues
Institute of Ophthalmology, University College London, London, UK
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Development, February 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#neuroscience#eyes#eye development#glial cells#glia#muller cells#retina#zebrafish#ophthalmology#immunofluorescence#image analysis
23 notes
·
View notes
Text




1 note
·
View note
Text
#glia #shoegaze
11 notes
·
View notes
Photo

MINI_REVIEW(s):
The review template of choice for the TL;DR Tribe…

‘NEVER ENDING SPACE’ (@_a_l_t_e_r_) is the latest LP from @chainofflowersband & it finds the London by way of Cardiff based outfit “reunited & rejuvenated” across 10 tracks that combine brooding post_punkisms w/ new_waving sheen while packing plenty of brassy textures, chiming six-strings, & muscled low-ends as witnessed on the (early) U2 meets The Cure meets Blitz vibing of “Old Human Material”

‘HAPPENS ALL THE TIME’ (@candlepin_records) is the latest LP from @glia_music & it finds the group solidifying their “Houston SlackerGaze” brand across an 11-track spread that dutifully incorporates all kinds of buzz_binning appeal thru a sheer force of head lowering haze, swirled psychedelia & melancholic moodiness… & a whole lotta MBV levels of pedal_assisted dissonance as evidenced on album highlight “Turn”

‘IT CAN’T RAIN ALL THE TIME’ (@rainy_day_music) is the latest EP from @s.green_rdm & it finds the Philadelphia-based project turning its attention towards the halcyon days of the 90s across 5 tracks that suitably combine elements of goth’d-out Emo, buzzed electronica & Nu_gazing heaviness under one blown-out & dream_popping umbrealla which is on full display throughout “Sometimes I Disappear”

‘OF GOOD FORTUNE’ (@candlepin_records) is the latest LP from @wiring____ & it finds vocalist/guitarist Connor Gibson’s project (rounded out here by a whole slew of friends) bringing all kinds of nostalgia-laced, emotionally earnest & Midwest 90s vibing across an aesthetically apt 8-track spread that’s chockful of CollegeRawk jangle, angular skramz’d Emo & slow_coring IndieRawk as heard on “He’s Without”
////
#albumreview#music#bands#artist#chainofflowers#glia#sgreen#wiring#rock#indie#indierock#shoegaze#alternative#altrock#alternativemusic#alternativerock#dreampop#newwave#goth#screamingforyears
6 notes
·
View notes
Text


I bet he has a fast metabolism with all that fighting
#genshin hunger#gen//shin im//pact#gutshin impact#hunger kink#stomach kink#stomach growling#hunger#tummy kink#tummy#gen//shin#chil//de#tar//ta//glia#tar//taglia#a//jax
174 notes
·
View notes
Link
“Taken together, our study presents the highest resolution, multi-cohort and multi-omics analysis to date, providing an important resource to facilitate mechanistic hypotheses of host-microbiome interactions in ME/CFS.”
Highlights:
Spike protein infusion into mouse brain induces late cognitive dysfunction
Spike protein induces late hippocampal microgliosis and synapse loss
Blockage of TLR4 renders mice resistant to Spike-induced cognitive dysfunction
TLR4-2604G>A GG genotype was related to poor cognitive outcomes in COVID-19 patients
Cognitive dysfunction is often reported in patients with post-COVID, but its underlying mechanisms are not completely understood. Evidence suggests that SARS-CoV-2 Spike protein or its fragments are released from the cells during infection, reaching different tissues, including the CNS, irrespective of the presence of the viral RNA.
Here, we demonstrate that brain infusion of Spike protein in mice has a late impact on cognitive function, recapitulating post-COVID syndrome. We also show that neuroinflammation and hippocampal microgliosis mediates Spike-induced memory dysfunction via complement-dependent engulfment of synapses.
Genetic or pharmacological blockage of TLR4 signaling protects animals against synapse elimination and memory dysfunction induced by Spike brain infusion. Accordingly, in a cohort of 86 patients recovered from mild COVID-19, the genotype GG TLR4 -2604G>A (rs10759931) is associated with poor cognitive outcome. These results identify TLR4 as a key target to investigate the long-term cognitive dysfunction after COVID infection both in humans and rodents.
TLRs are activated by different pathogen-associated molecular patterns (PAMPs) and are crucial for evoking the innate immune responses to infection, stress or injury. Studies have predicted that SARS-CoV-2 Spike protein binds to TLR4 with higher affinity than it binds to ACE2, and its aberrant signaling is involved in the hyperinflammatory response of patients with COVID-19. In vitro studies also demonstrated that SARS-CoV-2 Spike protein activates TLR4 in cultured phagocytic cells, stimulating production of proinflammatory mediators.
Although TLR4 has already been implicated in microglial activation and cognitive dysfunction of Alzheimer’s disease, the impact of TLR4 signaling in COVID-related neurological dysfunction is still unknown.
Further indicating that late but not early microgliosis was induced by Spike protein, we found significantly higher TMEM119 immunoreactivity in the DG hippocampal subregion of Spike-infused mice. Notably, the mRNA levels of the inflammatory mediators TNF, IL-1β, IFNα and IFNβ as well as the IFN receptor IFNAR2 were higher in the hippocampus of Spike-infused mice at this late time point.
We also found increased serum levels of TNF only in the late stage of the model, which returned to the control levels at 60 days post-infusion (Supplementary Fig.5T29 V), correlating with the cognitive dysfunction (Fig. 1B-E). Altogether, our results indicate that the cognitive impairment induced by Spike protein is accompanied by microglial activation and neuroinflammation.
SARS-CoV-2 Spike protein induces synaptic phagocytosis by microglia in mice. Remarkably, C1q blockage rescued object recognition memory impairment in Spike protein-infused mice without any effect on locomotion or exploration. Similarly, neutralizing C1q antibody treatment also prevented spatial memory dysfunction induced by Spike protein infusion. We found that the C1q blockage also prevented the late decrease in hippocampal synaptic puncta and reduced microglial synaptic engulfment in mice infused with the Spike protein. Together, these data suggest that C1q-mediated microglial phagocytosis underlie long-term cognitive dysfunction induced by Spike protein, as seen for other viral encephalitis.
Studies have described that Spike protein induces toll-like receptor 4 (TLR4) activation in cultured immune cells. Additionally, TLR4 has been implicated in microglial activation and cognitive dysfunction in degenerative chronic disease of CNS such as Alzheimer’s disease. In agreement with these observations, despite no changes found in TLR4 expression levels at the early time point after Spike protein infusion (Fig. 4A), we found a late upregulation of TLR4 gene in the hippocampus of Spike protein-infused mice that matches the late cognitive dysfunction.
To evaluate the role of TLR4 in Spike-induced cognitive impairment, we used either a pharmacological approach or a TLR4 knockout mouse model (TLR4First, to investigate whether activation of TLR4 is an early event that could impact cognition later on, mice were treated with the TLR4 inhibitor TAK242 1h before Spike protein brain infusion and once a day for 7 days.) Remarkably, early inhibition of TLR4 greatly prevented late memory dysfunction induced by Spike protein.
Together, these data suggest that TLR4 activation mediates cognitive deficit and synaptic pruning induced by Spike protein in mice.
Importantly, the early treatment with TLR4 inhibitor prevented the late neuronal damage, indicating that the TLR4 pathway is central to induce neurodegeneration and long-term cognitive impairment in the present model. Single nucleotide polymorphism within TLR4 gene is associated with increased risk of cognitive dysfunction after COVID-19.
Several lines of evidence have suggested that polymorphisms in TLR4 gene is a risk factor for developing inflammatory diseases, including sporadic Alzheimer's disease.
Considering our clinical findings demonstrating that SNP (rs10759931) is associated with poor cognitive function after COVID-19, we have performed functional analysis aimed to strengthen the link between this genetic variant and the levels of TLR4 mRNA after Spike stimuli.
Spike stimulation of cultured GG genotype cells resulted in increased levels of mRNA TLR4 when compared with GA genotype cells (*p = <0.0001) (Figure 4X). Our findings suggest that polymorphisms in TLR4 gene are associated with altered Spike-induced host immune responses, increasing the risk to develop long-term cognitive deficit in genetically susceptible individuals.
Post-COVID syndrome comprises a myriad of symptoms that emerge after the acute phase of infection, which include psychiatric symptoms, and dementia-like cognitive dysfunction.
Clinical studies have largely mapped the spectrum of neurological symptoms in patients with post-COVID, but do not provide significant advance in describing the molecular mechanisms that trigger this condition or targets for preventive/therapeutic interventions. On the other hand, studies involving COVID-19 preclinical models have focused mostly on the acute impacts of viral infection. Therefore, it is mandatory to develop novel tools to dissect the mechanisms underlying the neurological deficits in post-COVID, especially the direct effect of the virus and/or viral products on the brain.
Synapse damage is a common denominator in a number of memory-related diseases, often preceding neurodegeneration. It has been shown that neuroinvasive viruses, such as West Nile virus (WNV), Borna disease virus (BDV) and Zika virus (ZIKV), are also associated with synapse impairment.
Likewise, we found that the late cognitive dysfunction induced by Spike protein was accompanied by prominent synapse loss in mice hippocampus. Recent data have revealed the upregulation of genes linked to synapse elimination in SARS-CoV-2-infected human brain organoids and in post-mortem brain samples from patients with COVID-19. In line with these observations, we found that infusion of Spike protein into the mouse brain induces a late elevation in plasma levels of NFL, an axonal cytoskeleton protein identified as a component of pre- and postsynaptic terminals. Plasma NFL [Neurofilament light] increase can be employed as a marker of synapse loss and disease progression in neurodegenerative diseases, including Alzheimer's disease.
Collectively, these findings suggest that brain exposure to Spike protein induces the synapse loss and behavioral alterations typical of viral encephalitis, leading to a prolonged neurological dysfunction that can persist long after recovery from the infectious event.
Microglia are the most abundant immune cell type within the CNS and play a critical role in most of the neuroinflammatory diseases. In viral encephalitis, microglial cells have both protective and detrimental activities depending on the phase of infection. Previous studies showed that human coronaviruses can reach the CNS and induce neuroinflammation and/or gliosis both in mature and immature brain tissues. Here we found that microglial cell lineage BV19 2 was impacted by Spike protein, corroborating recent data showing an increase in proinflammatory mediators in S1-stimulated microglia. Since cultured primary cortical neurons were not directly affected by Spike stimulation, our in vitro results indicate that microglia could be seen as the main cell type affected by exposure to SARS-CoV-2 Spike protein.
It is well known that viral infections are often associated with excessive activation of inflammatory and immune responses, which may in turn elicit and/or accelerate brain neurodegeneration. Here, we found that Spike protein-infused mice presented late microglial activation, but not astrocyte reactivity, similar to observed in other animal models of viral encephalitis. Hippocampal and serum increased levels of proinflammatory mediators were found only at late time points after Spike infusion, showing a temporal correlation with synaptic loss and cognitive dysfunctions. Conversely, we found that the downregulation of IFNAR2 gene occurred shortly after Spike injection, similar to what is observed in neuronal cells of post-mortem samples from patients with COVID-19. This finding corroborates recent evidence demonstrating that SARS-CoV-2 may evade innate immune through modulation of type-I IFN responses.
Altogether, our results show that brain exposure to Spike protein induces an early negative modulation of the main receptor involved in type-I IFN response followed by a late proinflammatory process in the hippocampus.
Together, our findings strongly suggest that brain dysfunction in post-COVID is associated with Spike-induced TLR4 signaling in microglial cells.
The engagement of complement and TLRs in signaling crosstalk has been proposed to regulate immune and inflammatory responses in neurodegenerative diseases. Indeed, it was shown that TLR4 activation induces the upregulation of complement components in the mouse hippocampus. Given the role of complement activation in synaptic pruning, we hypothesized that TLR4 is the molecular switch that regulates microglial synaptic engulfment.
Notably, our hypothesis is in agreement with emerging evidence showing a role for TLR4 in Spike-induced microglial responses. Olajide et al. found significant inhibition in TNF and IL-6 release in S1 Spike-stimulated BV-2 microglia using the same pharmacological inhibitor used in our study (TAK-242) or in cells transfected with TLR4 small interfering RNA. Similar results using TLR4 pharmacological or genetic blockade were found in both murine and human
macrophages.
Our animal model provides evidence of the ability of SARS-CoV-2 Spike protein to induce synapse dysfunction.
Using brain organoids, Samudyata and colleagues described that SARS-CoV-2 infection is able to increase microglial engulfment of postsynaptic termini 72 hours after virus inoculation. Thus, it is plausible to assume that TLR4 activation can induce either acute or delayed synaptic dysfunction depending on the agonist/proinflammatory insult. In light of this, we speculate that this possible uncommon ability of SARS-CoV-2 Spike protein to induce delayed synapse loss could account for the occurrence of the intriguing delayed-onset post-COVID cognitive impairment.
...longitudinal data indicates that mild SARS‐CoV‐2 infection is associated with persistent cognitive symptoms with delayed symptom onset not only in individuals with pre-existing cognitive risk factors, but also in young individuals in the absence of comorbidities.
#long covid#science journal#covid effects#neuroinflammation#TLR4#glia#microglia#encephalitis#synapse#phagocytosis
0 notes

