Text
Electric discharge in aluminum powder in castor oil | source
594 notes
·
View notes
Text
Mimicking Plant Movement

Many plants control the curvature of their leaves by selectively pumping water into cells that line the outer surface. This swelling triggers bending. Engineers created their own version of this structure. (Image credit: T. Gao et al.; via GoSM)
Read the full article
206 notes
·
View notes
Text






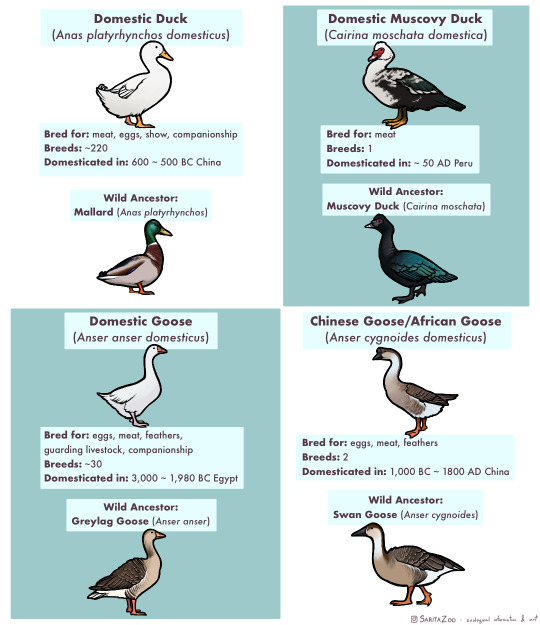



Phew. This one took, uh… a bit longer than expected due to other projects both irl and art-wise, but it’s finally here. The long-awaited domestic animal infographic! Unfortunately, I didn’t have enough space to cover every single domestic animal (I’m so sorry, reindeer and koi, my beloveds) but I tried to include as many of the “major ones” as possible.
I made this chart in response to a lot of the misunderstandings I hear concerning domestic animals, so I hope it’s helpful!
Further information I didn’t have any room to add or expand on:
🐈 “Breed” and “species” are not synonyms! Breeds are specific to domesticated animals. A Bengal Tiger is a species of tiger. A Siamese is a breed of domestic cat.
🐀 Different colors are also not what makes a breed. A breed is determined by having genetics that are unique to that breed. So a “bluenose pitbull” is not a different breed from a “rednose pitbull”, but an American Pitbull Terrier is a different breed from an American Bully! Animals that have been domesticated for longer tend to have more seperate breeds as these differing genetics have had time to develop.
🐕 It takes hundreds of generations for an animal to become domesticated. While the “domesticated fox experiment” had interesting results, there were not enough generations involved for the foxes to become truly domesticated and their differences from wild foxes were more due to epigenetics (heritable traits that do not change the DNA sequence but rather activate or deactivate parts of it; owed to the specific circumstances of its parents’ behavior and environment.)
🐎 Wild animals that are raised in human care are not domesticated, but they can be considered “tamed.” This means that they still have all their wild instincts, but are less inclined to attack or be frightened of humans. A wild animal that lives in the wild but near human settlements and is less afraid of humans is considered “habituated.” Tamed and habituated animals are not any less dangerous than wild animals, and should still be treated with the same respect. Foxes, otters, raccoons, servals, caracals, bush babies, opossums, owls, monkeys, alligators, and other wild animals can be tamed or habituated, but they have not undergone hundreds of generations of domestication, so they are not domesticated animals.
🐄 Also, as seen above, these animals have all been domesticated for a reason, be it food, transport, pest control, or otherwise, at a time when less practical options existed. There is no benefit to domesticating other species in the modern day, so if you’ve got a hankering for keeping a wild animal as a pet, instead try to find the domestic equivalent of that wild animal! There are several dog breeds that look and behave like wolves or foxes, pigeons and chickens can make great pet birds and have hundreds of colorful fancy breeds, rats can be just as intelligent and social as a small monkey (and less expensive and dangerous to boot,) and ferrets are pretty darn close to minks and otters! There’s no need to keep a wolf in a house when our ancestors have already spent 20,000+ years to make them house-compatible.
🐖 This was stated in the infographic, but I feel like I must again reiterate that domestic animals do not belong in the wild, and often become invasive when feral. Their genetics have been specifically altered in such a way that they depend on humans for optimal health. We are their habitat. This is why you only really see feral pigeons in cities, and feral cats around settlements. They are specifically adapted to live with humans, so they stay even when unwanted. However, this does not mean they should live in a way that doesn’t put their health and comfort as a top priority! If we are their world, it is our duty to make it as good as possible. Please research any pet you get before bringing them home!
31K notes
·
View notes
Text
Molecule of the Day: Galaxolide (& other musks)
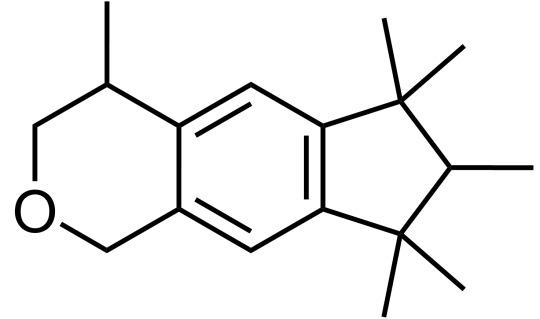
What smells do you associate with “cleanness”? Is it the clean, dewy freshness after a thunderstorm (due to geosmin - Day 14)? Or is it that warm, enveloping smoothness of clean linen?
If you’re thinking about the latter, Galaxolide (C18H26O) is probably responsible for that. It belongs to a class of compounds called “musks”, which are extremely commonly used in fragrances, from functional (detergents and soaps) to fine (perfumes).
Musks used to be obtained from musk pods - a gland of musk deer - since ancient times, when they were discovered to not only have an aroma of their own, but also acted as a fixative for fragrances, which is to say that it prolonged the lasting power of fragrances that it was added to. Natural musk can also be obtained from civet cats and muskrats, amongst other animals.

However, in recent decades, the industry has turned to synthetic musks due to sustainability and ethical concerns regarding the harvesting of musk pods.
The first synthetic musks were discovered serendipitously in the late 19th century; a chemist, Albert Bauer, was trying to find a more explosive analogue to trinitrotoluene (TNT - Day 58) when he noticed that the products of his experiments had a pleasant musky odour. These “nitro musks”, as they were called, found widespread use due to their relatively low costs and similarity to natural musk. Chanel famously used nitro musks in many of their perfumes, including No. 5.

However, nitro musks were eventually suspected of neurotoxicity, and were phased out; only one nitro musk, musk ketone, is currently approved to be used in the fragrance industry.
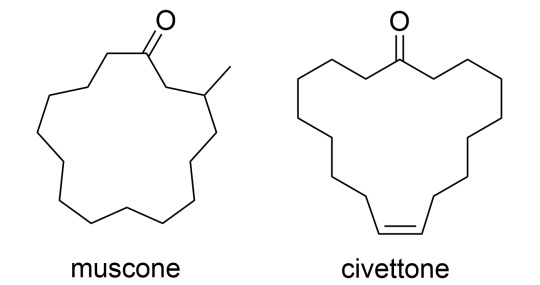
Other types of musks - macrocyclic musks, such as muscone and civettone, were isolated from natural musk from musk deer and civet cats. However, industrial synthesis proved too complicated and commercially unfeasible for the early 20th century.
The hunt for a new type of synthetic musk - one that was safe and cheap - was on again. In 1965, Galaxolide was first synthesised by International Flavors and Fragrances (IFF) in an attempt to make existing musks more chemically stable and hydrophobic, and was quickly utilised in many functional and fine fragrances due to its crisp, clean connotations; it was completely free of any animalic nuances, unlike molecules like muscone.
Why does the molecule have to be hydrophobic? Consider its purpose - when used in detergents, it would be a desirable quality to be able to adsorb onto fabric fibres and not be washed off by water. Its relatively large size meant that strong intermolecular forces of attractions would lead to low volatility too, thus allowing it to impart a long-lasting pleasant smell.

While Galaxolide was not the first polycyclic musk to be discovered, it is by far the most well-known one and is produced on a scale of 1,000 tons a year as of 2015.
However, with its ubiquity, its persistence in nature and its tendency to bioaccumulate has been brought under scrutiny. While no toxic effects to humans have been reported, it has still been a cause for concern as its effects on wildlife are not well understood.
And the search for new affordable, safe, and biodegradable musks continues…
My dear readers - what chemicals have you tried sniffing? Which ones had a nice aroma, and which ones smelled awful? Let me know in the comments!

160 notes
·
View notes
Link
As the sun moves across the sky, sunflowers continually orient themselves to soak up the most light (SN: 8/4/16). Now a type of human-made material can do that, too.
This is the first artificial material capable of phototropism, researchers report November 4 in Nature Nanotechnology. Stemlike cylinders of the material, dubbed SunBOTs, can maneuver to capture about 90 percent of available sunlight, even when the sun comes in at an oblique angle, materials scientist Ximin He of UCLA and her colleagues found. The technology could someday be used to optimize solar panels, desalinate water or move robots, the researchers say.
Other scientists have made artificial substances that can bend toward light, but those materials stop arbitrarily. SunBOTs can self-regulate, moving into the optimal position needed to absorb the sun’s rays, then making small adjustments to stay there as the sun shifts.
That ability comes from a SunBOTs’ configuration: a stemlike polymer about 1 millimeter in diameter embedded with a nanomaterial that responds to light. The nanomaterial absorbs light and converts it into heat; the polymer shrinks in response to increased temperatures.
When He and colleagues trained a beam of light on one of these artificial stems, the illuminated side heated up and contracted. That caused its top to bend toward the light. The newly shaded underside of the stem then cooled, stopping the SunBOT’s movement in a position best oriented to soak up the light. The process repeated as the angle of the light beam changed.

SunBOTs are designed to bend to meet different angles of a beam of light (arrows indicate the direction of incoming light), and can shift position as the light moves. That ability lets the devices capture more solar energy than stationary devices.
CREDIT: XIAOSHI QIAN, YUSEN ZHAO, YOUSIF ALSAID AND XIMIN HE
To build their initial SunBOTs, the researchers used gold nanoparticles and a hydrogel. But tests with other materials — such as carbon black nanoparticles and liquid crystalline polymers — revealed that the components could be mixed and matched.
“If we have this big repertoire of materials working with the same principle … scientists can use it in different environments for different applications,” says Seung-Wuk Lee, a bioengineer at the University of California, Berkeley who was not involved in the study. For instance, hydrogel SunBOTs work in water, He’s team found.
SunBOTs can be lined up in rows to cover an entire surface, creating a “mini sunflower forest,” she says. Coating surfaces with this material could solve one of the biggest problems in solar energy: As the angle of direct sunlight changes as the sun moves overhead, conventional materials can’t keep up.
Materials that stay in one position — like solar cells on a solar panel — capture about 24 percent of available energy from the sun. Simply by moving to face the sun, He says, an array of SunBOTs can harvest about 90 percent of energy from sunlight.
By creating a material that can follow sunlight, the researchers were able to keep near-maximum solar absorption as the sun moved overhead, Lee says. “That is a major thing that they achieved.”
252 notes
·
View notes
Video

These are $30 for one but stitches at the hospital are more expensive so this is pretty damn great
143K notes
·
View notes
Text
How was the shape of DNA discovered?
Many of you may recognize this photo of the x-ray diffraction pattern of DNA found by Rosalind Franklin and her PhD student, Raymond Gosling. But, you may wonder how one could figure out from this image that DNA is structured as a double helix and even how x-ray crystallography works.
X-Ray Crystallography
X-ray crystallography is a method of determining the positions and arrangements of atoms in a crystal. Crystals are usually defined to be a highly ordered and repeating microscopic structure of a solid rather than the macroscopic crystals we know like quartz which actually tend to be “polycrystals” because at a microscopic level they do have the highly ordered structure required. Ice is also a polycrystal composed of many smaller ice crystals.
1.) X-ray beams are shot at the crystals
The x-rays interact with electrons of the atoms. This interaction or collision is typically modeled by Thomson scattering where the energy and thus frequency of the x-rays do not change after diffraction. This is similar to light going through a diffraction grating.
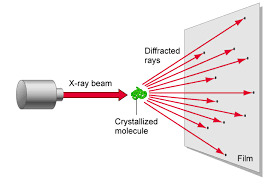
2.) Beam is diffracted
The x-rays are diffracted based on the crystal lattice structure of the substance. This is dependent on the characteristics of the bonds between atoms like the bond angles and bond lengths. Also the spacing between molecules also determines the diffraction.

3.) Diffraction pattern
The diffracted x-rays are light waves so they interfere both constructively and destructively. The resulting intensities of the x-rays are recorded on a screen behind the sample to create a diffraction pattern. The sample is rotated to take more data. After sufficient data is taken a model for the crystal structure for the sample can be developed. With a diffraction pattern an electron density map can be made which depicts the location and size of electron clouds in the substance.
Above is an example of an electron density map.
Sources & Read more: (1) (2)
228 notes
·
View notes
Text
50 years of Polymer International – Top 5 Articles
Our SCI journal, Polymer International is celebrating it’s 50th publication year in 2019. Volume 1, Issue 1 of Polymer International was first published in January 1969 under the original name British Polymer Journal. The journal, published by Wiley, continues to publish high quality peer reviewed demonstrating innovation in the polymer field.
Today, we look at the five highest-cited Polymer International papers and their significance.
Biodegradable Plastic
Article: A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies – Wendy Amass, Allan Amass and Brian Tighe. 47:2 (1998)
In the last few years, much of environmentalists’ focus has been on our plastic waste issue, particularly the issue of plastic build up in the oceans, and searching for alternatives. This review, published in 1998, was ahead of its time, describing biodegradable polymers and how they could help to solve our growing plastics problem. Research in this area continues to this day.
youtube
Here’s how much plastic trash Is littering the Earth. Video: National Geographic
The life of RAFT
Article: Living free radical polymerization with reversible addition – fragmentation chain transfer (the life of RAFT) – Graeme Moad, John Chiefari, (Bill) Y K Chong, Julia Krstina, Roshan T A Mayadunne, Almar Postma, Ezio Rizzardo and San H Thang. 49:9 (2000)
This research article by Moad et al., published in 2000, looks to answer questions about free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). RAFT polymerization is a type of polymerization that can be used to design polymers with complex architectures including comb-like, star, brush polymers and cross-linked networks. These complex polymers have application in smart materials and biological applications.
Sugar Biomaterials
Article: Main properties and current applications of some polysaccharides as biomaterials – Marguerite Rinaudo. 57:3 (2008)

Biomaterials made from sugar polymers have huge potential in the field of regenerative medicine
The review by Marguerite Rinaudo looks at polysaccharides – polymers made from sugars – and evaluates their potential in biomedical and pharmaceutical applications. They concluded that alginates, along with a few other named examples, were promising. Alginate-based biomaterials have since been used in the field of regenerative medicine, including would healing, bone regeneration and drug delivery, and have a potential application in tissue regeneration.
Supramolecular Chemistry
Article: Supramolecular polymer chemistry—scope and perspectives – Jean-Marie Lehn. 51:10 (2002)
This 2002 paper reviews advances in supramolecular polymers – uniquely complex structured polymers. They have a wide range of complex applications. Molecular self-assembly – the ability of these polymers to assemble into the correct structure without input – can be used to develop new materials. Supramolecular chemistry has also been applied in the fields of catalysis, drug delivery and data storage. Jean-Marie Lehn won the 1987 Nobel Prize in Chemistry for his work in supramolecular chemistry.
Flexible Screens
Article: Organic light‐emitting diode (OLED) technology: materials, devices and display technologies – Bernard Geffroy, Philippe le Roy and Christophe Prat. 55:6 (2006)

Organic light-emitting diode (OLED) technology could be used to make flexible screens and displays
This review looks at organic light-emitting diode (OLED) technology, which can be made from a variety of materials. When structured in a specific way, these materials can result in a device that combined in a specific red, green, blue colour combination, like standard LED builds, can form screens or displays. Because of the different structure of the material, these displays may have different properties to a standard LED display including flexibility.
Discover the latest advances in polymer chemistry for free in the first issue of this anniversary year.
Read more about Polymer International’s 50th Anniversary at soci.org
92 notes
·
View notes
Text
50 years of Polymer International – Top 5 Articles
Our SCI journal, Polymer International is celebrating it’s 50th publication year in 2019. Volume 1, Issue 1 of Polymer International was first published in January 1969 under the original name British Polymer Journal. The journal, published by Wiley, continues to publish high quality peer reviewed demonstrating innovation in the polymer field.
Today, we look at the five highest-cited Polymer International papers and their significance.
Biodegradable Plastic
Article: A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies – Wendy Amass, Allan Amass and Brian Tighe. 47:2 (1998)
In the last few years, much of environmentalists’ focus has been on our plastic waste issue, particularly the issue of plastic build up in the oceans, and searching for alternatives. This review, published in 1998, was ahead of its time, describing biodegradable polymers and how they could help to solve our growing plastics problem. Research in this area continues to this day.
youtube
Here’s how much plastic trash Is littering the Earth. Video: National Geographic
The life of RAFT
Article: Living free radical polymerization with reversible addition – fragmentation chain transfer (the life of RAFT) – Graeme Moad, John Chiefari, (Bill) Y K Chong, Julia Krstina, Roshan T A Mayadunne, Almar Postma, Ezio Rizzardo and San H Thang. 49:9 (2000)
This research article by Moad et al., published in 2000, looks to answer questions about free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). RAFT polymerization is a type of polymerization that can be used to design polymers with complex architectures including comb-like, star, brush polymers and cross-linked networks. These complex polymers have application in smart materials and biological applications.
Sugar Biomaterials
Article: Main properties and current applications of some polysaccharides as biomaterials – Marguerite Rinaudo. 57:3 (2008)

Biomaterials made from sugar polymers have huge potential in the field of regenerative medicine
The review by Marguerite Rinaudo looks at polysaccharides – polymers made from sugars – and evaluates their potential in biomedical and pharmaceutical applications. They concluded that alginates, along with a few other named examples, were promising. Alginate-based biomaterials have since been used in the field of regenerative medicine, including would healing, bone regeneration and drug delivery, and have a potential application in tissue regeneration.
Supramolecular Chemistry
Article: Supramolecular polymer chemistry—scope and perspectives – Jean-Marie Lehn. 51:10 (2002)
This 2002 paper reviews advances in supramolecular polymers – uniquely complex structured polymers. They have a wide range of complex applications. Molecular self-assembly – the ability of these polymers to assemble into the correct structure without input – can be used to develop new materials. Supramolecular chemistry has also been applied in the fields of catalysis, drug delivery and data storage. Jean-Marie Lehn won the 1987 Nobel Prize in Chemistry for his work in supramolecular chemistry.
Flexible Screens
Article: Organic light‐emitting diode (OLED) technology: materials, devices and display technologies – Bernard Geffroy, Philippe le Roy and Christophe Prat. 55:6 (2006)

Organic light-emitting diode (OLED) technology could be used to make flexible screens and displays
This review looks at organic light-emitting diode (OLED) technology, which can be made from a variety of materials. When structured in a specific way, these materials can result in a device that combined in a specific red, green, blue colour combination, like standard LED builds, can form screens or displays. Because of the different structure of the material, these displays may have different properties to a standard LED display including flexibility.
Discover the latest advances in polymer chemistry for free in the first issue of this anniversary year.
Read more about Polymer International’s 50th Anniversary at soci.org
92 notes
·
View notes
Photo

One major problem that has plagued supersonic aircraft is their noise. The Concorde – thus far the only supersonic commercial airliner – was plagued with noise complaints that ultimately restricted its usability. Noise reduction is a major area of inquiry in aerospace, and the video below shows one experiment trying to understand the connections between supersonic flow and noise.
Above you see a supersonic, Mach 1.5 microjet emanating from a nozzle at the top of the image. The jet is hitting a flat plate at the bottom of the image. Just beyond nozzle’s exit, you can see the X-shape of shock waves inside the jet. The position of that X is oscillating up and down.
In the background, you can see horizontal light and dark lines traveling up and down. Those horizontal lines in the background are acoustic waves. When they hit the bottom plate, they reflect and travel upward until they hit another surface (outside the picture) and reflect back down. As they travel, they interact with the jet, causing those X-shaped shock waves to move up and down. This coupling between flow and acoustic waves makes the jet much louder – up to 140 dB – than it would be otherwise.
Researchers hope that unraveling the physics of simpler systems like this one will help them quiet more complicated aircraft. (Image and video credit: F. Zigunov et al.)
youtube
155 notes
·
View notes
Text
polymorphs of other minerals: aragonite, vaterite
polymorphs of ice:

2K notes
·
View notes
Photo

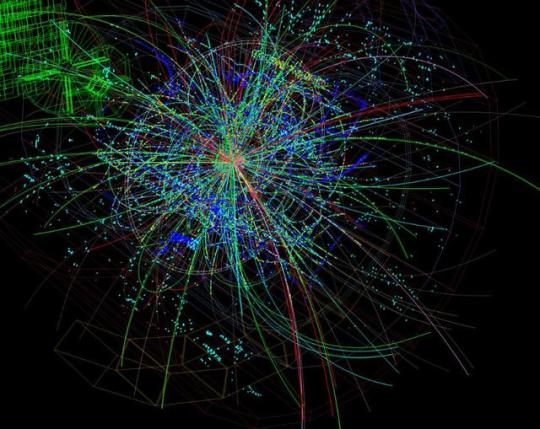
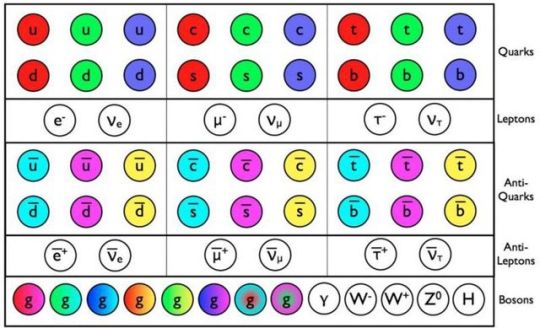



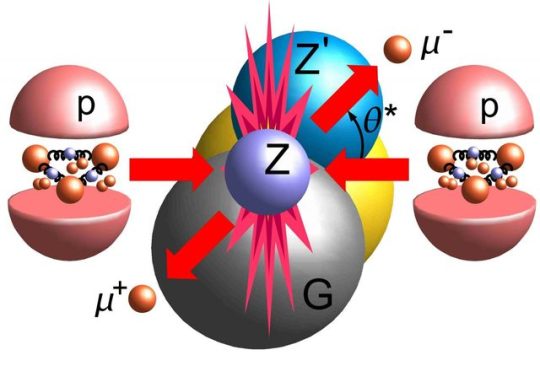
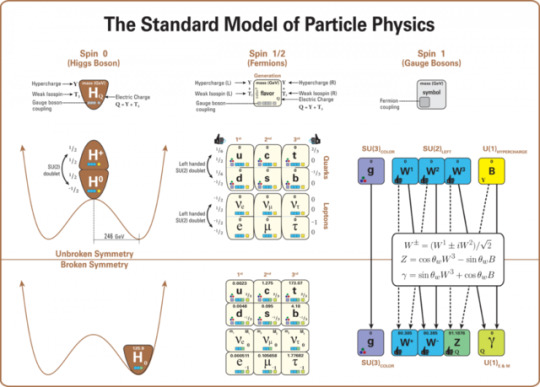
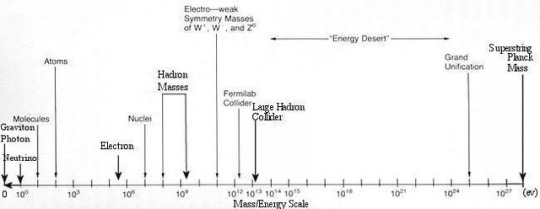

The Simple Case For Why Physics Needs A Particle Collider Beyond The LHC
“But how can you compare the cost of a new collider to the cost to humanity of not even trying to comprehend the great unknowns before us? There may come a day where we give up on what science can teach us, but today is not that day. So long as there’s a frontier to push in terms of energy, precision, or the amount of data we can collect, it is our duty as a curious species to push those boundaries as far as we possibly can.
The brute force approach isn’t the only one we should take, of course, just as surely as astronomers don’t invest everything in building a single telescope with as much light-gathering power as possible. But to abandon it now, after it’s taken us so far, would be the worst mistake we could make.
The low-hanging fruit may be gone, and we don’t know what may be up there in the tree-tops. We can build a cherry-picker good enough to take us there. Don’t you want a chance to taste the sweetest fruit of all?”
Do you want to know what lies beyond the Standard Model? To have a chance at peering beyond the current frontier that’s limiting our understanding of all that exists in the Universe? Or is it simply time to state that we know what we know, and there’s no point in looking further?
There’s a lot to unpack, but there’s a simple case to be made for building a new particle collider beyond the LHC. I’m not ready to give up yet; are you?
250 notes
·
View notes
Photo

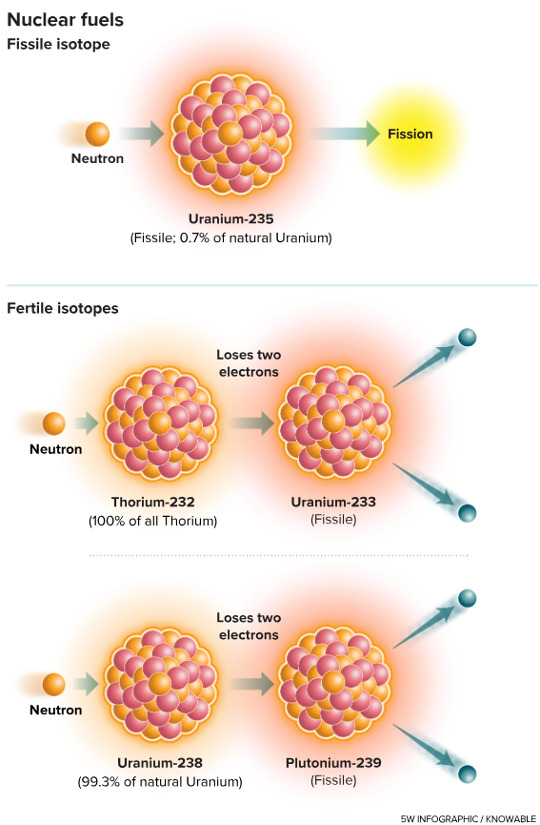

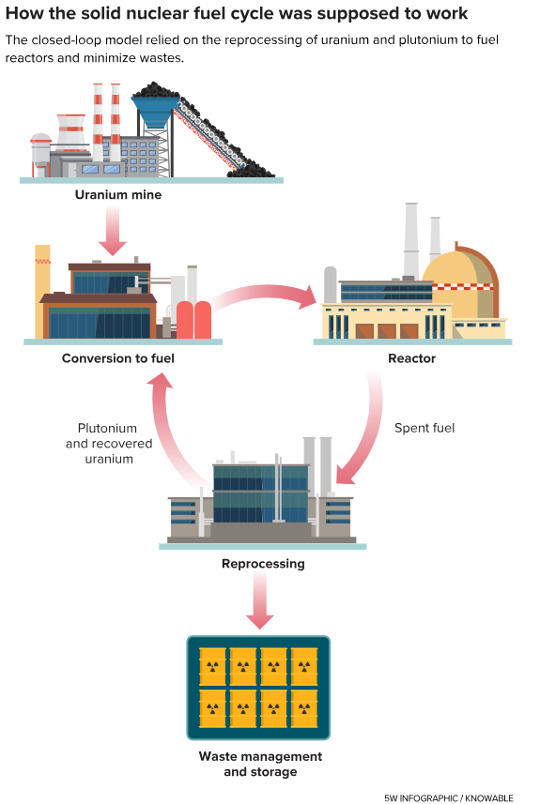
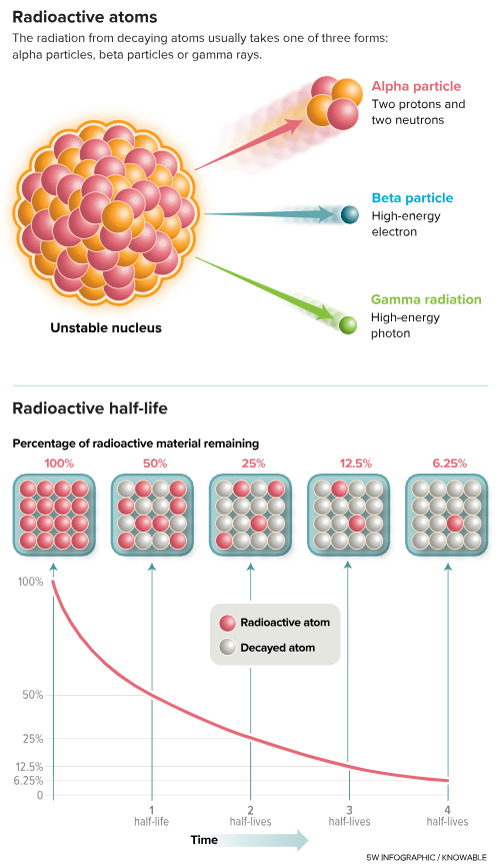
A very much-needed article and a wonderful imagery. Perhaps the only benefit of the demonization of CO2 will be the decriminalization of nuclear fission, in this case preventively, about molten salt reactors. It was time. Don’t miss it:
Nuclear goes retro — with a much greener outlook
Great work from Knowable Magazine and 5wgraphics.com
494 notes
·
View notes
Photo
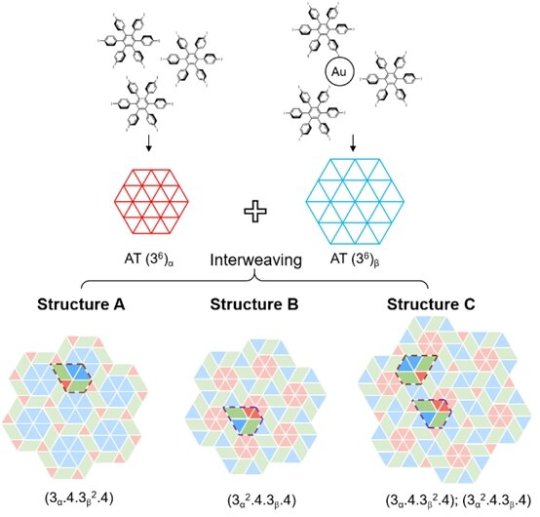
Highly complex two-dimensional tessellation in the molecular world
Tessellation is a repeating pattern made of one or more shapes, without the formation of gaps or overlaps. An example is the periodic arrangement of hexagonal cells found in honeycombs. Tessellation can also be found at the molecular level, where single molecule units act as a tile (repeating pattern) to tessellate a surface through spontaneous and reversible interactions between them. It is challenging to build complex molecular tessellation involving more than one type of tile. Most research studies in the past decade have focused on tessellation using a specific tile type.
A research team led by Prof Loh Kian Ping from the Department of Chemistry, NUS has demonstrated that highly complex periodic tessellation can be constructed from the tiling of two molecular phases that possess the same geometric symmetry but different packing densities. The two molecular phases, a high-density phase and a low-density phase, arise from the different intermolecular and molecule-substrate interactions. The high-density phase is formed by halogen bonds, while the low-density phase is formed via a halogen-gold coordination network. The geometric similarity between these two molecular phases allows the molecular units to serve as tiles to tessellate and form highly complex molecular tessellations.
Read more.
20 notes
·
View notes

