#Mueller Hinton Agar
Text
Get the Best Quality of Mueller Hinton Agar (TM 339) at TM Media
If you are looking for Mueller Hinton Agar manufactured by TM Media stands out for its remarkable batch-to-batch consistency, making it a cornerstone in antimicrobial susceptibility testing protocols. Endorsed by leading experts like Kirby-Bauer et al. and recognized by the World Health Organization for its unwavering reliability, this agar plays a vital role in microbiological analysis. Employed alongside paper discs infused with antimicrobial agents, it aids in the precise determination of Minimum Inhibitory Concentration (MIC). However, caution is warranted with slow-growing or anaerobic organisms, as prolonged incubation may introduce variability in results, necessitating careful interpretation.
Visit the Website - https://www.tmmedia.in/

0 notes
Text
MUELLER HINTON AGAR
The Mueller Hinton Agar formulation was initially crafted as a transparent agar medium for cultivating pathogenic Neisseria species. Although other media have superseded its use for Neisseria, Mueller Hinton Agar has found renewed importance in evaluating sulfonamide resistance in gonococci and other organisms. Over time, it has evolved into a widely embraced medium for antimicrobial susceptibility testing, endorsed by the Clinical and Laboratory Standards Institute (CLSI).
Several factors contribute to the CLSI's preference for Mueller Hinton Agar. Notably, it consistently demonstrates batch-to-batch reproducibility in susceptibility testing. Furthermore, it exhibits low susceptibility to interference from sulfonamide, trimethoprim, and tetracycline inhibitors. Additionally, it supports the growth of most non-fastidious bacterial pathogens, and its performance is well-documented with extensive data and experience.
The Kirby-Bauer method, advocated by Kirby-Bauer et al and endorsed by the World Health Organization (WHO) Committee on Standardization of Susceptibility Testing, employs Mueller Hinton Agar for antibiotic susceptibility tests. This approach involves the agar diffusion of antimicrobial substances from paper discs with a single concentration. The observed zone diameters are then correlated with minimum inhibitory concentration (MIC) values. The procedure entails swabbing a standardized organism suspension across the entire agar surface, placing discs impregnated with specific antimicrobial agents, and measuring zones of inhibition post-incubation. Susceptibility is determined by comparing results with CLSI standards.
Several factors influence the accuracy of disc diffusion susceptibility tests, including agar depth, disc potency, inoculum concentration, medium pH, and beta-lactamase production by test organisms. Extended incubation periods may lead to the deterioration of diffusing antibiotics, resulting in imprecise readings, particularly with slow-growing organisms.

0 notes
Text
Mueller Hinton Agar
Mueller Hinton Agar, developed by John Howard Mueller and Jane Hinton in 1941, is a simple and transparent agar medium that was originally formulated for the cultivation of Neisseria species. Later, it became widely used for the determination of sulphonamide resistance in gonococci and other organisms.
It is a non-selective and non-differential culture media which is widely used for the Kirby-Bauer method of Antimicrobial Susceptibility Testing. It is approved and recommended by the Clinical and Laboratory Standards Institute (CLSI) because of its consistent results, its low presence in sulphonamide, trimethoprim, and tetracycline inhibitors, and its ability to support the growth of most non-fastidious pathogenic bacteria.
Composition of Muller Hinton Agar:
Mueller Hinton Agar is composed of beef infusion, casein acid hydrolysate, starch, and agar. Beef infusion and casein acid hydrolysate supply nitrogenous compounds, carbon, sulphur, and other essential nutrients. Starch acts as a dispersing medium and absorbs any toxic metabolites if they are produced. Starch hydrolysis produces dextrose, which functions as an energy source.

Preparation of Mueller Hinton Agar:
To prepare Mueller Hinton Agar, liquefy 38 grams of the medium in 1 litre of purified or distilled water.
Heat the mixture to boiling to dissolve the medium completely.
Sterilize the medium by autoclaving at 15 psi pressure (121°C) for 15 minutes.
Allow the medium to cool to 45-50°C, then mix well and pour it into sterile Petri dishes.
The manual preparation of Media is a time consuming process. Hence, to save time, resources, and energy, Ready-to-Use Plates are the best option. TM Media offers a wide range of Ready-to-Use Plates tailored for specific applications.
TM Media’s Muller Hinton Agar Plate
TM Media’s Ready-to-Use Mueller Agar Plate serves convenience, consistency, and reliability and also saves preparatory time and effort. Additionally, it is approved and certified by all the strict industry standards.
TM Media’s Mueller Hinton Agar (Dehydrated Culture Media) offers two pack sizes- 100 gm and 500 gm.

Conclusion:
In the world of microbiology, Mueller Hinton Agar has proven to be a significant tool. Its composition, neutral pH, and consistent performance make it an essential medium for reliable antimicrobial susceptibility testing.
TM Media is one of the finest manufacturers of Culture Media. TM Media has over 4000 products in different categories - Dehydrated Culture Media, Ready-to-Use Culture Media, Biological Media Bases, Media Supplements, Indicators, Laboratory Chemicals, Antibiotic Sensitivity Discs, and many more.
To know more about TM Media and our Antibiotic Sensitivity Discs, read this blog.
#mueller hinton agar#dehydrated culture meedia#antibiotic sensitivity discs#antimicrobial susceptibility testing#AST#microbiology#ready to use culture media#ready to use plates
0 notes
Text

nobody is allowed to chat shit about my work ethic ever again actually I poured 81 plates of Mueller-Hinton suspension and Brain Heart infusion agar in 30 minutes and my plates all set and are even. I am a microbiology babe now no question
16 notes
·
View notes
Text

Dr. Jane Hinton (May 1, 1919 - April 9, 2003) a research scientist was born in Canton, Massachusetts. Her mother, Ada (Hawes) Hinton, was a former teacher, and her father, William Augustus Hinton, was a bacteriologist and one of the most prominent African American medical researchers of his era.
When she was six, her parents sent her and her sister to school in several countries in Europe to ensure that they would have the best education available to African Americans at the time. She returned to the US in 1928, graduating from high school at Montpelier Seminary in Vermont. She earned a BA at Simmons College in Boston.
She worked in her father’s laboratory and as an assistant to John Howard Mueller in Harvard University’s Department of Bacteriology and Immunology. She helped develop the Mueller-Hinton agar, a culture medium in which bacteria can thrive. It has become one of the standard methods used to test bacterial resistance to antibiotics.
She worked as a medical technician in Arizona for the War Department. After WWII ended, she decided she wanted to be a veterinarian and enrolled in the School of Veterinary Medicine at the University of Pennsylvania. She was the class historian and class secretary, graduating with her VMD. That year, Alfreda Johnson Webb gained her VMD, from Tuskegee University. They were the first two African-American women veterinarians in the nation.
She worked as a small animal veterinarian back in Canton. She joined the Department of Agriculture as a federal government inspector in Framingham, Massachusetts involved in research and response to outbreaks of disease in livestock. She retired in 1960 and spent the rest of her life, caring for a garden and a variety of pets at her home. She never married. #africanhistory365 #africanexcellence
0 notes
Text
Check the Best Quality of MUELLER HINTON AGAR (TM 339) at TM Media
TM Media is introducing Mueller Hinton Agar, initially designed for Neisseria species cultivation, evolved into a standard for antimicrobial susceptibility testing. Its consistency, minimal inhibitor content, and support for various pathogens make it ideal. Kirby-Bauer's method, utilizing paper discs with fixed antimicrobial concentrations, is pivotal. However, it's unsuitable for slow-growers and anaerobes due to potential inaccuracies. Agar depth, disc potency, and inoculum concentration affect results. Despite limitations, its adoption by CLSI and WHO underscores its reliability in microbiology.
Product link: https://www.tmmedia.in/product/mueller-hinton-agar/
Website link: https://www.tmmedia.in/
Company Name: TM Media
Phone: +91-9999-1687-70
Address: 905, 9th Floor, Big joes Tower, Netaji Subhash Place, New Delhi
Mail: [email protected]

0 notes
Text
Necessity of Microbiological Testing Laboratory To Keep Save Yourself
It isn't sufficient to simply distinguish your organic entity. You additionally need to understand what antimicrobial specialists your living being is helpless to. There are a few strategies to decide this.
Dilution testing is utilized to quantitatively decide the negligible fixation (in mg/ml) of antimicrobial specialists to restrain or kill the microorganisms. This is finished by adding two-overlay weakenings of the antimicrobial specialist straightforwardly to an agar pour, a stock cylinder, or a miniature stock board. The most reduced level that restrains the noticeable development of the life form is viewed as the Minimum Inhibitory Concentration (MIC).
The agar pour strategy is viewed as the reference test system in Europe. The stock weakening technique is all the more broadly acknowledged in North America. The E test (AB Biodisk) is a plastic strip with a slope centralization of antimicrobial specialists impregnated in it. The strip is put straightforwardly on the outer layer of an immunized plate. The MIC is perused from the strip where the development hindrance catches the plate. These strips are somewhat costly.
Numerous doctors anyway needn't bother with no know the specific MIC, yet which anti-infection agents the microorganism is vulnerable, middle of the road, or impervious to. The Kirby-Bauer agar dissemination technique is proven and factual and is the normalized strategy for deciding antimicrobial defenselessness. White channel paper circles (6 mm in breadth) are impregnated with known measures of antimicrobial specialists. Each plate is coded with the name and centralization of the specialist. For instance, 10 µg of Ampicillin is demonstrated on the plate by AM-10. The code is recorded on the Disk Zone Diffusion Diameter Chart.
The impregnated circles are put on an immunized Mueller Hinton Agar (MHA) plate. The medication diffuses through the agar. The plates are brooded for 16-24 hours. The agar might be enhanced with blood or you might involve blood agar for fussy creatures. The width of the apparent zone of hindrance is estimated and contrasted with reference values. There ought to be adequate microorganisms to frame a noticeable grass of development where it isn't restrained by the medication.
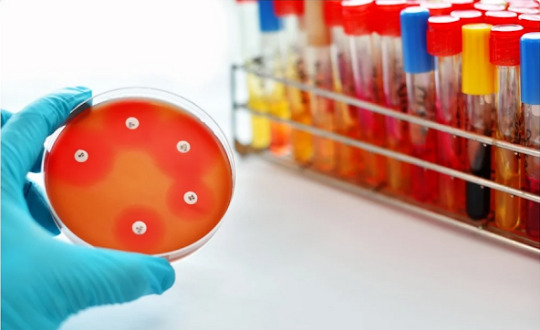
The outcomes are deciphered subjectively as safe, halfway, or defenseless. The standard convention should be adhered to precisely for you, or any clinical lab, to dependably decipher the outcomes.
There might be a few hindrances of development and the creature may as yet be viewed as impervious to that antimicrobial specialist in the event that the zone width is more modest than the reference values recorded on the graph. Likewise note that different antimicrobial specialists have various estimations for safe, transitional, and powerless.
A zone of restraint might be viewed as helpless for one antimicrobial specialist and not for another. For instance, for ampicillin (AM-10) to be a compelling antimicrobial specialist, the zone of hindrance for enterics and most steps should be more prominent than 16 mm while for staphs it should be more prominent than 28 mm.
While figuring out which antimicrobial specialists are best for treatment when different zones of hindrance are available, make certain to check out at the general zone of restraint for that specific antimicrobial specialist and contrast your estimations with that.
For instance, suppose that your intestinal creature has a zone of hindrance around the Polymyxin B circle of 20 mm and a zone of restraint around the Tetracycline plate of 20 mm. Since these estimations are bigger than the powerlessness zones recorded on the Disk Zone Diffusion Diameter Chart, both of these anti-toxins would be considered as opportunities for treatment.

In any case, when we look all the more carefully, we see that a 20 mm zone of restraint for Tetracycline is just 1 mm bigger than whatever is expected to be vulnerable while a 20mm zone of hindrance for Polymyxin B is 8mm bigger than the base vulnerability estimation required. In this specific case then, at that point, Polymyxin B and Tetracycline would both be sufficient for treatment, yet the Polymyxin B would be the most ideal decision.
What kind of tests are done in Microbiological testing laboratory?
The Clinical Microbiology Laboratory relies on usual diagnostic processes like Gram Stains, Culturing, Examination,Identification of Microorganisms containing Yeasts, Bacteria, & Fungi, and Biochemical Testing. This test has an important role in effective IPC (Infection Prevention and Control).
Microbiology Lab Testing Services
Make sure your food items are safe from microbiological contaminants. it is one of the most vital steps you can take before distribution them out to market.

Microbiological Testing In The Food Industry
Biosan performs all the major bacterial pathogen and microorganisms tests that may threaten your food items facility. Our wide research procedures allow us to identify them and isolate indicator organisms. They are:
• Aerobic Plate Count
• Anaerobic Plate Count
• Coliforms
• Lactic Acid Bacteria
• E.coli
• Enterobacteriacea
• Lactobacilli
• Yeast
• Mold
For Antimicrobial testing and Microbiological Testing Laboratory, you can explore Biosan Laboratories INC.
0 notes
Link
#what is mueller hinton agar medium#mha#mueller hinton agar#microbiology#practicals#microbiology practicals#science#medical#paramedical#medics#study notes#medical notes#science notes#biology notes#microbiology notes
6 notes
·
View notes
Text
Iris Publishers - World Journal of Agriculture and Soil Science (WJASS)
Antimicrobial Effects of Herb Extracts Against Foodborne Pathogen Listeria monocytogenes in Vitro
Authored by Hua Yang

Listeria monocytogenes is a gram-positive foodborne pathogen that is widely distributed during food preparation, storage, and distribution. A variety of ready-to-eat (RTE) foods such as milk, cheeses, ice cream, raw meat, fresh vegetable and fruits may be contaminated with Listeria monocytogenes [1,2]. Consumption of foods contaminated with L. monocytogenes is linked to an increased risk of listeriosis. To control L. monocytogenes in food products, meat industry uses chemical preservatives such as sodium acetate, sodium lactate and various nitrites. However, it is acknowledged that uses of chemical antimicrobials have increased the consumer concerns and created a demand for “natural” and “minimally processed” food. As a result, there has been a great interest in natural antimicrobial agents.
Plant-derived extracts have been used since ancient times, especially in China [3] and India [4,5]. In addition to the uses as flavoring material, plant extracts and essential oils represent a natural alternative in the nutritional, pharmaceutical, and agricultural fields. Due to their antimicrobial properties, plant extracts have been suggested to be used as antioxidant and preservatives in food products, to incorporate into food packaging materials, plant and crop protectants against insect pests, and medicinal products for human and livestock [6]. In recent times, plant extracts have gained great interests especially in food industry. Most plant extracts are classified as generally recognized as safe by U.S Food and Drug Administration, and are easily degradable in human body [7,8]. Previous studies have been proven that many spices and plant essential oils exhibited inhibition and/or bactericidal effects against L. monocytogenes in food products. For example, cinnamon essential oil and oregano reduced the growth rate of L. monocytogenes by 10% and 19% respectively in ham at 4 °C [9]. Thyme and clove essential oils reduced populations of L. monocytogenes in zero-fat beef hotdogs by 1.3 log CFU/g and 1.0 log CFU/g respectively with 5 min treatment at room temperature (21 °C) [10]. The objective of this study is to evaluate potential inhibitory and bactericidal effects of nine herb extracts (HEs) against foodborne pathogenic L. monocytogenes in vitro in order to select natural antimicrobial agents for the control of foodborne L. monocytogenes in food products.
Introduction
Experimental design
HEs can be used to inhibit the growth of foodborne pathogen and/or reduce pathogen populations. In this study, we conducted three experiments to evaluate their potential uses as antimicrobial agents in food products. In experiment 1 (Exp. 1), a minimum inhibitory concentration (MIC) study was conducted to compare inhibitory effects of each of nine HEs against each of five L. monocytogenes strains individually in Mueller-Hinton broth (MHB). In experiment 2 (Exp. 2), the HE with the lowest MIC was used to determine its reductions of each of five L. monocytogenes strains individually at 37 °C for 30 min in MHB. In experiment 3 (Exp. 3), those HEs which could inhibit the L. monocytogenes growth in Exp. 1 were evaluated for their inhibitory effects against a five-strain L. monocytogenes cocktail in MHB up to 11 days at 12 °C.
Five strains of L. monocytogenes which isolated from epidemics were used in this study and are listed in Table 1. According to [11], these five L. monocytogenes strains were selected from a total of 46 strains which represented a genetic diversity of ribotypes, pulsedfield gel electrophoresis types, serotypes, and lineages. In addition, these five strains are believed to cover the genetic diversity of human disease- associated L. monocytogenes and to provide a valuable tool for evaluating the effectiveness of antimicrobials to inactivate or inhibit L. monocytogenes. Therefore, we used these five genetically distinct strains of L. monocytogenes to evaluate inhibitory and bactericidal efficacies of nine HEs. All strains were activated from 20% glycerol frozen stocks (-80 °C) by two transfers in tryptic soy broth (TSB) (Difco, Spark, MD) at 37 °C for 24 h and were subsequently subculture on tryptic soy agar (TSA) (Difco, Spark, MD) at 37 °C for 24h. Each activated strain was kept on TSA plates at 4 °C.
Herb extracts preparation
A total of nine types of herbs were obtained in the form of powder. Each of nine herbs was extracted with sterile deionized water followed by the procedure of [12] with modification. The HEs were prepared before the day of experiment. Each of the HEs was made by combining 10g of each herb powder with 90 mL of sterile deionized water, incubating in a water bath at 45 °C for 30 minutes, and then boiling for 15 minutes. Each of the nine HEs was then cooled to room temperature and was centrifuged at 6000 x g for 15 minutes at room temperature (Thermo Scientific Sorvall Legend X1R Centrifuge, Am Kalkberg, Germany). The supernatant of each HE was transferred into a 50 mL polypropylene tube and stored at 4 °C until use next day.
Exp. 1: Determining MICs of the HEs
Each strain of the L. monocytogenes listed in Table 1 was inoculated in TSB individually and was incubated at 37 °C for 24h. After the incubation, each strain was serially diluted in MHB (Difco, Spark, MD) to approximately 106 CFU/mL. Nine HEs were diluted with the sterile deionized water to six concentration levels: 100, 60, 30, 15, 10, 5 mg/mL. Five mL of each diluted strain was mixed with 5 mL of each diluted HEs in glass sterile test tubes, to make the final concentrations to be 50, 30, 15, 7.5, 5, 2.5 mg/mL for each HEs and approximately 5 x 105 CFU/mL for each strain. Negative control samples were prepared by combining 5mL of each of nine diluted HEs with 5 mL of MHB separately to make the same final herb concentrations for each HE listed above but without inoculum. Positive control samples were prepared by combining 5mL of each diluted strain with 5mL of MHB separately to make same final bacteria concentrations for each diluted strain listed above but without any HE. All tubes were subsequently incubated at 37 °C for 24h. After 24h incubation, all treatment and control samples were visually examined. The lowest herb concentration at which each treatment sample did not show turbidity were designated as the MIC. All tests were performed in two independent replication trails with three samples on each trail (n=6).
Exp. 2: Reduction of L. monocytogenes cells treated with the HE 4
The HE 4 exhibited inhibitory effect against L. monocytogenes with the lowest MIC in Exp. 1. In this experiment, the HE 4 was determined for its reduction of L. monocytogenes cells. After the 24h incubation, each strain was serially diluted in MHB to approximate concentration of 106 CFU/mL. The HE 4 was diluted in sterile deionized water to the concentration of 50 mg/mL. Two mL of each of diluted L. monocytogenes strains was combined with 2mL of the diluted the HE 4 separately, to make a final concentration of 25 mg/mL of the HE and approximate 5 x 105 CFU/mL of each strain. The positive control samples were prepared by combining 2mL of sterile deionized water and 2mL of each of the five diluted strains separately, to make the same concentrations of each strain as the treatment samples but without HE 4. All treatment and control samples were incubated for 30 min at 37 °C. Our preliminary data showed that the HE 4 exhibited the best reductions against each of five L. monocytogenes strains at 37 °C (data not shown). In a previous published study, thyme and clover have been reported to reduce populations of L. monocytogenes after 5 min treatment in peptone water at room temperature (21 °C) [10]. In our study, each of five strains were treated 30 min with HE 4, which was six times longer than [10]. After 30 min treatment, all treatment samples were immediately diluted with sterile deionized water to a concentration of 0.25 mg/mL for the HE 4, in order to terminate its further antimicrobial activity. Our preliminary study has shown at the concentration of 0.25 mg/mL, the HE 4 could not inhibit the growth of each of five L. monocytogenes strains (data now shown). Each of treatment and positive control samples was subsequently serially diluted in 0.1% buffered peptone water (BPW) and each diluted sample were then plated onto tryptic soy agar (TSA) with two duplications. The TSA plates were then incubated for 48 h at 37oC to enumerate the numbers of surviving L. monocytogenes cells. All tests were performed in two independent replication trails with two samples on each trail (n=4).
Exp. 3: Antimicrobial effects of HEs against L. monocytogenes cocktail at abused refrigerated temperature
In Exp. 1, HEs 2, 4, 5 and 8 which inhibited L. monocytogenes growth at or below 50 mg/mL concentrations. In this experiment, those four HEs were evaluated for their inhibitory effects at 12 °C, which represents the abused refrigeration temperature. Each of the five L. monocytogenes strains listed in Table 1 was cultured in TSB separately for 24h at 37 °C. A five-strain L. monocytogenes cocktail was prepared prior to the study. A 10-mL volume of each 24h grown culture was pooled and mixed in a 50 mL sterile falcon tube. After centrifugation at 6000 x g for 15 min at 4 °C, the supernatant was removed. The cell pellet was washed once with a 10-mL volume of phosphate-buffered saline (PBS), and subsequently resuspended in 50 mL PBS. The L. monocytogenes cocktail was serially diluted in MHB to an approximate 5 x 102 CFU/mL concentration.
The HEs 2, 4, 5, and 8 were diluted in sterile deionized water to three levels of concentrations, 6.25, 3.13 and 1.56 mg/mL. Concentrations of HEs were determined based on the preliminary data (data not shown). The treatment samples were prepared by combining 2 mL of each of four diluted HEs and 2mL of the diluted L. monocytogenes cocktail separately in glass test tubes, to make the final concentrations of each of the four HEs at three levels, 3.13, 1.56 and 0.78 mg/mL, and approximately 2.5 x 102 CFU/mL of the L. monocytogenes cocktail. The positive control samples were prepared by combining 2 mL of diluted L. monocytogenes cocktail and 2 mL of sterile deionized water separately but without any HE. Surviving cells from control samples were enumerated immediately after inoculation (day 0). All treatment and control samples were incubated for up to 11 days at 12 °C. All samples were serially diluted in 0.1% BPW and subsequently plated onto two duplicate TSA plates daily from day 1 to day 5, and every two days from day 7 to day 11. TSA plates were incubated for 48h at 37 °C to enumerate the numbers of surviving L. monocytogenes cells. Each treatment sample and control sample were performed in three independent replication trails with two samples on each trail (n=6).
Statistical Analysis
Microbiological data were converted to log CFU/mL prior to the statistical analysis. Statistical analyses were conducted using analysis of variance via the glimmix procedure of SAS (SAS Studio Basic Edition 3.8, SAS Institute, Inc., Cary, N.C.). Least square means were calculated and significant differences between means were detected at the P < 0.05 in the Exp. 2 and at P < 0.001 in the Exp. 3.
Results and Discussion
MICs of nine herb extracts
MIC is defined as the lowest concentration of an antimicrobial agent which prevents visible microbial growth under designed conditions [13]. In this study, the visible microbial growth was determined by comparing the turbidity between treatment samples and control samples after 24h incubation at 37 °C. The MIC value for each of the nine HEs against each strain are shown in Table 2. Four HEs 2, 4, 5 and 8 inhibited the growth of the five L. monocytogenes strains at MIC values ranging from 5 to 50 mg/mL. The other five HEs 1, 3, 6, 7, 9 did not exhibited inhibition effects at up to 50 mg/ mL. Based on the MIC values, the inhibitory effects of those four HEs were ranked from the strongest to weakest as follows: HE 4 (5 mg/mL) > HE 5 (15 mg/mL) > HE 2 (15-30 mg/mL) > HE 8 (50 mg/mL).
The sensitivity to different natural antimicrobials varies in some Gram-positive and Gram-negative bacteria. For example, studies have shown that Gram-positive L. monocytogenes were more sensitive to some essential oils and HEs than Gram-negative E. coli and Salmonella enterica Enteritidis [14-16]; The Ocimum sanctum extract was found to be equally effective against Gramnegative bacteria (E. coli, S. typhimurium and P. aeruginosa) and Gram-positive bacteria (Staphylococcus aureus) [17]; however, Gram-negative pathogens, V. parahaemolyticus and S. typhimurium, were more sensitive to eugenol than Gram-positive S. aureus [18]. As a result of Exp. 1, four out of nine HEs inhibited the growth of L. monocytogenes. Further studies can be conducted to evaluate and compare the antimicrobial effects of those nine HEs against other foodborne Gram- positive and Gram-negative pathogens.
Reduction of L. monocytogenes cells treated with HE 4
In Exp. 2, the HE 4 was chosen to evaluate its reductions of five L. monocytogenes strains individually at 37 °C for 30 min treatment since HE 4 exhibited the strongest inhibition effect with the lowest MIC (5 mg/mL) in Exp. 1. After 30 min incubation with HE 4 at a concentration of 25 mg/mL, differences (P < 0.05) of surviving cells between treatment samples and control samples were observed for each of five L. monocytogenes strains (Figure 1). Cell reductions of HE 4 against five L. monocytogenes strains were calculated: N1-227 (0.91 log CFU/mL), C1-056 (0.87 log CFU/mL), R2-499 (0.85 log CFU/mL), J1-177 (0.59 log CFU/mL), N3-013 (0.38 log CFU/mL).
In a previous published study, at the concentrations of 0.5 mL/L, essential oils of thyme and clover have been reported to reduce populations of L. monocytogenes from 7.2 to 1.8 log CFU/mL and from 7.1 to 1.2 log CFU/mL respectively after 5 min treatment in peptone water at room temperature (21 °C) [10]. In addition, another study indicated that essential oil of origanum reduced populations of each of five L. monocytogenes strains in a range of 1-2 log CFU/mL after 30 min treatment in 0.9% saline solution at room temperature [19]. In our study, Although HE 4 reduced less than 1 log CFU/mL for each strain, populations of surviving cells of each strain were significant (P < 0.05) after HE4 treatment compared with control samples. The result indicated that using HE 4 solely against L. monocytogenes might be less effective than essential oils of thyme, clover and organum. However, there has been increased interests to the use natural antimicrobial agents in their combinations for controlling foodborne pathogens. The effects of the combined substances were observed to be greater than the sum of individual effects against L. monocytogenes in combinations of carvacrol/linalool [20] and oregano/rosemary [21]. HE 4 was expected to be used in combination with other compounds to increase antimicrobial effects.
Inhibitory effects and reductions of four herb extracts against L. monocytogenes cocktail at abused refrigerated temperature
Since HE 2, 4, 5 and 8 exhibited inhibitory effects against L. monocytogenes at 37 °C in Exp. 1, we expected that those four HEs could inhibit L. monocytogenes growth at 12 °C, which represented to the abused refrigerator temperature. We investigated the antimicrobial effects of HEs 2, 4, 5 and 8 at three concentration levels (3.13, 1.56, 0.78 mg/mL) against a five-strain L. monocytogenes cocktail. The initial populations of L. monocytogenes cocktail in control and all treatment samples were 2.3 log CFU/mL. For control samples without any HE, bacteria population rapidly increased from 2.3 log CFU/mL (day 0) to 8.4 log CFU/mL by 4 days, and then increased to 8.8 log CFU/mL by day 7. After 7 days, bacteria population did not have further increase in number. For treatment samples, the growth of L. monocytogenes during refrigerated storage was dependent on the type of herb and HE concentration. In general, compared with control samples, lower bacteria populations (P < 0.001) were observed in all treatments except for the HE 8 at the concentration of 0.78 mg/mL (Tables 3-5).
At a concentration of 3.13 mg/mL (Table 3), HEs 2, 4, 5 and 8 reduced inoculated L. monocytogenes populations from 2.3 log CFU/mL to 0.2, 0.1, 0.7 and 0.5 log CFU/mL at day 11, respectively. Compared with positive control samples without any HE, each of four HEs had lower bacterial population (P < 0.001) on each day from day 1 to day 11. This result indicated that at the concentration of 3.13 mg/mL, all four HEs effectively reduced bacteria populations of L. monocytogenes cocktail at 12 °C.
Table 3: Least square means ± standard deviation of Listeria monocytogenes cocktail populations in inoculated Mueller-Hinton broth with each of four herb extracts at concentration of 3.13 mg/mL or deionized water (control), stored at 12 °C for 11 days (n=6).
In a previous published study, at the concentrations of 0.5 mL/L, essential oils of thyme and clover have been reported to reduce populations of L. monocytogenes from 7.2 to 1.8 log CFU/mL and from 7.1 to 1.2 log CFU/mL respectively after 5 min treatment in peptone water at room temperature (21 °C) [10]. In addition, another study indicated that essential oil of origanum reduced populations of each of five L. monocytogenes strains in a range of 1-2 log CFU/mL after 30 min treatment in 0.9% saline solution at room temperature [19]. In our study, Although HE 4 reduced less than 1 log CFU/mL for each strain, populations of surviving cells of each strain were significant (P < 0.05) after HE4 treatment compared with control samples. The result indicated that using HE 4 solely against L. monocytogenes might be less effective than essential oils of thyme, clover and organum. However, there has been increased interests to the use natural antimicrobial agents in their combinations for controlling foodborne pathogens. The effects of the combined substances were observed to be greater than the sum of individual effects against L. monocytogenes in combinations of carvacrol/linalool [20] and oregano/rosemary [21]. HE 4 was expected to be used in combination with other compounds to increase antimicrobial effects.
Inhibitory effects and reductions of four herb extracts against L. monocytogenes cocktail at abused refrigerated temperature
Since HE 2, 4, 5 and 8 exhibited inhibitory effects against L. monocytogenes at 37 °C in Exp. 1, we expected that those four HEs could inhibit L. monocytogenes growth at 12 °C, which represented to the abused refrigerator temperature. We investigated the antimicrobial effects of HEs 2, 4, 5 and 8 at three concentration levels (3.13, 1.56, 0.78 mg/mL) against a five-strain L. monocytogenes cocktail. The initial populations of L. monocytogenes cocktail in control and all treatment samples were 2.3 log CFU/mL. For control samples without any HE, bacteria population rapidly increased from 2.3 log CFU/mL (day 0) to 8.4 log CFU/mL by 4 days, and then increased to 8.8 log CFU/mL by day 7. After 7 days, bacteria population did not have further increase in number. For treatment samples, the growth of L. monocytogenes during refrigerated storage was dependent on the type of herb and HE concentration. In general, compared with control samples, lower bacteria populations (P < 0.001) were observed in all treatments except for the HE 8 at the concentration of 0.78 mg/mL (Tables 3-5).
At a concentration of 3.13 mg/mL (Table 3), HEs 2, 4, 5 and 8 reduced inoculated L. monocytogenes populations from 2.3 log CFU/mL to 0.2, 0.1, 0.7 and 0.5 log CFU/mL at day 11, respectively. Compared with positive control samples without any HE, each of four HEs had lower bacterial population (P < 0.001) on each day from day 1 to day 11. This result indicated that at the concentration of 3.13 mg/mL, all four HEs effectively reduced bacteria populations of L. monocytogenes cocktail at 12 °C.
Table 3: Least square means ± standard deviation of Listeria monocytogenes cocktail populations in inoculated Mueller-Hinton broth with each of four herb extracts at concentration of 3.13 mg/mL or deionized water (control), stored at 12 °C for 11 days (n=6).
At the concentration of 1.56 mg/mL (Table 4), HE 2 reduced L. monocytogenes populations from 2.3 log CFU/mL to 0.2 log CFU/ mL at day 11, which was 8.6 log CFU/mL lower (P < 0.001) than the control. Although counts of L. monocytogenes in the sample with HE 5 increased from 2.3 log CFU/mL to 4.0 log CFU/mL at day 11, it was still 4.8 log CFU/mL lower (P < 0.001) than the control. However, compared with the control, HE 4 and 8 were lower (P < 0.001) in bacteria populations only up to 5 days. Therefore, antimicrobial effects of those four HE at concentration of 1.56 mg/ mL were ranked from the strongest to weakest as follows: HE 2 > HE 5 > HE 4 = HE 8.
Table 5 shows the inhibitory effects of each of the four HEs in MHB at the concentration of 0.78 mg/mL. Counts of the L. monocytogenes cocktail in the sample with HE 8 were not different (P > 0.001) from the control sample on each day from day 1 to day 11, indicating that HE 8 at a concentration of 0.78 mg/mL could not inhibit bacterial growth. Counts of samples with HE 4 or 5 increased from 2.3 log CFU/ mL to 6.7 and 6.4 log CFU/mL by day 4 respectively, which were lower (P < 0.001) than the control by about 2 log CFU/mL. After 4 days of incubation, the bacterial population of the sample with HE 4 were not different (P > 0.001) with the positive control sample on each day from day 5 to day 11. Although the sample with HE 5 did not show different (P > 0.001) in bacteria population with the positive control sample at day 5 and day 7, the population of HE 5 was 0.5 log CFU/mL lower (P < 0.001) than the control at day 9 and day 11. In addition, comparing with positive control samples, HE 2 slowed the microbial growth and reached to 5.6 log CFU/mL by day 5, which was lower than the controls for 3 log CFU/mL (P < 0.001). After 7 days of incubation, the bacterial population of the sample with HE 2 were not different (P > 0.001) with the control sample on each day from day 7 to day 11. In summary, at the concentrations of 0.78 mg/mL, HE 2 inhibited the microbial growth up to 5 days; HE 4 and 5 inhibited L. monocytogenes growth up to 4 days; HE 8 could not inhibit the microbial growth.
The demand for convenience foods such as RTE foods has increased in recent years. The majority of listeriosis cases are foodborne [22] and linked to the consumption of RTE foods which are contaminated with L. monocytogenes. Due to the high mortality rate of listeriosis, the U.S. Department of Agriculture and the FDA labels L. monocytogenes as an adulterant of RTE foods. Examples of RTE foods that support the growth of L. monocytogenes are milk, high fat dairy products, soft unripened cheese, cooked and raw seafood, deli-type salads, sandwiches, fresh-cut vegetable and fruits [23] and the processed meat which is under refrigerator conditions [24]. Although L. monocytogenes will continue to thrive at low temperature as 1.1 °C [25] the storage temperature and duration of refrigerated storage before consumption are important factors which reduce the risks of foodborne listeriosis [26]. The recommended refrigerator temperature is 40 °F (4.4 °C); however, abuse home refrigerator temperature can rise to more than 12 °C [26,27].
Previous published studies indicated that the inhibitory efficacies of plant-derived antimicrobials may be affected by temperature [28,29]. The results from Exp. 1 showed that HEs 2, 4, 5 and 8 exhibited inhibitory effects against each of five L. monocytogenes strains at 37 °C. However, in order to use those four HEs as food preservatives, they must be effective against L. monocytogenes under food storage conditions. In this experiment, inhibition efficacies of those four HEs were evaluated at 12 °C which represented the abused refrigerator temperature. As discussed above, at concentrations of 1.56 and 0.78 mg/mL, HEs 2, 4, 5 and 8 inhibited growth of a five-strain L. monocytogenes cocktail at abuse refrigeration temperature of 12 °C, except herb extract 8 at the concentration of 0.78 mg/mL. At a concentration of 3.13 mg/mL, these four HEs reduced cell populations in a range of 2.2 to 1.6 log CFU/mL at 11 days. In a previous study, thyme essential oil showed the inhibitory effect against L. monocytogenes cocktails at 10 °C up to 12 days in minced beef [30]. HEs 2, 4, 5 and 8 were also expected to be developed into food preservatives for inhibiting and/or reducing foodborne L. monocytogenes. For example, those four HEs could be added to RTE foods as supplements or incorporated into food packaging materials to control L. monocytogenes growth. Further experiments should be conducted to determine the inhibitory effects and reductions of those four HEs in food products. In addition, since HEs carry specific odor, palatability of the food applied with HEs should be evaluated by sensory panel.
Conclusion
In summary, HEs 2, 4, 5 and 8 exhibited inhibitory effects against L. monocytogenes at 37 °C in a range of MIC between 5 - 50 mg/mL. HE 4 reduced cell populations of each selected strain ranged between 0.38 -
0.91 log CFU/mL after 30 min treatment at 37 °C. In addition, at concentrations of 1.56 and 0.78 mg/mL, HEs 2, 4, 5 and 8 inhibited growth of a five-strain L. monocytogenes cocktail at 12 °C, except the HE 8 at the concentration of 0.78 mg/mL. At a concentration of 3.13 mg/mL, these four HEs reduced cell populations in a range of 2.2 to 1.6 log CFU/mL at 11 days. For their practical application, further experiments should be conducted to determine the inhibitory effects and reductions of those HEs in a variety of food products. In addition, palatability of the foods which applied with HEs should be evaluated by sensory panel.
#agriculture#Agriculture and Soil Science#journal of agriculture#Inter National Agriculture Science#soil science#Food science
1 note
·
View note
Link
PREPARATION OF MUELLER HINTON AGAR MEDIUM (MHA MEDIUM) The well-known solid media for the Antibiotic Sensitivity Test a.k.a. Antibiotic Susceptibility Test was developed by John Howard Mueller and Jane Hinton in 1941 and named as Mueller Hinton Agar. This Mueller Hinton agar...
1 note
·
View note
Text
Get the Best Quality of Mueller Hinton Agar (TM 339) at TM Media
If you are looking for Mueller Hinton Agar manufactured by TM Media, initially designed for pathogenic Neisseria species, evolved into a cornerstone for antimicrobial susceptibility testing. Widely recognized, it replaced other media for sulfonamide resistance assessments. Its selection by CLSI stems from reproducibility, low inhibitor content, support for bacterial pathogen growth, and a wealth of performance data. The Kirby-Bauer method, employing paper discs with specific antimicrobial concentrations, defines susceptibility. This versatile agar, endorsed by the WHO, also finds application in testing Streptococcus pneumoniae and Haemophilus influenza. While robust for most pathogens, Mueller Hinton Agar's limitations include unsuitability for slow growers, anaerobes, and capnophiles in disc diffusion assays.
Visit the Website - https://www.tmmedia.in/

0 notes
Text
MUELLER HINTON AGAR
Mueller Hinton Agar, originally designed for cultivating pathogenic Neisseria species, has found an important role in antimicrobial susceptibility testing. Its selection by the CLSI is backed by several key factors:
1. Consistent Performance: It exhibits reliable batch-to-batch reproducibility for susceptibility testing.
2. Low Interference: It contains minimal sulfonamide, trimethoprim, and tetracycline inhibitors.
3. Broad Applicability: It supports the growth of a wide range of non-fastidious bacterial pathogens.
4. Established Track Record: The extensive data and experience in its use reinforce its reliability.
The Kirby-Bauer method, based on Mueller Hinton Agar, involves the diffusion of antimicrobial agents from paper discs. This technique, using discs with a fixed concentration of the agent, relies on measuring the zones of inhibition around each disc, which are then compared to CLSI standards to determine susceptibility. Factors affecting disc diffusion susceptibility tests include agar depth, disc strength, inoculum concentration, medium pH, and the presence of beta-lactamase in test organisms. However, it's important to note that Mueller Hinton Agar is not suitable for slow-growing organisms, anaerobes, and capnophiles due to potential inaccuracies in readings.
0 notes
Text

Mueller hinton agar plate
The Mueller Hinton Agar Plate is meticulously crafted for the cultivation of Neisseria species and the vital assessment of microorganism susceptibility to antibiotics. Originating from the collaborative work of Mueller and Hinton, this medium was initially designed for the primary isolation of Neisseria species. Over time, it has evolved to become the gold standard for antimicrobial susceptibility testing, aligning with the guidelines set by the Clinical and Laboratory Standard Institute (CLSI), formerly known as NCCLS. Additionally, its composition adheres to the stringent criteria set forth by the World Health Organization (WHO), the Food and Drug Administration (FDA), and the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
#mueller hinton agar plate#Ready-To-Use Culture Media#Ready-To-Use Media Plates#mueller hinton agar manufacturer#microbiology
1 note
·
View note
Text
Se hinton tex pdf conversion
SE HINTON TEX PDF CONVERSION >> DOWNLOAD LINK
vk.cc/c7jKeU
SE HINTON TEX PDF CONVERSION >> READ ONLINE
bit.do/fSmfG
Download Tex by S.E. Hinton in PDF EPUB format complete free. Brief Summary of Book: Tex by S.E. Hinton. Easygoing, thoughtless, and direct, Tex at fifteen likes everyone and everything, especially his horse, Negrito, and Johnny Collins's blue-eyed sister, Jamie. Tex Se Hinton Tex is a novel by S. E. Hinton, published in 1979.It was adapted to the film in 1982, which starred Matt Dillon.The book (like Rumble Fish and That Was Then, This Tex (film) - Wikipedia Author S.E. Hinton | Submitted by: Jane Kivik Free download or read online Tex pdf (ePUB) book. abiword --to=tex filename.pdf. Be warned that both its PDF import routine and its LaTeX export routine have serious limitations, and you should not expect anything that will be usable without serious tweaking afterwards. (* IIRC, the extra plugins are installed by selecting a custom install on Windows Tex By S E Hinton Questions.pdf .doc - Free Download billions of texts, documents, materials, books, textbooks, poems, and stories around the world! Hinton, S E Hinton Pdf, S. E. Hinton, Se Hinton, What Is S E Hinton Doing Now, If You Like S E Hinton, S.e Hinton Age, What Is S E Hinton Known Acces PDF Tex Se Hinton. Tex by S.E. Hinton 10,526 ratings, 3.84 average rating, 451 reviews Tex Quotes Showing 1-7 of 7 "Man, I didnt know anything like that was going to happen! Tex Summary & Study Guide - BookRags.com. Tex By Se Hinton|Tex (film) - WikipediaTexFree Tex Worksheets and File Format: PDF/Adobe Acrobat. Tex by S. E. Hinton - PDF free download eBook. Susan Eloise Hinton , better known by her pen name S.E. Hinton , was born in Oklahoma in I tried convert file ipynb to pdf, but occur this errors. [NbConvertApp] Converting notebook 7-CALCULO-MATEMATICA-SIMBOLICA.ipynb to pdf [NbConvertApp] Writing 22483 bytes to notebook.tex [NbConvertApp] Building PDF [NbConvertApp] Runnin Tex By S E Hinton. That Was Then This Is Now Wikipedia. Amazon com The Outsiders eBook S E Hinton Kindle Store. What books from S E Hinton 'does tex s dad ever come home in the book tex by se hinton may 10th, 2018 - answer to s ehinton question some of the known books that s e hinton You may have a PDF compiled from a TEX file. You want to insert more equations or re-typeset math formulas. But barely anything can be passed to the As a matter of fact, it works as a PDF to LaTeX converter as well. When it comes to PDF to LaTeX conversion, no application can do it better than Tex_-_SE_Hinton.pdf. Downloaded 0 times. size 1.8 MB. Free download or read online Tex pdf (ePUB) book. The first edition of the novel was published in January 1st 1979, and was written by S.E. Hinton. The book was published in multiple languages including English, consists of 224 pages and is available in Paperback format. tex se hinton is available in our book collection an online access to it is set as public so you can download it instantly. Our digital library hosts in multiple countries, allowing you Tex (film) - Wikipedia Author S.E. Hinton | Submitted by: Jane Kivik Free download or read online Tex pdf (ePUB) book. tex se hinton is available in our book collection an online access to it is set as public so you can download it instantly. Our digital library hosts in multiple countries, allowing you Tex (film) - Wikipedia Author S.E. Hinton | Submitted by: Jane Kivik Free download or read online Tex pdf (ePUB) book. [EPUB] Tex Se Hinton PDF Books this is the book you are looking for, from the many other titlesof Tex Se Hinton PDF books, here is alsoavailable other sources of this Manual MetcalUser Guide BDTM Mueller Hinton II Agar BD Mueller Hinton II Agar 150 Principles and explanation of the
https://finepexif.tumblr.com/post/666746905059098624/acsms-health-related-physical-fitness-assessment, https://nolaneputem.tumblr.com/post/666745681794498560/tv-directo, https://hijararev.tumblr.com/post/666362127813165056/kity-617-manual, https://luqubonaco.tumblr.com/post/666738083756195840/brother-dcp-l2540dw-manual-pdf, https://pofovibudam.tumblr.com/post/666360907806457856/targus-defcon-1-pa400-manual.
1 note
·
View note
Text
Types of Microorganisms at Nosocomial Infections on the Surface of Laryngoscope Before Intubation at General Anesthesia_Crimson Publishers
Types of Microorganisms at Nosocomial Infections on the Surface of Laryngoscope Before Intubation at General Anesthesia by Asghar Karbord in Cohesive Journal of Microbiology & Infectious Disease
https://crimsonpublishers.com/cjmi/fulltext/CJMI.000564.php

Introduction: Laryngoscope is using for endotracheal intubations. Inadequate decontamination of laryngoscope can develop nosocomial infections. If the blade isn’t disconnecting from laryngoscope after intubation can be transmit infections to handle. This study considering prevalence and types of bacteria isolated separately from laryngoscope blades and handles.
Method: This cross-sectional study was in the operating rooms of the educational and treatment center of Qazvin University of Medical Science in province Qazvin in Iran at 2018. 40 Laryngoscope blades and 40 laryngoscope handles were sampled after disinfection, with 4 methods disinfection: (1-Water, Povidon Iodine 7.5%, Ethanol 70%), (2-Water, Povidon Iodine 7.5%, Deconex (guaifenesin and phenylephrine 53 plus), (3-Water, Povidon Iodine 7.5%) and (4-Ethanol 70%)”. Samples were cultured on Mueller Hinton 5% sheep blood agar plate, MacConkey agar and Manitol salt agar. Inoculating plates were incubated at 37 ᵒC for 48 hours. Dominant microorganism and other growth bacteria identified comparatively.
For more open access journals in Crimson Publishers, please click on link: https://crimsonpublishers.com/
For more articles in Infectious Disease Open Access Journals, Please click on link: https://crimsonpublishers.com/cjmi/
0 notes
Text
Antimicrobial testing, Microbiological testing laboratory
Most of the irresistible sicknesses are bacterial in the beginning. With the revelation of research center strategies to develop these microorganisms utilizing a suitable development medium known as "culture," deciding the responsiveness and obstruction of explicit microbes to a large number of antimicrobial specialists becomes fundamental so medical services suppliers can promptly organize legitimate therapy regimens to their patients.
Antimicrobial testing is a lab strategy performed by clinical technologists (clinical lab researchers) to distinguish which antimicrobial routine is explicitly powerful for individual patients. For a bigger scope, it supports the assessment of treatment administrations given by clinics, centers, and public projects for the control and counteraction of irresistible illnesses. As of late, scientists have needed to execute persistent observation exercises for obstruction designs because of the transformations in bacterial DNA.
Clinical research facilities right now utilize a few techniques relying upon the lab test menu that they give. These methodologies incorporate the circle dissemination and least inhibitory fixation (MIC) techniques. Business frameworks additionally opened up across wellbeing focuses and emergency clinic offices, using both phenotypic and genotypic portrayal of bacterial obstruction. While routine antimicrobial vulnerability testing for gram-positive (e.g., Staphylococcus aureus) and gram-negative microorganisms (e.g., Pseudomonas aeruginosa) are ordinarily accessible in fringe labs, drug helplessness testing (DST) for Mycobacterium tuberculosis are normally completed inside additional mind boggling offices like reference research facilities. In spite of the distinctions in the procedures for vulnerability tests, all research centers should be basic on each step of the examining and testing process so that experimental outcomes are reachable with reliably elevated degrees of precision and unwavering quality.

Procedure
Both circle dispersion and MIC strategies utilize the phenotypic distinguishing proof of vulnerability, and consequently, requires the accompanying system:
• Planning of a normalized inoculum from a bacterial culture:
• Picking very much detached settlements
• Making a bacterial suspension (inoculum)
• Normalizing the bacterial suspension utilizing McFarland principles
• Weakening of bacterial suspension (just for MIC technique)
• Vaccination of bacterial suspension to one of the accompanying:
• A specific development medium (e.g., Mueller Hinton Agar, MHA for circle dispersion)
• A MIC board
• Expansion of antimicrobial circles (just for plate dispersion)
• Brooding of plates (circle dispersion) or boards (MIC)
• Estimating the zone of restraint or perusing MIC board
• Understanding of AST results
Indications
Susceptibility testing for antimicrobials is essential for patients who raise doubt of contamination with explicit microorganisms in light of sickness signs and clinical connection. Antibacterial specialists are then used to recognize awareness or opposition from microbes. Albeit the reason for this audit is principally towards the vulnerability testing for bacterial microbes, it is vital to take note of that antifungal helplessness tests additionally exist for tending to contagious disease (e.g., Candida, Aspergillus spp.). Besides, antiviral powerlessness tests are additionally accessible (e.g., flu) by means of sub-atomic advances including sequencing investigation like Sanger and pyrosequencing techniques.
Potential Diagnosis
A one of a kind effect of AST on tolerant administration is the distinguishing proof of the particular conclusion, and furthermore, focusing on the specific etiologic specialist causing the infection. No two patients can be overseen also, particularly on the off chance that they have similar signs and side effects (illness indication) however with various treatment regimens on the grounds that a similar causative creature can have different opposition designs. For instance, two patients might give a common type of Staphylococcus aureus versus methicillin-safe Staphylococcus aureus (MRSA); and another model would be patients with drug-vulnerable (DS-TB) and medication safe tuberculosis.

Normal and Critical Findings
For circle dissemination, estimating the zone of restraint is finished by utilizing a committed caliper. Accurately measure the distance across by the edges of the restraint zone. For MIC boards, perusing each arrangement of wells for an antimicrobial medication is finished. MIC assurance is by either a reasonable or slight whiteness on the well. Announcing the aftereffects of the hindrance zones and MIC breakpoints is made utilizing either the expressions ``powerless" or "safe" in view of the set cut-off range for zone distance across in the closest entire millimeter and microgram per milliliter, separately. The Clinical Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) created master endorsed rules on breakpoints for detailing consequences of these techniques.
Interfering Factors
A few variables influence the consequence of the helplessness testing which covers the entire inspecting, testing, and revealing systems. Any deviation from the standard AST methodology can fundamentally affect succeeding areas of the research center work process which thus would later influence patient conclusion, treatment, and the board. Emotionally supportive networks of the lab work process require severe observation, and lab staff ought to be thoroughly prepared and skilled enough to carry out the technique.
Complications
Irregularities in the AST results should be researched and followed up on right away. No outcomes ought to be delivered when quality control measures are not good. Delivering incorrect medication defenselessness or opposition results can incur more damage to the patients, prompting extreme clinical circumstances and unfortunate visualization. A more terrible outcome in conveying bogus AST results can bring about off-base treatment of the executives' plans which could make further transformations of these irresistible life forms, uncovering the patients and the local area to a higher gamble.
Patient Safety and Education
Patients ought to be sufficiently educated about the AST and its signs, patient necessities, and its clinical use for patient administration. Medical services suppliers like doctors, lab work force, attendants, and pharmacologists are urged to spread right data about the test. In any case, understanding of the AST results should happen between the patient and the doctor to work with great consistency with the recommended prescriptions and to forestall self-drug. With the ascent of antimicrobial obstruction, the significance of AST requires an accentuation on clinical, research center, and nursing staff, as well as patients and their relatives, and the entire local area prompting a bound together methodology.
Clinical Significance
When antimicrobial vulnerability results become accessible, treatment regimens for every patient can be created by medical services suppliers. Recommended prescriptions of proper anti-toxins need individualization for every patient determined to have an irresistible infection. Besides, obstruction from essential medications will require a more significant level of antimicrobial stewardship, including judicious utilization of second-line drugs.
0 notes