#edit: the wa y i already changed the colouring -___-
Photo




Bang Chan for Men’s Folio
#chan#bang chan#stray kids#skz#cb97net#createskz#*gifs#*m#; ______________ ;#im so annoying this didnt have to be this long but it is <3#bestie said support by getting a copy today.. babie i;ll get all the copies if it means you get that bag#im really sitting here crying at my laptop at 7:18 am someone should seek help (me its me !!!!!!!!!!!!!!!!!!!!!!! im someone!!!!!)#these are ugly but i had 2 do it </3#i dont even know why i got so fucking emotional i just DID <3 !#im so sad.. he looks so soft n tiny and cute...#edit: the wa y i already changed the colouring -___-#whatever bro </3#ill fix this later in the day idc rn#im tired
1K notes
·
View notes
Text
Allergic to the Sun?: A Genetic Look into X-Linked Protoporphyria
Advancement in genetics have assisted doctors and scientists around the world in identifying the genetic causes behind some of the most puzzling disorders. These advancements have been most helpful with Mendelian disorders – those that are caused by a mutation in only one gene. While these disorders are rare, the results of a single mutation can be dramatic. I am going to focus on one in particular: X-Linked Protoporphyria (XLP). No, not the porphyria from which King George III allegedly suffered. This porphyria’s main symptom is photosensitivity, colloquially known as a sun allergy.
The name sun allergy is actually a misnomer. An allergy refers to an immune system response (ex. hay fever) while XLP is actually a chemically induced skin irritation (similar to a sun burn, pictured below) caused by an overabundance of a compound called a protoporphyrin. In XLP, this overabundance is caused by a single mutation in one gene. For those whose high school biology days are a bit too far away to remember, a mutation is an alteration in the DNA code. A gene is a portion of the DNA that gives the instructions for the creation of proteins. The protein affected by XLP is called ALAS2.
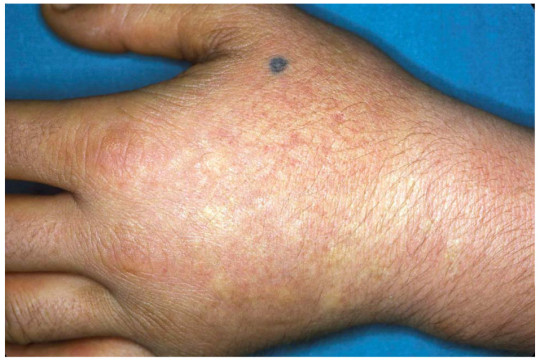
Acute photosensitivity reaction
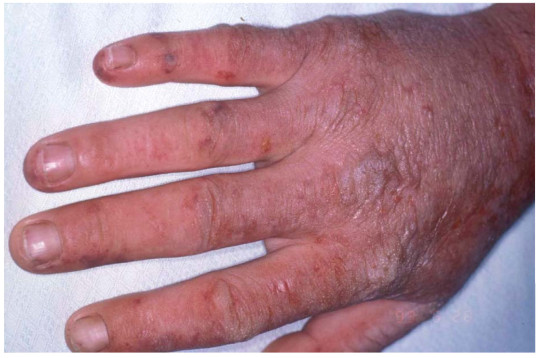
Another acute photosensitivity reaction
Symptoms of XLP show at infancy but vary in severity between males and females. The symptoms include photosensitivity (a painful skin reaction to sunlight), anemia, and low iron levels in the blood. Uncommonly, those affected can have liver damage which can eventually lead to the need for a liver transplant. Unfortunately, the number of people affected with XLP is hard to determine since it is so rare and very similar to another porphyria, Erythropoietic Protoporphyria. So far, a dozen or so families have been identified and there seems to be no correlation to geographic location or ethnicity. It affects everyone equally.
Knowing that other porphyrias are related to a mutation in the DNA, doctors aimed to find the mutation that caused XLP. This relied on many past studies, the largest of which was the Human Genome Project. It amassed a huge amount of information on what many of our genes do and where they are located.
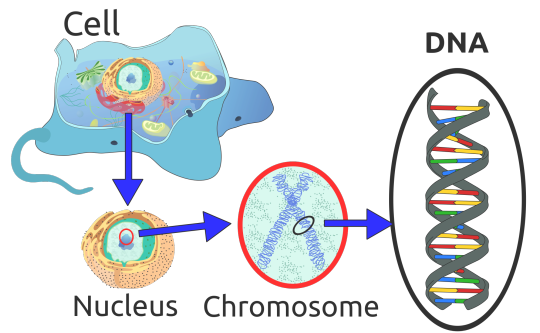
DNA in Human Cell

Human chromosomes, coloured by UCSC browser default colours
As you can see above, each human cell contains all 23 pairs of chromosomes within it’s nucleus. Each pair contains part of your DNA and it’s own unique genes. Which chromosome and gene would XLP be found in? Using previous studies, Whatley et al made an educated guess that is was in a gene called ALAS2 (5-aminolevulinic acid synthase), named after the protein it’s “blueprints” create. ALAS2 is located on the X chromosome, number 23. Females have two X chromosomes and males have one X and one Y, hence the name sex chromosomes. The fact that it is only on the X chromosome means that it is “linked” to that chromosome, so the mutation only shows up in offspring if the affected X chromosome is inherited. Daughters get one X chromosome from their father and one from their mother. Sons, however, get one Y chromosome from their father and one X chromosome from their mother. Depending upon which parent’s X chromosome is affected, and which one gets passed on, affects which children inherit the mutation.

X-Linked Dominant Inheritance
Since XLP doesn’t get passed to every child, the image above helps to show part of why XLP was difficult to identify. The first scientists to do so was a team lead by Sharon D. Whatley that published the find in 2008.
While everyone has an ALAS2 gene, as it is part of blood cell production, not everyone has the mutation. However, scientists know that the ALAS2 gene is found on chromosome X, so that’s where Whatley et al looked. Below is an X portion of chromosome 23 with the area containing the ALAS2 gene highlighted. It turns out that Whatley et al was right and further studies have identified other mutations within the ALAS2 gene that also cause XLP.
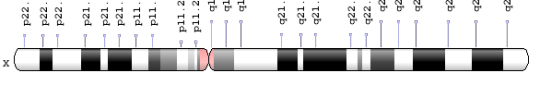
ALAS 2 Gene Location
After identifying the location of the mutation, scientists attempted to find why the mutation causes the multitude of symptoms. They started by looking at what the ALAS2 protein does. It had already been found that this protein is involved in your blood cell production, which is involved in creating protoporphyrins. It turns out that the ALAS2 mutations change the “blueprints” (DNA) enough to change the shape of the ALAS2 protein. Below is an example structure of non-mutated ALAS2 protein.

Crystal Structure of ALAS2 Protein from R. capsulatus
The change in shape, or conformation, causes the protein to do it’s work faster. Due to this, a chemical chain reaction occurs and eventually leads to too many protoporphyrins within a patient’s body. The main pathway involved is called the heme pathway, and as you may have guessed, it’s involved in blood cell production.
Since blood cell production and it’s related compounds are so important, there are many other diseases related to them, including the porphyria King George III was theorized to suffer from. Many of the other diseases and porphyrias have distinct symptoms, but one in particular, Erythropoietic Protoporphyria (EPP), is so similar to XLP that it is nearly impossible to tell them apart without a genetic test. In fact, many people diagnosed with EPP may have XLP but have never been tested. Currently, there are only 3 testing locations that can identify XLP through a genetic test: the Mayo Clinic in Rochester, MN, Icahn School of Medicine at Mount Sinai in New York, NY, and Odense University Hospital in Odense, Denmark.
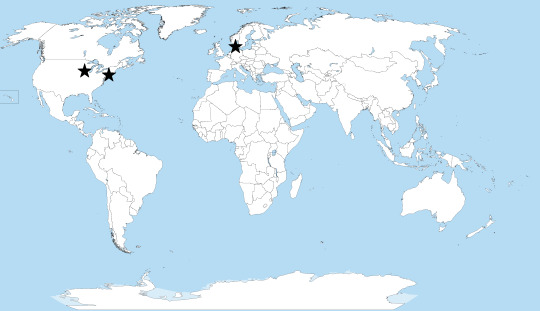
Locations of XLP Testing Locations (edited from original)
Even with the identification of XLP as the cause of symptoms, doctors have not found a cure for it or an FDA-approved treatment. However, doctors have figured out numerous ways to manage the symptoms. The main method is sunlight avoidance which prevents the chemical reaction with porphyrins in a patient’s skin from occurring. There are many UV-protective clothes now, but staying inside is key. Some drugs have been shown to improve tolerance to sunlight, such as Lumitene or Cysteine and another drug has been shown to absorb some porphyrins, Cholestyramine. A new medication, Afamelanotide, is in clinical trials and may provide another option for sunlight protection.
None of these treatments would have been possible if the underlying mutation and subsequent chemicals had not been identified. Since the 90s and the Human Genome Project, technology has advanced rapidly. Sequencing, the method of reading DNA, used to cost millions. Now, it costs hundreds to thousands depending upon how much of the DNA you want to read. This decrease led to the birth of genomics, a discipline that uses genetics and molecular biology to identify the structure, function, and evolution of entire genomes (all of the genes within one person or species). So many different diseases and disorders have been identified, and it was mainly possible due to what is called open-source software. Doctors and scientists worked together by posting their findings on the internet, which are freely available for anyone to view. In sharing this knowledge, they were able to build upon each other’s work. This is exactly how Whatley and her team were able to identify the XLP mutation.
Identifying the genetic components of diseases and disorders is the first step in treating and curing them. The birth of genomics was a direct result of advancement in computer technologies. Without the internet, increased computing capabilities, and larger data storing methods, the human genome may not have been mapped. The faster our technology advances, the faster we can understand the role our genes play in our lives. This leads to identifying cures not only for rare disorders like XLP, but for more common diseases/disorders, such as cancer, Alzheimer’s, and Huntington’s.
References:
1. “X-Linked Protoporphyria – NORD (National Organization For Rare Disorders)”. NORD. N. p., 2013 & 2016. Web. 12 June 2017.
2. Whatley, Sharon D. et al. “C-Terminal Deletions in the ALAS2 Gene Lead to Gain of Function and Cause X-linked Dominant Protoporphyria Without Anemia or Iron Overload”. The American Journal of Human Genetics 83.3 (2008): 408-414. Web. 12 June 2017.
3. “Homologene – NCBI”. Ncbi.nlm.nih.gov N.p., 2017. Web. 12 June 2017.
4. “OMIM Entry – “300752 – PROTOPORPHYRIA, ERYTHROPOIETIC, X-LINKED; XLEPP”. Omim.org. McKusick, Victor A., and Kniffin, Cassandra L.,2017. Web. 12 June 2017.
5. Balwani M, Bloomer J, Desnick R; Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. X-Linked Protoporphyria. 2013 Feb 14. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Web. 12 June 2017.
6. Balwani, Manisha et al. "Loss-Of-Function Ferrochelatase And Gain-Of-Function Erythroid-Specific 5-Aminolevulinate Synthase Mutations Causing Erythropoietic Protoporphyria And X-Linked Protoporphyria In North American Patients Reveal Novel Mutations And A High Prevalence Of X-Linked Protoporphyria.". Mol Med 19 (2013): 26-35. Web. 21 June 2017.
7. Fratz, Erica J. et al. "Human Erythroid 5-Aminolevulinate Synthase Mutations Associated With X-Linked Protoporphyria Disrupt The Conformational Equilibrium And Enhance Product Release". Biochemistry 54.36 (2015): 5617-5631. Web. 21 June 2017.
8. Fratz-Berilla, Erica J. et al. "Isoniazid Inhibits Human Erythroid 5-Aminolevulinate Synthase: Molecular Mechanism And Tolerance Study With Four X-Linked Protoporphyria Patients". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1863.2 (2017): 428-439. Web. 21 June 2017.
9. Minder, Elisabeth I. "Afamelanotide, An Agonistic Analog Of Α-Melanocyte-Stimulating Hormone, In Dermal Phototoxicity Of Erythropoietic Protoporphyria". Expert Opinion on Investigational Drugs 19.12 (2010): 1591-1602. Web. 21 June 2017.
10. Minder, El., Schneider-Yin, X., Steurer, J., Bachmann, L.M. "A Systematic Review Of Treatment Options For Dermal Photosensitivity In Erythropoietic Protoporphyria". Cell Mol Biol (Noisy-le-grand) 55.1 (2009): 84-97. Web. 21 June 2017.
11. "ALAS2 - 5-Aminolevulinate Synthase, Erythroid-Specific, Mitochondrial Precursor - Homo Sapiens (Human) - ALAS2 Gene & Protein." Uniprot.org. N.p., 2017. Web. 21 June 2017.
12. Team, EBI. “ALAS2 Homo Sapiens P22557”. Ebi.ac.uk. N.p., 2017. Web. 27 June 2017.
13. “Porphyrin”. En.wikipedia.org. N.p., 2017. Web. 27 June 2017.
14. Shipman, Alexa R., and Kate E. Shipman. “X-Linked Dominant Protoporphyria: Response To “Cutaneous Porphyrias Part 1””. International Journal of Dermatology 54.3 (2014): e87-e88. Web. 28 June 2017.
15. Brancaleoni, V. et al. “X-Chromosomal Inactivation Directly Influences The Phenotypic Manifestation of X-Linked Protoporphyria”. Clinical Genetics 89.1 (2015): 20-26. Web. 29 June 2017.
16. "Transcript: ALAS2-201 (ENSLACT00000016428.1) - Summary - Latimeria Chalumnae - Ensembl Genome Browser 89". Ensembl.org. N.p., 2017. Web. 1 July 2017.
17. Benoff, S., Skoultchi, A. I. “X-linked control of hemoglobin production in somatic hybrids of mouse erythroleukemic cells and mouse lymphoma or bone marrow cells.” Cell. 12: 263-274, 1977. Web. 1 July 2017.
18. Astrin, K. H., Desnick, R. J., Bishop, D. F. “Assignment of human delta-aminolevulinate synthase (ALAS) to chromosome 3.” (Abstract)Cytogenet. Cell Genet. 46: 573, 1987. Web. 1 July 2017.
19. Astrin, K. H., Bishop, D. F. “Assignment of human erythroid delta-aminolevulinate synthase (ALAS2) to the X chromosome.” (Abstract)Cytogenet. Cell Genet. 51: 953-954, 1989. Web. 1 July 2017.
20. Cotter, P. D., Baumann, M., Bishop, D. F. “Enzymatic defect in 'X-linked' sideroblastic anemia: molecular evidence for erythroid delta-aminolevulinate synthase deficiency.” Proc. Nat. Acad. Sci. 89: 4028-4032, 1992. Web. 1 July 2017.
21. Surinya, K. H., Cox, T. C., May, B. K. “Identification and characterization of a conserved erythroid-specific enhancer located in intron 8 of the human 5-aminolevulinate synthase 2 gene.” J. Biol. Chem. 273: 16798-16809, 1998. Web. 1 July 2017.
22. Bishop, D. F., Henderson, A. S., Astrin, K. H. “Human delta-aminolevulinate synthase: assignment of the housekeeping gene to 3p21 and the erythroid-specific gene to the X chromosome.” Genomics. 7: 207-214, 1990. Web. 1 July 2017.
23. O’Connor, Leigh, Jane Gilmour, and Constanze Bonifer. “The Role of the Ubiquitously Expressed Transcription Factor Sp1 in Tissue-Specific Transcriptional Regulation and in Disease.” The Yale Journal of Biology and Medicine 89.4 (2016): 513–525. Web. 1 July 2017.
24. Merika, M, and S H Orkin. “DNA-Binding Specificity of GATA Family Transcription Factors.” Molecular and Cellular Biology 13.7 (1993): 3999–4010. Web. 1 July 2017.
25. Han L, Lu J, Pan X. et al. “Histone acetyltransferase p300 regulates the transcription of human erythroid-specific 5-aminolevulinate synthase gene.” Biochem Biophys Res Commun. 2006;348(3):799–806. Web. 1 July 2017.
26. Wei, Chunlan et al. “Effects Of Psychological Stress On Serum Iron And Erythropoiesis.” International Journal of Hematology 88.1 (2008): 52-56. Web. 10 July 2017.
27. Tian, Xue et al. “Psychological Stress Induced Zinc Accumulation And Up-Regulation of ZIP14 And Metallothionein In Rat Liver.” BMC Gastroenterology 14.1 (2014): n.p. Web. 10 July 2017.
28. Ninomiya, Yukiko et al. “X-Linked Dominant Protoporphyria: The First Reported Japanese Case.” The Journal of Dermatology 43.4 (2015): 414-418. Web. 10 July 2017.
29. Livideanu, Cristina Bulai et al. “Late-Onset X-Linked Dominant Protoporphyria: An Etiology Of Photosensitivity In The Elderly.” Journal of Investigative Dermatology 133.6 (2013): 1688-1690. Web. 10 July 2017.
30. "Variation Viewer." Ncbi.nlm.nih.gov. N.p., 2017. Web. 11 July 2017.
31. "NM_000032.4(ALAS2):C.1757A>T (P.Tyr586phe) AND Not Specified - Clinvar - NCBI." Ncbi.nlm.nih.gov. N.p., 2017. Web. 11 July 2017.
32. "Gene: ALAS2 (ENSG00000158578) - Transcript Comparison - Homo Sapiens - Ensembl Genome Browser 89." Ensembl.org. N.p., 2017. Web. 11 July 2017.
33. "GWAS Central - Gene/Region." Gwascentral.org. N.p., 2017. Web. 11 July 2017.
34. Balwani, Manisha et al. "Clinical, Biochemical, And Genetic Characterization Of North American Patients With Erythropoietic Protoporphyria And X-Linked Protoporphyria." JAMA Dermatology��(2017): n. pag. Web. 13 July 2017.
35. Stadhouders, Ralph et al. "Control Of Developmentally Primed Erythroid Genes By Combinatorial Co-Repressor Actions." Nature Communications 6 (2015): 8893. Web. 13 July 2017.
36. Guberman, Alejandra S., María E. Scassa, and Eduardo T. Cánepa. "Repression Of 5-Aminolevulinate Synthase Gene By The Potent Tumor Promoter, TPA, Involves Multiple Signal Transduction Pathways." Archives of Biochemistry and Biophysics 436.2 (2005): 285-296. Web. 13 July 2017.
37. "Porphyrin And Heme Synthesis And Bilirubin Metabolism." Themedicalbiochemistrypage.org. N.p., 2017. Web. 15 July 2017.
38. Tanimura N, Miller E, Igarashi K, Yang D et al. Mechanism governing heme synthesis reveals a GATA factor/heme circuit that controls differentiation. EMBO Rep (2016): 249-65. PMID: 26698166 Web. 18 July 2017.
39. Database, GeneCards. "ALAS2 Gene - Genecards | HEM0 Protein | HEM0 Antibody." Genecards.org. N.p., 2017. Web. 21 June 2017.
40. Das, Devika, Ahmed Murad, and Ayman Saad. "Erythropoietic Porphyria – Role of Curative Hematopoietic Stemcell Transplantation." Jscimedcentral.com. N.p., 2017. Web. 20 July 2017.
41. "Genome Decoration Page." Ncbi.nlm.nih.gov. N.p., 2017. Web. 20 July 2017.
42. Astner, Isabel et al. "Crystal Structure Of 5-Aminolevulinate Synthase, The First Enzyme Of Heme Biosynthesis, And Its Link To XLSA In Humans." The EMBO Journal 24.18 (2005): 3166-3177. Web. 20 July 2017.
43. "Porphyrin And Heme Synthesis And Bilirubin Metabolism." Themedicalbiochemistrypage.org. N.p., 2017. Web. 21 July 2017.
44. "Genetests.Org." GeneTests.org. N.p., 2017. Web. 20 July 2017.
45. "List Of DNA Testing Companies - ISOGG Wiki." Isogg.org. N.p., 2017. Web. 20 July 2017.
46. "Heme Biosynthesis - Biosystems - NCBI." Ncbi.nlm.nih.gov. N.p., 2017. Web. 21 July 2017.
47. Reference, Genetics. "ALAS2 Gene." Genetics Home Reference. N.p., 2017. Web. 20 July 2017.
48. Scheiner, Dr. et al. "Research Proves You Can Reverse Aging With BBL Treatment – Adam Scheiner MD." Adam Scheiner MD. N.p., 2017. Web. 20 July 2017.
49. "File:Acute Photosensitivity Reaction In EPP.Jpg - Wikimedia Commons." Commons.wikimedia.org. N.p., 2017. Web. 21 July 2017.
50. "Erythropoietic Protoporphyria = البروتو بورفيريا المكونة للدم." Dermaamin.com. N.p., 2017. Web. 21 July 2017.
51. Landefeld C, et al. "X-Linked Protoporphyria: Iron Supplementation Improves Protoporphyrin Overload, Liver Damage And Anaemia. - Pubmed - NCBI." Ncbi.nlm.nih.gov. N.p., 2017. Web. 24 July 2017.
52. Stafford, R. et al. "The Impact Of Photosensitivity Disorders On Aspects Of Lifestyle." British Journal of Dermatology 163.4 (2010): 817-822. Web. 24 July 2017.
53. "Erythropoietic Protoporphyria (EPP) - Clinuvel Pharmaceuticals." Clinuvel.com. N.p., 2017. Web. 24 July 2017.
54. Hunter, Gregory A., and Gloria C. Ferreira. "Molecular Enzymology Of 5-Aminolevulinate Synthase, The Gatekeeper Of Heme Biosynthesis." PubMed Central Canada. N.p., 2017. Web. 2 Aug. 2017.
55. Heinemann, Ilka U., Martina Jahn, and Dieter Jahn. "The Biochemistry Of Heme Biosynthesis." Archives of Biochemistry and Biophysics 474.2 (2008): 238-251. Web. 2 Aug. 2017.
0 notes