#biozentrum
Text

Abbruch Biozentrum Basel. Das neue Gebäude ist links sichtbar.
0 notes
Text
Fwd: Graduate position: UMainz.BehaviouralEvolutionAnts
Begin forwarded message:
> From: [email protected]
> Subject: Graduate position: UMainz.BehaviouralEvolutionAnts
> Date: 2 March 2024 at 05:56:00 GMT
> To: [email protected]
>
>
>
> PhD position Behavioral neurobiology of ants
>
> We are recruiting a motivated and talented student interested in
> understanding the evolution and organization of the olfactory system in
> ants (and other insects). The student will join an interdisciplinary
> team composed by Carlotta Martelli (neurobiology and computational
> biology),Susanne Foitzik (behavior and evolution) and Hugo Darras
> (evolution and genomics), two PhD students and one postdoc.
>
> The project aims to unravel the organizational logic of the olfactory
> system in Temnothorax ants, from genes to neurons to behavior. The
> long-term goal is to identify evolutionary signatures of non-canonical
> organizations of the olfactory system and to understand the computational
> consequences of different architectures for odor coding and behavior. This
> innovative, interdisciplinary project combines neurobiological
> and behavioral experiments, molecular genetic analysis, genomics,
> transcriptomics, and theory.
>
> This call is intended to fill a PhD position that will focus on the
> analysis of behavior, with the goal to link molecular and anatomical
> features of the olfactory system to the behavior of individual ants
> within a colony.
>
> The project will be co-supervised by S. Foitzik and C. Martelli, in
> collaboration with H. Darras.
>
> The PhD student should have a strong interest in behavior and
> neurobiology, ideally with experience in quantitative behavioral
> analysis. Training in evolutionary biology, neuroanatomy and experience
> in handling insects would also be an advantage but are not a prerequisite.
>
> Funding for this position is secured over 3 years, with potential of
> extension.
>
> The PhD student will have the opportunity to be integrated into the
> GenEvo graduate program (https://ift.tt/Kda8CNP), which offers
> a close-knit community of graduate students and provides training in
> molecular and evolutionary biology, as well as methodological courses
> such as on bioinformatics.
>
> To apply, please send a letter of motivation, CV and contact information
> of two referees to [email protected] and [email protected]
>
> DEADLINE: 27.03.2024
> For additional information, please contact us!
> Carlotta Martelli
> [email protected],
> https://ift.tt/FZaBg5u
> Susanne Foitzik
> [email protected], Group Foitzik | Behavioural Ecology and Social
> Evolution
> Hugo Darras
> [email protected], Group Darras | Behavioural Ecology and Social
> Evolution
> iDN and iomE, Johannes Gutenberg University Mainz, Germany
>
> Prof. Dr. Susanne Foitzik
> Institute of Organismic and Molecular Evolution
> Johannes Gutenberg University Mainz
> Biozentrum
> Hanns Dieter H??sch Weg 15
> D-55128 Mainz
> Germany
> Tel: +49 (0) 6131 39 27 840
> Fax: +49 (0)6131 39 27 850
> Email: [email protected]
>
>
>
> "Foitzik, Susanne"
0 notes
Text
A Virus That Kills Sleepers - Technology Org
New Post has been published on https://thedigitalinsider.com/a-virus-that-kills-sleepers-technology-org/
A Virus That Kills Sleepers - Technology Org
ETH Zurich researchers have found a virus that kills dormant bacteria. This rare discovery could help to combat germs that can’t be treated with antibiotics alone.
The paride phage (purple) is one of the few phages to attack dormant bacteria. Image credit: Fabienne Estermann & Enea Maffei / ETH Zurich
In nature, most bacteria live on the bare minimum. If they experience nutrient deficiency or stress, they shut down their metabolism in a controlled manner and go into a resting state. In this stand-by mode, certain metabolic processes still take place that enable the microbes to perceive their environment and react to stimuli, but growth and division are suspended.
This also protects bacteria from, say, antibiotics or from viruses that prey exclusively on bacteria. Such bacteria-infecting viruses, known as phages, are considered a possible alternative to antibiotics that are no longer (sufficiently) effective due to drug resistance. Until now, expert consensus held that phages successfully infect bacteria only when the latter are growing.
Researchers at ETH Zurich asked themselves whether evolution might have produced bacteriophages that specialise in dormant bacteria and could be used to target them. They began their search in 2018. Now, in a new publication in the journal Nature Communications, they show that such phages, though rare, do indeed exist.
Phage catcher in action: The researcher takes a water sample from which he will isolate bacteriophages in the laboratory. Image credit: Enea Maffei / ETH Zurich
A lucky strike in a compost heap
When ETH Professor Alexander Harms and his team at the Biozentrum of the University of Basel began their project in 2018, they assumed that within the first year, they would be able to isolate around 20 different phages that attack dormant bacteria.
But this wasn’t the case: it wasn’t until 2019 that Harms’ doctoral student Enea Maffei isolated a new, previously unknown virus. Found in rotting plant material from a cemetery near Riehen (Canton of Basel-Stadt), this virus can infect and destroy dormant bacteria.
“This is the first phage described in the literature that has been shown to attack bacteria in a dormant state,” Maffei says. Harms adds: “In view of the huge number of bacteriophages, however, I was always convinced that evolution must have produced some that can crack into dormant bacteria.” They have named their new phage Paride.
Active against widespread bacteria
The virus the researchers found infects Pseudomonas aeruginosa, a bacterium commonly found in many environments. Various strains colonise bodies of water, plants, the soil – and people. In the human body, certain strains can cause serious respiratory diseases such as pneumonia, which can be fatal.
How the new phage takes dormant P. aeruginosa germs by surprise, however, is not yet clear to the researchers. They suspect that the virus uses a specific molecular key to awaken the bacteria, and then hijacks the cell’s multiplication machinery for its own reproduction. However, the ETH researchers have not yet been able to clarify exactly how this works.
They thus aim to elucidate the genes or molecules that underlie this awakening mechanism. Based on this, they could develop substances in a test tube that take over the wake-up process. Such a substance could then be combined with a suitable antibiotic that completely eliminates the bacteria. “But we’re just at the beginning. The one thing we know for sure is that we know almost nothing,” Harms says.
Initial tests show an effect
To test the efficacy of the Paride phage, the researchers paired it with an antibiotic called meropenem. This disrupts cell wall synthesis and so it interferes only with cellular processes that don’t damage the phages. The antibiotic has no effect on dormant bacteria, as these don’t synthesise a new cell wall.
When tested in cell culture dishes, the virus was able to kill 99 percent of all dormant bacteria but left 1 percent alive. Only the combination of Paride phages and meropenem was able to eradicate the bacterial culture completely, even though the latter had no detectable effect on its own.
In a further experiment together with Nina Khanna, a doctor at Basel University Hospital, Maffei tested this combination on mice with a chronic infection. Neither the phage nor the antibiotic alone worked particularly well in the mice, but the interaction between phages and antibiotics proved to be very effective in living organisms as well. “This shows that our discovery is not just a laboratory artefact, but could also be clinically relevant,” Maffei says.
A glimmer of hope – but never more than that?
Experts have been intensively discussing phage therapy for many years. Researchers and physicians hope that one day they can use phages to replace ineffective antibiotics. However, broad applications are still lacking, as no comprehensive studies have been conducted. “What we have at present is mostly individual case studies,” Harms says.
Studies by researchers at the Queen Astrid Military Hospital in Brussels showed that the treatment improved the condition of three-quarters of patients and that it was able to eliminate the bacteria in 61 percent. However, this also means that in four out of ten patients, the germs could not be removed with phage therapy, even though the bacteria in question were phage-sensitive in the lab.
“This may be because many bacteria in the body are in a dormant state, especially in the case of chronic infections, and so phages can’t penetrate them,” Harms says. Dormant bacteria could also play an important role in infections with non-resistant strains.
“In the case of infections, that means it would be important to know the physiological state of the bacteria in question. Then the right phages, combined with antibiotics, could be used in a targeted manner. However, you need to know exactly how a phage attacks a bacterium before you can select the right phages for a particular treatment. This hasn’t happened yet because we still know too little about the phages,” Harms explains.
That’s why in the years ahead, the researchers will investigate precisely how the new phage brings bacteria out of deep sleep, infects them and makes them susceptible to antibiotics. This work is funded by an SNSF Starting Grant to Alexander Harms and by NCCR AntiResist.
Source: ETH Zurich
You can offer your link to a page which is relevant to the topic of this post.
#amp#antibiotic#Antibiotics#applications#Bacteria#bacteriophages#Biotechnology news#cell#communications#comprehensive#deep sleep#Diseases#drug#drug resistance#Environment#ETH Zurich#Evolution#Featured life sciences news#genes#germs#growth#Heap#how#human#indeed#infection#infections#interaction#it#Link
0 notes
Link
0 notes
Photo

Chillen mit Freunden am Rhein in Basel. . . #streetphotography #streetsofbasel #basel #people #rhein #rhine #biozentrumbasel #biozentrum @biozentrum #unibasel #fujix100v #blackandwhite @streetsnappers @streetphotographyinternational @streetphotographers @street.grammers @streetphotographyglobally @classic.polly (hier: Biozentrum der Universität Basel) https://www.instagram.com/p/CGZoa5TltNF/?igshid=1ryufhhavisvf
#streetphotography#streetsofbasel#basel#people#rhein#rhine#biozentrumbasel#biozentrum#unibasel#fujix100v#blackandwhite
0 notes
Photo

• Calmate Basel • 📷www.martinvogt.ch #basel #clouds #bluesky #switzerland #rhein #river #water #sky #skyline #city #summertime #herzogdemeuron #lockdown #afternoon #xmas #iphoneography #sunday #martinvogt #birds #watch #walk #novartis #city #covid_19 #winter #calm #biozentrum #streetstyle (hier: Basel, Switzerland) https://www.instagram.com/p/CJB3EZ9BE75/?igshid=1o02r2onizh47
#basel#clouds#bluesky#switzerland#rhein#river#water#sky#skyline#city#summertime#herzogdemeuron#lockdown#afternoon#xmas#iphoneography#sunday#martinvogt#birds#watch#walk#novartis#covid_19#winter#calm#biozentrum#streetstyle
33 notes
·
View notes
Photo
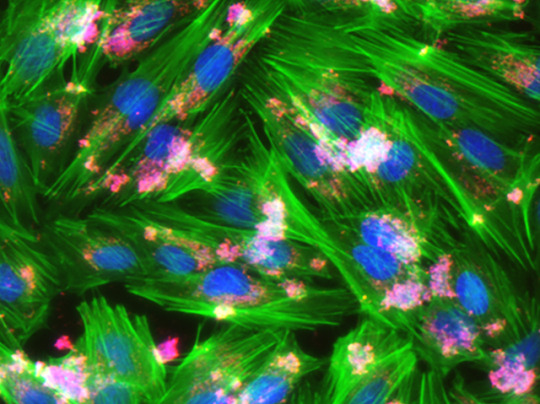
Getting Stressed
Catching a bacterial infection can be stressful, not just for you but for your cells too. These lab-grown human cells have been infected with Bartonella bacteria (pink) – an increasingly important infectious bug that causes several diseases in humans. Bartonella can be transmitted by biting insects, such as fleas or lice. It can also be caught through animal scratches, leading to so-called ‘cat-scratch disease’, which can be very serious in people with weakened immune systems. Researchers have discovered that Bartonella bacteria produce chemicals that hijack the normal processes inside cells, activating the formation of stress fibres (green). These fibres disrupt the normal structures inside cells so they break up into pieces when they try and move. In turn, this disrupts the regular structure of tissues, allowing the infection to spread deeper into the body. Understanding how Bartonella interact with our cells could point towards targets for developing better antibacterial drugs.
Written by Kat Arney
Image from work by Simon Marlaire and Christoph Dehio, on January 2021 cover of PLOS Pathogens
Biozentrum, University of Basel, Basel, Switzerland
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in PLOS Pathogens, January 2021
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#stress#cellular stress#bartonella#bacteria#cat-scratch#immunofluorescence#stress fibres#antibiotics
10 notes
·
View notes
Text
¿Estamos a punto de perder la batalla de la salud por la mutación bacteriana?
Una de las aportaciones a la medicina moderna y un enorme salto para la salud lo fue sin duda el descubrimiento de la penicilina hace ya casi 100 años, cuando Alexander Fleming en 1928 descubrió un moho que produce una sustancia natural con efectos antibacterianos que destruía las bacterias del staphylococcus aureus.
Fleming no patentó su descubrimiento creyendo que así sería mas fácil la difusión de un antibiótico necesario para el tratamiento de las numerosas infecciones que azotaban a la población, y hoy en día, la falta de interés en la búsqueda de mejores antibióticos podría en un futuro poner en riesgo la salud publica.
Esta problemática esta siendo considerada por el profesor Christoph Dehio, del Biozentrum de la Universidad de Basilea, quien dirige el Centro Nacional de Competencia en Investigación (NCCR) AntiResist, y que defiende la necesidad de facilitar el desarrollo de nuevos medicamentos que superen la resistencia actual de los antibióticos a través de una mejor comprensión de la fisiología de las bacterias en los humanos.
Bacterias y virus no son lo mismo.
En la actualidad estamos tan acostumbrados a la accesibilidad de los medicamentos y en algunos casos incluso a la autoreceta de los mismos, que no tomamos en cuenta que es en realidad lo que estamos haciendo en nuestro organismo al decidir la medicación para males tales como las gripas comunes. Con el surgimiento de nuevos Virus como el Covid-19 hemos comenzado a tomar interés nuevamente en la importancia y las diferencias que debemos considerar al utilizar los medicamentos que consumimos.
“Los resfriados son causados principalmente por virus” (comenta el microbiólogo Christoph Dehio), y los antibióticos no ayudan en estos casos. A no ser que se haya tenido una infección secundaria con bacterias, es posible que el medico recete un antibiótico. A lo mejor haya mejorado rápidamente, pero no sabemos si realmente era necesario usar un antibiótico en esta circunstancia”.
Según afirma la clínica mayo en su articulo “Antibióticos: ¿Los estás usando de manera incorrecta?” (https://www.mayoclinic.org/es-es/healthy-lifestyle/consumer-health/in-depth/antibiotics/art-20045720) los antibióticos tratan las infecciones bacterianas y son adecuadas en el tratamiento de enfermedades tales como la amigdalitis estreptocócica causada por la bacteria Streptococcus Pyogenes y no es el tratamiento correcto para la mayoría de los dolores de garganta provocados por virus.
Es decir, las infecciones virales frecuentes que NO requieren antibiótico son:
Resfriado, gripe (influenza), bronquitis, la mayoría de los casos de tos, algunas infecciones del oído, algunas infecciones de los senos paranasales y la gastroenteritis viral.
Por otra parte, tomar antibiótico para una infección viral no solo no curará la infección ni evitará que las personas se contagien, y si puede provocar efectos secundarios innecesarios y perjudiciales.
Sin embargo, aquí el tema mas critico para el empleo indiscriminado del consumo de antibacteriales, radica en un tema que preocupa de sobremanera a los científicos y que es la resistencia del organismo al antibiótico.
Tomar antibióticos cuando no es necesario, no solo ataca las bacterias beneficiosas de nuestro organismo, sino que pueden hacer que las bacterias inofensivas desarrollen propiedades resistentes que puedan traspasar a otras bacterias, o que sean reemplazadas por bacterias potencialmente dañinas, afirma el articulo de la clínica mayo.
¿Pero que podría pasar en un futuro con esta situación?, según el Dr. Dehio es probable que tarde o temprano, las intervenciones médicas que hoy en día son tradicionalmente seguras podrían convertirse en operaciones de riesgo, incluyendo las operaciones de todo tipo como quimioterapias para el cáncer, trasplante de órganos o incluso el tratamiento de la neumonía bacteriana producida por una infección por influenza o Covid.
“Es urgente desarrollar nuevos antibióticos basados en nuevos principios que puedan matar los gérmenes resistentes a múltiples fármacos existentes… Necesitamos nuevas sustancias activas de vez en cuando para adelantarnos a los gérmenes resistentes en la carrera contra la evolución bacteriana”. Afirma el Dr. Dehio.
¿Cual es la problemática actual para desarrollar nuevos antibióticos?
Según el Dr Dehio, uno de los problemas principales para desarrollar nuevas sustancias activas es económico, es decir, que no hay negocio en los antibióticos por que el precio es ridículamente bajo debido a que los derechos de las patentes ya expiraron, esto no apoya a contar con fondos suficientes para la investigación necesaria, por otra parte, aquellos intentos para desarrollar nuevos antibióticos no han tenido éxito, esto debido a que las condiciones en laboratorio son diferentes a las que se presentan en los gérmenes de nuestros cuerpos.
Actualmente el Dr. Dehio esta intentando simular de una manera mas realista las condiciones de los tejidos infectados de nuestro cuerpo, utilizando muestras de tejido infectado de operaciones ortopédicas, orina o secreciones bronquiales, simulando un proceso infeccioso en escala miniatura para la búsqueda de nuevas sustancias activas. Con ello busca crear las bases para el desarrollo de nuevos fármacos que permitan a la industria farmacéutica apoyar en la producción de nuevos medicamentos para el mercado.
Fuente: Wikipedia, https://www.unibas.ch/en/Research/Uni-Nova/Uni-Nova-135/Uni-Nova-135-In-conversation.html
2 notes
·
View notes
Photo

After four years, during which I've carried out four trips to Madagascar, and published >40 papers, I have today defended my doctoral thesis in front of friends and family! Thrilled to have been awarded a Summa Cum Laude! . . . . . . . . . #phd #phdlife #phdstudent #phdgraduation #gradstudent #academia #thesis #thesisdefense #thesisdefended #science #frog #frogs #animal #animals #zoology #herpetology #biology #systematics #taxonomy (at LMU Biozentrum) https://www.instagram.com/p/Bzyjj4PBe4z/?igshid=1vepu3sdt0jhw
#phd#phdlife#phdstudent#phdgraduation#gradstudent#academia#thesis#thesisdefense#thesisdefended#science#frog#frogs#animal#animals#zoology#herpetology#biology#systematics#taxonomy
118 notes
·
View notes
Text
Fwd: Conference: Olhao_Portugal.HostMicrobeSymbiosis.Jun10-13
Begin forwarded message:
> From: [email protected]
> Subject: Conference: Olhao_Portugal.HostMicrobeSymbiosis.Jun10-13
> Date: 24 February 2024 at 05:45:05 GMT
> To: [email protected]
>
>
> SymbNET International Conference on Host-Microbe Symbiosis
>
> Webpage:
> https://ift.tt/4ifJA7G
>
> Where: Real Marina - Hotel & Spa, Olhão, Portugal
>
> When: 10-13 June 2024
>
> About: The "SymbNET International Meeting on Host-Microbe Symbiosis"
> will bring together researchers working on a diverse range of questions,
> approaches, and model systems. The meetingwill cover different host
> systems such as plants, animals and humans and different molecular
> mechanisms, functional understanding and ecological models of the
> interactions. The purpose is to highlight the most recent advances in
> the field, common principles between systems,and future directions to
> explore. This meeting is organized in the context of the EU twinning
> grant SymbNET with specific sessions organised by NCCR Microbiomes
> and CRC Metaorganisms.It will host 200 participants, with all sessions
> being plenary.
>
> Confirmed Speakers:
> Marjolein Bruijning| Universiteit van Amsterdam, The Netherlands
> Luisa De Sordi | Sorbonne University,France
> Ellen Decaestecker| KU Leuven, Belgium
> Médéric Diard | Biozentrum, University of Basel, Switzerland
> Isabel Gordo | Instituto Gulbenkian de Ciência, Portugal
> Nancy Moran| University of Texas at Austin, USA
> Markus Ralser | Charité Universitätsmedizin Berlin, Germany
> Eduardo Rocha | Institut Pasteur, France
> Olivia Roth| Kiel University, Germany
> Pascale Vonaesch| University ofLausanne, Switzerland
> Michael Zimmermann| European Molecular Biology Laboratory, Germany
> Sebastian Pfeilmeier|
> Universiteit van Amsterdam, The Netherlands
>
> Registration Fee and Sponsoring:
> Register by March 15 -
> https://ift.tt/4ifJA7G
> Selected applicants will pay a registration fee of 650 EUR.
>
> We will sponsor participants from members ofthe SymbNET partner
> institutes, from Widening countries in Horizon2020 or Horizon Europe,
> or countries with low performance in Research and Innovation.
>
> Organisers:
> Philipp Engel| University of Lausanne, Lausanne, Switzerland
>
> Migla Miskinyte| Católica Biomedical Research Centre, Portugal; Instituto
> Gulbenkian de Ciência, Portugal
>
> Hinrich Schulenburg| Kiel University and Metaorganisms CRC, Kiel, Germany
>
> Luís Teixeira| Católica Biomedical Research Centre, Portugal; Instituto
> Gulbenkian de Ciência, Portugal
>
> Maria Zimmermann-Kogadeeva| European Molecular Biology Laboratory,
> Heidelberg, Germany
>
> SymbNET, EU-funded Twinning project
> Instituto Gulbenkian de Ciência (FCG-IGC),Portugal
> Origin and Functions of Metaorganisms Collaborative Research Centre 1182
> (CRC Metaorganisms), Germany
> NCCR Microbiomes, Switzerland
> Católica Biomedical Research Centre, Portugal
>
via Email
February 24, 2024 at 07:00AM
0 notes
Link
Biozentrum PhD Fellowships Program Life Sciences 2020 International PhD Program “Biozentrum PhD Fellowships” 2018 Winter Call has been announced. Exceptional students from the world over are encouraged to apply for the prestigious “Fellowships for Excellence” program. Interested candidates can check out all of the details on the same below: This call expires in : Biozentrum […] The post Biozentrum PhD Fellowships Program Life Sciences 2020 appeared first on BioTecNika .
1 note
·
View note
Link
0 notes
Photo

Like a zipper -- how cells form new blood vessels
Blood vessel formation relies on the ability of vascular cells to move while remaining firmly connected to each other. This enables the vessels to grow and sprout without leaking any blood. Scientists now describe how this works. In this process, the cytoskeleton pushes the cell forward, while an adhesion protein subsequently closes the gap to the neighboring cell, like a zipper.
Vascular system of a two days old zebrafish embryo (magenta: endothelial cells, light blue: blood cells).Credit: University of Basel, Biozentrum
175 notes
·
View notes
Photo

Die RP #Gent auf dem #Rhein unter der #MittlerenBrücke in #Basel. #Elefanten #Rhine #рейн #базель #Schiffahrt #Rheinschifffahrt #Biozentrum @biozentrum #foto_wyser_ch #picoftheday #Feierabend (hier: Mittlere Brücke) https://www.instagram.com/p/BqkkM7FHTaM/?utm_source=ig_tumblr_share&igshid=21nwfchyxcl3
#gent#rhein#mittlerenbrücke#basel#elefanten#rhine#рейн#базель#schiffahrt#rheinschifffahrt#biozentrum#foto_wyser_ch#picoftheday#feierabend
0 notes
Photo
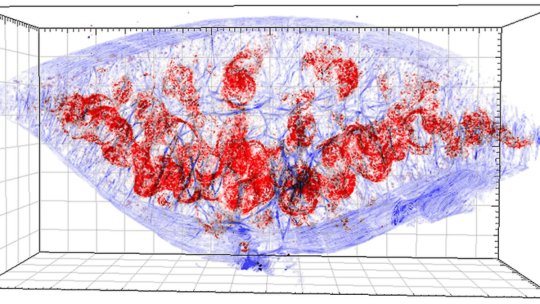
Mittwoch, 29. Dezember 2021 Am Antibiotikum vorbei Salmonellen können sich in der Milz verstecken Salmonellen kennt man meist als Verursacher von Durchfall, sie können aber auch hinter schwereren Erkrankungen wie Typhus stecken. Antibiotika können helfen - sie wirken aber manchmal kaum oder nicht gut genug. Die Erklärung dafür findet ein Forscherteam der Schweizer Uni Basel. Salmonellen können sich in bestimmten Bereichen der Milz verstecken und so der tödlichen Wirkung von Antibiotika entgehen. Dies erkläre, warum einige Patienten trotz zunächst wirksamer Antibiotika-Behandlung zu einem späteren Zeitpunkt einen Rückfall erleiden, berichten Wissenschaftler um Dirk Bumann vom Biozentrum der Universität Basel in den "Proceedings" der US-amerikanischen Akademie der Wissenschaft ("PNAS"). In ihren Tierexperimenten half eine gleichzeitig eingesetzte Immuntherapie, die Salmonellen zu vertreiben. Salmonellen sind Bakterien, die beim Menschen und vielen Tieren Erkrankungen verursachen können. Die werden als Salmonellosen zusammengefasst. Häufig verursachen Salmonellen Durchfallerkrankungen, etwa nach dem Verzehr von mit Bakterien verunreinigten Lebensmitteln wie Eiern oder Geflügelfleisch. Bestimmte Varianten von Salmonellen können Typhus und Paratyphus auslösen - Infektionskrankheiten, die unbehandelt schwer verlaufen können. Bakterien überlebten trotz Antibiotika Grundsätzlich sind Salmonellen-Infektionen mit Antibiotika gut zu therapieren, sofern die Bakterien keine Resistenzen gegen die Mittel entwickeln. Rätselhaft war bisher, warum auch ohne Resistenzen bei einigen Menschen die Antibiotika-Behandlung nicht alle Bakterien vertreibt, die Erkrankung dann teils erneut auftritt. Die Forschergruppe um Bumann suchte nun nach einer Erklärung. Die Forscher nutzten dazu ein spezielles Verfahren, die Zwei-Photonen-Tomographie. "Nach einer Antibiotika-Therapie überlebt nur etwa jedes 100. Bakterium", sagt Studienleiter Bumann. "Diese wenigen Salmonellen in Geweben aufzuspüren und zu untersuchen, ist wie die Suche nach der Nadel im Heuhaufen." Bei dem Verfahren wird die jeweils oberste Schicht eines Gewebes untersucht, die Schicht dann abgetragen und die nächst darunterliegende Schicht gescannt. Am Ende entsteht eine dreidimensionale Ansicht des Gewebes, die zeigt, wo Bakterienherde versteckt sind. In weißer Pulpa blieben Bakterien zurück Die Forschenden wendeten das Verfahren bei der Milz von Mäusen an, die zuvor gezielt mit Salmonellen infiziert und dann mit Antibiotika behandelt worden waren. In der Milz fanden sich die meisten Salmonellen in der sogenannten roten Pulpa. "Hier werden die Salmonellen während der Antibiotika-Behandlung fast vollständig eliminiert", erläutert Erstautor Jiagui Li. In der weißen Pulpa blieben hingegen einige wenige Bakterien zurück - die Antibiotika-Behandlung erreichte sie nicht. Mehr zum Thema Weitere Untersuchungen zeigten, dass der Körper für die vollständige Beseitigung der Erreger auf Zellen des Immunsystems angewiesen ist, vor allem auf sogenannte Neutrophile. Das sind spezialisierte weiße Blutkörperchen, die Bakterien bekämpfen. In der weißen Pulpa nimmt die Zahl dieser Zellen stark ab, sobald die Antibiotika zu wirken beginnen. Ihre Zahl reiche dann nicht mehr aus, um die Bakterien wirkungsvoll zu bekämpfen, berichten die Wissenschaftler. Um das Problem zu beheben, versuchten die Forscher mit einer Immuntherapie die Abwehrkräfte zu verbessern. "Dieser Ansatz kann dazu beitragen, das Immunsystem zu stimulieren und eine hohe Dichte an Neutrophilen über einen längeren Zeitraum aufrechtzuerhalten", erläutert Bumann. Die Ergebnisse eröffneten neue Möglichkeiten für die Behandlung wiederkehrender Salmonellosen, schreiben die Wissenschaftler.
0 notes
