#funthem
Text









Mycelium, the word for the conglomerate growths of hyphae of any given fungal organism! These are a single organism isolate in each jar! Took some regular close ups then pulled out my macro attachment for better views of what surely would be a slow and lovely embrace (of dissolving under the chemicals exudes by the hyphae to break down cell walls for nutritious consumption :)
#mycology#lgbtqia2s#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s+#myc#enby#fungus#funthey#funthem#mycelium#fungusamongus#beauty#art#macro#gay
64 notes
·
View notes
Photo

𝙷𝚊𝚍 𝚜𝚞𝚌𝚑 𝚊𝚗 𝚊𝚠𝚎𝚜𝚘𝚖𝚎 𝚝𝚒𝚖𝚎 𝚊𝚝 𝚝𝚑𝚎 𝚁𝚎𝚗𝚊𝚒𝚜𝚜𝚊𝚗𝚌𝚎 𝙿𝚕𝚎𝚊𝚜𝚞𝚛𝚎 𝙵𝚊𝚒𝚛𝚎! 𝚁𝚊𝚗 𝚒𝚗𝚝𝚘 𝚊 𝚖𝚘𝚍𝚎𝚕 𝚏𝚛𝚒𝚎𝚗𝚍 𝙸'𝚟𝚎 𝚜𝚑𝚘𝚝 𝚠𝚒𝚝𝚑 𝚊𝚝 𝚝𝚑𝚎 𝚏𝚞𝚗 𝚝𝚑𝚎𝚖𝚎𝚍 𝚐𝚛𝚘𝚞𝚙𝚜𝚑𝚘𝚘𝚝𝚜 𝙸 𝚊𝚝𝚝𝚎𝚗𝚍, @johns_path. ⚔️ ☪�� ☪︎ ☪︎ ☪︎ ☪︎ ☪︎ ☪︎ ☪︎ #renaissancefestival #getoutandexplore #dowhatyoulovetodo #photooftheday #liveamplified #artofvisuals #createyourownreality #artist #photography #goodvibes #models #beautiful #hot #fun #renaissancepleasurefaire #renaissancefaire #faire #medieval #posing #themed #funthemes (at Renaissance Pleasure Faire) https://www.instagram.com/p/CdLlH1kLmqZ/?igshid=NGJjMDIxMWI=
#renaissancefestival#getoutandexplore#dowhatyoulovetodo#photooftheday#liveamplified#artofvisuals#createyourownreality#artist#photography#goodvibes#models#beautiful#hot#fun#renaissancepleasurefaire#renaissancefaire#faire#medieval#posing#themed#funthemes
0 notes
Video
Tone it up Tuesdays - Zumba Toning - 6:15PM w/Nikki (First Session is FREE, Register online or arrive early) Theme of the week - Bright Colors, Neon - Light It Up & Smile! Making Halloween Month Fun at STBB #selfietime #STBBfitness #Halloweenthemes #fitnessfamily #funthemes #ZumbaFitness #Zumbatoning #stbbfitness #glenburnie Zumba is for everyone, try a class with a cool group TODAY! www.strivingtobebetterfitness.com/classes.html 10000000_674053493074532_5502620118077451683_n.mp4 (at Striving To Be Better Fitness & Yoga Studios) https://www.instagram.com/p/B3pYsV1jJQR/?igshid=1b5efqdkhlyl3
0 notes
Photo

Mardi Gras #originalart #originalartwork #multimedia #acrylic#abstractart #visualsoflife #funtheme #artjournal
0 notes
Photo

1st May 2017 #FUNTASIA #a_miracle_calunary_experiences wish #everybody #success #lightoflife #culinary #lovemusic #foodlover #funtheme #clientele #wonderfull #RoyceSaayan 💃🏿🎤 (at Hotel Bangi-Putrajaya)
#lightoflife#funtheme#everybody#a_miracle_calunary_experiences#clientele#success#culinary#lovemusic#wonderfull#foodlover#funtasia#roycesaayan
0 notes
Text

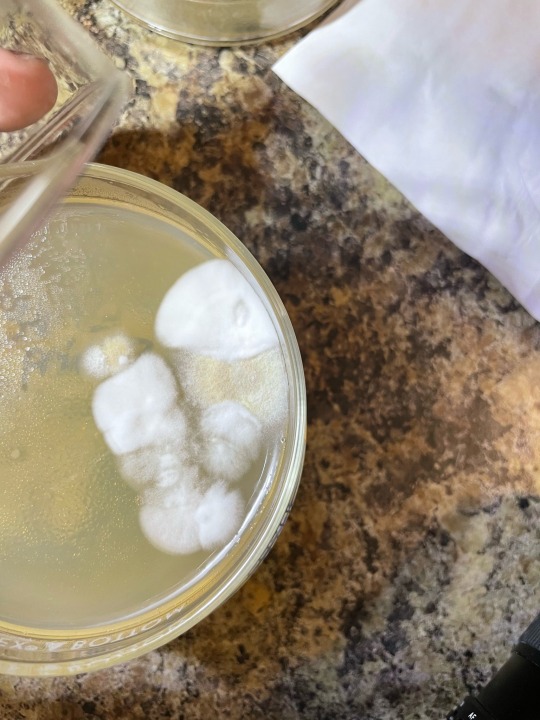
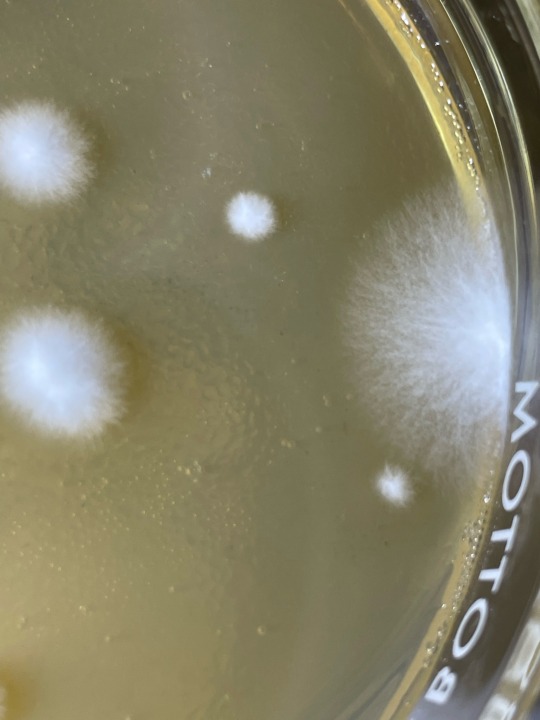
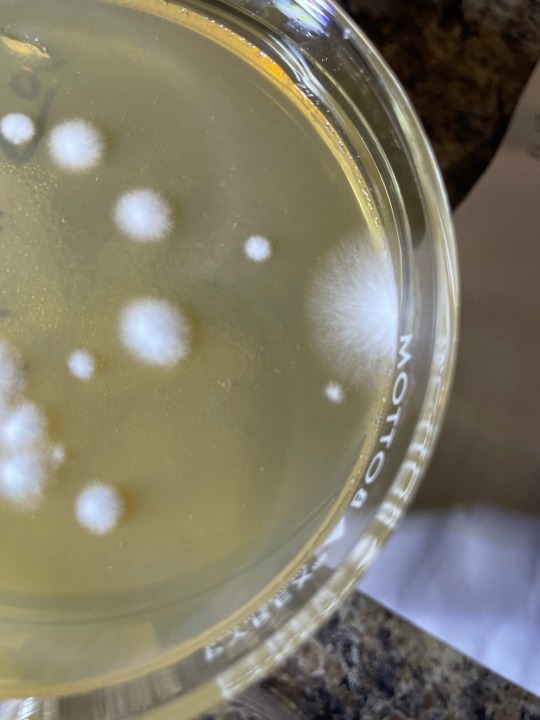
my wittle baby inoculated mushroom spore swab colonies, all have two fungal friends that I dont want there xD- Recent fungal contam close ups, so you dont have to do it <3 —- if we compare morphologies of both contam colonies in question to the expected smaller mushroom fungus’ mycelium, its easy to tell they are troublesome fungal molds!
The mold in last two photos gives itself away by its location, its excessive growth speed, and its hyphae being overly filamentous compared to expected mushroom mycelium and esp conpared to inoculated germinations visible near it :) but as i watch the PE spores grow out the hyphae start to look similar to the edge outlier…. Hmmm. I wont toss this plate just isolate… would hate to have a particularly vigorous mushy isolate be wasted xD
first two photos are of umbo plate, with clearly a colorized mold colony, very dusty and powdery. I plan to do microscopic ID later today once I get scotch tape to try and ID it for real! Im thinking its am ascomycete at least!
Linktr.ee/mycochaotix
#mycology#lgbtqia2s#60s psychedelia#lgbtqia#lgbtqia2s+#magic mushies#microbiology#mold#myc#enby#fungi#fungus#fungusamongus#funthey#funthem#nb#gay#queer
29 notes
·
View notes
Text




two sets of plates inoculated at same time, all plates are on 2nd gen (meaning mss->1gen->2nd gen (each gen is a transfer from the mss plate which I call mother plates)
above images: These separate, 2nd gen, penis envy isolates are already so dang rhizoid in their growth!
Compared to my (below images) Huatuala plates which still need another run or two through agar over next few weeks to work towards growth that looks similar to whats shown here in these PE plates!


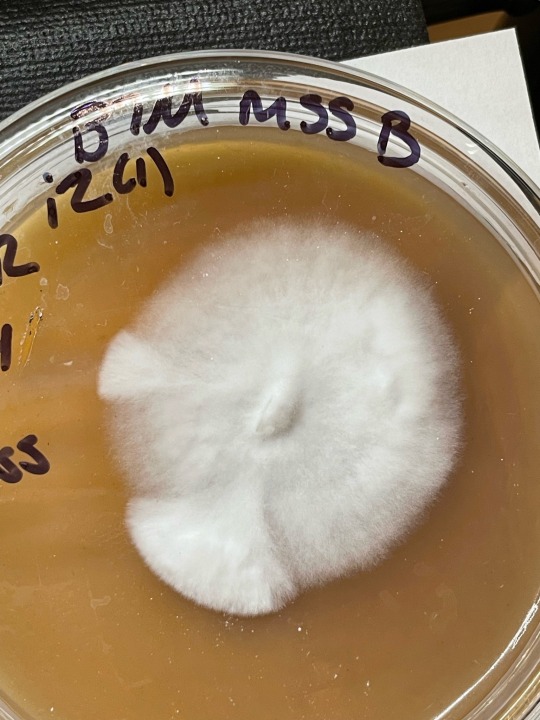
#mycology#lgbtqia2s#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s+#myc#enby#fungi#fungus#fungusamongus#funthey#funthem#nb#non binary#gay
22 notes
·
View notes
Text


The selected fruits:

Example of selective harvesting 🍄
Follow on this mycojourney! for more:
patreon.com/mycochaotix
youtube.com/myco_chaotix
Instagram: @myco_chaotix
www.linktr.ee/mycochaotix
#mycology#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s#lgbtqia2s+#myc#enby#huautla#fungi#fungus#fungusamongus#funthey#funthem#fungifinds#fungivore#queer#non binary
19 notes
·
View notes
Text
30 sec video short: Casual jar inspections 🍄 feeling the beauty of the fungi yall 🍄🧬🫨🕺🎶
#mycology#lgbtqia2s#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s+#myc#enby#mycelium#fungi#fungus#fungusamongus#funthey#funthem#gay#non binary
18 notes
·
View notes
Text
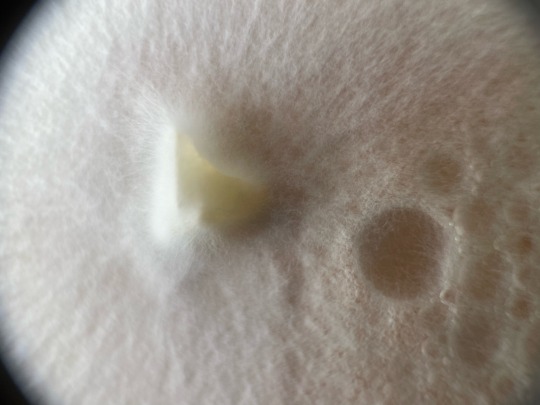
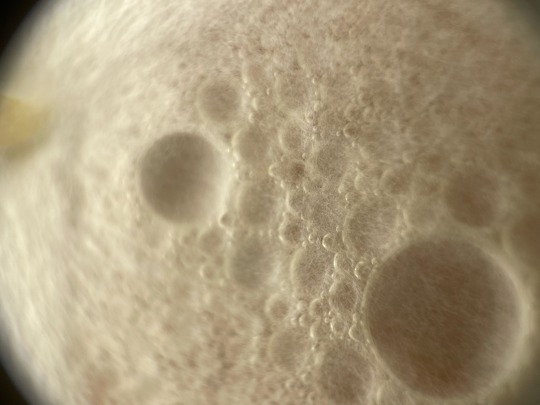



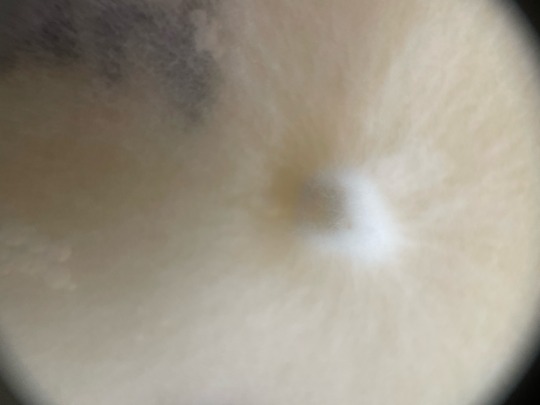


Some macro shots by muah, of my plates that ive been showcasing lately, random order :)
——-
Follow for more! linktr.ee/mycochaotix
#mycology#lgbtqia2s#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s+#myc#enby#mushroom#fungi#fungus#funthey#funthem#gay#non binary
19 notes
·
View notes
Text
Cool study to review about characteristics of fungal mycelium!
#mycology#lgbtqia2s#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s+#myc#enby#mycelium#fungi#fungus#funthey#funthem#nb#nonbinary#gay
23 notes
·
View notes
Text








Timelapse each is 12 hours apart !
McKennaii
#60s psychedelia#lgbtqia#lgbtqia2s#lgbtqia2s+#magic mushies#microbiology#mold#myc#mycelium#mycology#enby#fungi#fungus#gay#nb#substrate#mushroom#funthem#funthey#magic mushroom
29 notes
·
View notes
Text




12/11 tub updates-
End of first flush for far right tub, second flush for left two, gonna go ahead and harvest all because i meed the space for new tubs xD tossing with a salute, the two left tubs. Far right tub is lidded and up on top shelf for a few days for second flush rest.
-Also put first PE tub and newest Huautla iso into fruiting on new shelf set up



— most recent s2b tubs already showing signs of voracious growth :)

- top right tub is next to go to fruiting, is same iso as newest tub put into fruiting but with double the casing layer :)
#mycology#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s#lgbtqia2s+#myc#enby#queer#non binary#mushroom#fungi#fungus#fungusamongus#funthey#funthem#fungifinds
14 notes
·
View notes
Note
hi mycopal :) I'm enjoying your blog and admiring your set up, seems very hightech. I'm also reading about cultivating a local (Australian) psilocybin variety we often find in pine forests. Could you point me in the right direction for more info to get started with a simple at home set up for beginners? thanks again for your good work :)
Hello there mycopal! Thanks for reaching out! I appreciate your kind words :) I’m super proud of my lab area! It has taken two years to put together by saving 25-50 a paycheck lol!! Humorously my roommate doesn’t like that i commandeered a whole room … haha, but here we are ;) What mushy are you looking to cultivate? Pscilocybe Tampensis or P. subtropicalis maybe? :)
note: my opinions and advice are based on my own experience and studious research :) there are MANY ways to skin things with… skin… lol; as such, my way should work for most but may not and there are many other mycoeducators with varied approaches that offer great insight in their own right :) I have a few vids on youtube (still new to content creating there and working on adding more) if you wanna see more of what I do as I outline it below! At the end of this long winded MCX response I will provide supportive resources for your consideration in order they are addressed in this response.
🍄Tip: get some nasalpharyngeal swabs so you can actively swab and store mushroom genetics you find in the wild for later reararch ;)
Pretext: I live in a (technically) tropical area so what i do with my research areas may be different than you! I am not sure how much of what im about to detail has been considered by you… but if youve considered it then disregard :) i like to break things down like you’re new new new incase theres any detail to miss.
Introductory Answering your Q: Basically you have to consider a handful of generalized steps for cultivating any fungus; and find TEKs (ie: guides by other mycopal’s that have yielded them success) and make your own tek— I always say that the mycojourney is coming up with your own tek, cobbled together from current teks that speak to you and also are appropriate for your resource availability :) after you get through my response you’ll have homework of studying resources but also: finding TEKs for your specific strain you want to work with :)
My preferred supplies-
Genetics: Dont know about the laws and availability where you live, but formal vendors (like innoculatetheworld ; sporeworks) and informal mycoeducators (who sometimes vendor spores) like PGT and Boomershroomer; are the only spore sources I trust (outside of my own stock;).
Boomershroomer makes quality inflatable monotubs and if you order one of her tubs she sends genetics with it! A little ‘secret’ ;) to be aware of!
PGT has a shop online thats only open sometimes but has a cool collectible trading card system for his genetics (buy the card get the microscopy supply with the card).
ITW and SW are generally up 24/7 and are more formal vendors that also sell microscopy stuff.
I currently use and recommend using 6qt shoebox totes for grow container (the kind that you buy in 5-10 packs from Walmart that have gusseted lids that allow some air flow for ‘sneakers’ to be stored hehe). Note that you can use 12/24/48/72qt etc, but I have no experience with those and they require larger set ups with fans, humidifiers, etc. i prefer less is more :)
I always promote a company called Microppose :) they do amazing filters and just started their own monotub production I think :) my fave substrate is CocoBliss coconut coir pith, and I use lab grade nutritional additives like: gypsum, lime, malt extract, and yeast off amazon in various stages of my process. For grain bags, before I made my own, I only trust: spawnmagic.com ; for my grains I use Producers Pride: Whole Oats (like what is given to horses) from the feedstore :) a 50lb bag has lasted me two years, no lie. I dont use bags, but jars for grain: i use brand: Ball, glass mason jars for spawn containers prior to moving spawn into a tub with substrate. I use Aozita wide mouth masonjar plastic lids (off Amazon) for my jars as they can be modified with filters and then pressure cooked safely and come with rubber seals :)
Now to go into detail to answer your question:
Here we go-deep breath-: lets talk cultivation and research starting:
1. Genetics: (a) multi spore syringe (mss) (can be injected into a grow bag but isnt ideal and may not produce viable strains) (recomended to use mss on agar to isolate your own colonies), (b) spore print/swab (requires agar) or (c) liquid culture (LC) isolate syringe (best option for immediate injection-inoculation of grain containers/bags with best chance for healthy growth and fruiting without time and hassle of the steps I outline further :)
1a. If you are able to work with agar (either make your own or buy premade sterilized, one time use agar plates): then you will start your journey by MSS->Agar->Isolate separate germinating colonies off mss agar plate to new plates (those become your mother isolate plates for each specific strain isolate for whatever strain youre working with). The mother plate should become your cold storage, reference plate as you study growth and fruiting characteristics of the colonies you isolate :) at that pont: You can then use some excised pieces of the mother plate (if in a rush, or if able to wait, till gen1 plates (transfers from mother plate to new plates that become duplicate isolates of the mother plate)) to inoculate a jar or two and also inoculate other plates to continue to ‘run the mycelium out’ / ‘chase the mycelium’.
1b. If you are not able to do agar work then I strongly suggest you seek genetics that are LC syringe. Basically, LC syringes are when mycopal takes a 2nd gen+ plate and moves some of that mycelium to sterile sugar water and lets the mycelium grow out in that water till its all filled with reproduced mycelium and can be sucked up into syringes for better more assured propagation of genetics ;)
Side thought: Spore swabs and spore syringed are dice rolls :) [Spore germination discussion incl quote from TMC- https://at.tumblr.com/mycochaos/uscrybal-commented-on-a-comment-i-made-quoting/pjzr0c86nlyt]
2. Grain spawn: once you have genetics hammered out, next is grain spawn. Grain spawn can be … well, any grain. Really. Mushrooms can colonize and fruit off of wet cardbord… 💯🍄😂, so what “type” of spawn is more about whats available in your area imo. Youll need to sterilize any grain spawn, unless using a premade bag thats already sterilized or taking chances with uncle bens (or similar) rice baggies that arent sterilized but are arguably cleaner than grains scooped out of a bags of grains from a mill or feedstore.
2a. Grain bags: milo, millet, rye berries, corn kernals, rice, whole oats, really any grain or berry that has a husk can be used :) some species prefer specific grains most work on all kinds of grains with varying levels of efficiency. Some grains are more or less robust and some do better when moistened and or pressure cooked than others :) i make my own grain but exclusively use glass mason quart jars :)
2b. Grain jars: my preference. I have recent grai. Jar prep and creation reels/shorts on my instagram and some on this tumblr if you wanna see specifics :) generally I do 15psi, 10 minute venting, for 1.5 hours for my grain and I do not soak grains, only low boil them for 30 min to soften husks and extract some grain nutrients to then use that liquid ‘grain soak’ run off for agar nutrients :)
2c. Uncle Bens rice bags: i dont do this and dont have any good advice on it. I have a UB tek link or two at end for consideration and there is a whole reddit mushroom sub i think r/unclebens (?) for this
2d. All in one bags: i also do not use these and do not recommend them generally. If you have never had a flush and are literally first timing it, then all in one may work fine :) but as much of the process you can source or create yourself the cheaper and often better, imo!
3. Substrate & Spawn-to-bulk (S2B): many mycopals have their own substrate preferences, but for me I prefer shaved coconut coir pith. I generally do a coir block 650g, 500g vermiculite (from garden store), and 50g gypsum + 50g lime for my substrate. I do not sterilize it, but I do heat pasteurize my substrate for at least 12 hours. In an air tight, insulated cooler (like for sodas at a party). You can also cold pasteurize. I like how Boomershroomer and PGT do their sub and learned from them then tweaked it for my own preferences :)
When my jars are fully colonized and observably free from contam, I will S2B using a clean butter knife :) I kinda cut down into the grain in pizza slices then swirl the knife around as I let grains that spill out mix with my substrate and basically do 1qt spawn to 2qt of substrate, saving maybe 10-15% of the spawn and substrate till end to make a special psuedo casing layer once bulk of spawn and substrate are mixed and compacted. Then I do a last sprinkling of the remaining grain like a baby lasgana and cover that with substrate.
Casing layer explainer: A casing layer is a layer of material applied on top of the colonized substrate in mushroom cultivation. It can help improve yield, reduce certain types of surface contaminants, and maintain humidity around the fruiting bodies. In my experience, using an organic sphagnum peat moss mixed with lime powder and filtered water has produced successful tubs without the need for pasteurization or sterilization. Before I used that i just used left over substrate as a casing layer :) — While some species require a specific type of casing layer after substrate colonization, most do not require one. However, adding a casing layer can be beneficial for improving yield and humidity control.
There are different ways to apply a casing layer. Some people apply it as part of the spawn to bulk (S2B) process, while others apply it only after the substrate surface is fully colonized or slightly before pinning. Personally, I have used a casing layer when colonizing pasteurized wheat/rye straw to provide an even fruiting surface for mycelium. I have also experimented with an organic peat and lime dust casing layer (no pasteurization or sterilization), which has helped retain humidity and has not resulted in any contamination. Personally, I've worked some APEs in that past that had a casing layer applied in the same instance as the S2B occuring, essentially the compacted bits colonized faster than the looser casing layer. Where I always use casing layer, is if I use spawn to colonize pasteurized wheat/rye straw, if only to provide an even fruiting surface for the mycelium! But, even then... most cubensis can fruit solely on pasteurized straw, with no casing layer!
4. Colonization and Fruiting:
4a. Youll need to find a TEK based on whatever substrate container you settle on using :) what ive outlined so far is my own tek, using 6qt shoe boxes and the materials ive outlined above. The substrate chamber/container can be a flat container/tote or could be fruited out the top of a grain bag/all-in-one bag OR could fruit off the side of a bag (if its a species like Oyster mushies that prefer side fruiting). I personally use unmodified tubs and will leave lid on my tubs while colonizing and then take lid off and replace with cleaned, upside down, misted 6qt tub that rests on the edges of the right-side-up tub to create a mini climate that allows more passive air flow, allows light to filter in from high angles promoting fruits growing upwards towards the light. Light isnt needed until pinning, and is a secondary trigger to pinning but a primary factor in pigmentation of fruits and growth direction of fruits.
Colonization of most mushroom fruiting fungi is generally between 68-80F, every species and even some varieties within those species, may have specific temp needs. The way mycopals control for this is many things that I dont have to use fortunately :) ‘Martha Tents’ are something to consider. Some use heating pads and humidifiers depending on where they live and where they are compared to the sea💯. I dont have any experience with martha tents or doing more than using my home A/C, a closet, and a heppa room filter to control my temps in the closet and with lots of trial and error…. I now leave my home at 72F, my closet warms to about 74 with the door shut and a/c at that temp, so i let plates, jars and tubs colonize at 74F and then I will move the tubs to open closet with more air flow and is closer to 72F when I am moving to fruiting :) Ive tried to be clever in how I use my space… so i use wire racks and know higher up on the rack is hotter and less air flow whereas lower is cooler and often more air flow.
Something I havent really gone into yet in this response is about sterility, aseptic environments, personal and environmental hygiene. All important to condsider…
4a. Heres my explainer on that:
Strict aespetic and hygiene techniques are not 100% and even using fancy laminar flow is not 100% contamination free potentials! Common contaminant sources include airborne spores, dust, and environmental factors. Pets that roam around your cultivation area could carry spores on their fur or paws, which may be released when they move through your space. Additionally, some fungal contaminants, like Kahms yeast, can present in distinctive ways and there are dimorphic fungal molds that have one or more alternate morphologies, main dimorphism being mold with a secondary yeast form (whence the mold spores get into human lungs, for example with blastomycosis perhaps) as an alternative reproductive presentation within its life cycle (based on temperature and environmental variables). It's essential to understand that spores and other contaminant-genetic cells are incredibly tiny and (in the case of most spores) can suspend in the air, waiting for air currents, light, or vibrations to move them around. Wet spores and bacterial cells oftentimes require animal assistance or liquid splashing/spritzing/spraying to move around, but can often hitch rides of natural environmental variabes (currents of wind, water, dirt, etc). Humans also carry a range of bacteria and fungal organisms on their skin, which can contribute to localized environmental dust and potential contamination when working in hyper sterile or attempted hygienic environments while researching fungi :)
4b: Primordia, Pins and Fruiting: Pinning is a colony activity (that impacts all sides of your cake once colonized) that shifts metabolic processes of the mycelia to pinning and fruiting, this is why when you start getting heavy side pins you rarely get any flat-surface pins and fruits (all the energy goes into what pins form and fruit, first). Additionally, I believe my suggestions will be effective in controlling for environmental triggers to pinning, it is important to note that side pinning can also occur due to other factors such as genetics or substrate composition.

You can reduce the microclimate from being created that promotes side pins, during the process, by ensuring that the substrate is firmly compressed, once S2B occurs, and then sprinkled with a .25" casing layer of the same substrate material (or peat+lime casing). I press my base spawn+cvg mix firmly, and ensuring that the surface is even with least amount of inconsistently level substrate surface. This early compression, keeps the cake against the wall for as longer than doing little to no compression of your spawn+sub. You should ensure your colonizing mycelium isnt exposed to the same lighting you would for fruiting, but light is only a secondary trigger to pinning, FAE+Temp Drop+Humidity pooling/then drying are the primary triggers for pinning once colonization has completed.
5. Harvesting and Dehydrating: i twist and pull my fruits, some will cut at base, some will float their cake with water and then cut or twist and pull at that poimt :) harvesting is preferential imo. I dehydrate fruits 125F for 24 hours in Air Fryer o. Dehydrate mode :)
Resources and foundational TEKs:
Genetics: innoculatetheworld.com, sporeworks,com, boomershroomer.com, pgtmycology,com
Casing layer post w/screencaps: https://www.tumblr.com/mycochaotix/723941213220339712/mycochaos-oldacnt-plzfollownew-one-of-my
Growing gourmet (book): https://drive.google.com/file/d/1-CsyZenWzF8kHLviXM8pencZ4FAHDedh/view?usp=drivesdk
PF tek - Check this site out, gives a great layout of "PF TEK" and also BRF cakes as part of that TEK - https://www.fungifun.org/pmwiki.php/English/Pftek
HOw to make easy (cvg) bulk substrate (boomer shroomer): https://www.youtube.com/watch?v=7M6YHfaMyQ8&t=3s
how to make plates, slants, and LC - north spore - https://www.youtube.com/watch?v=a4bzQQkh71Q&t=487s
pgt LC basics - https://www.youtube.com/watch?v=gqwjUq31KgU&t=284s
Southwest mushrooms - mycelium grain spawn and LC - https://www.youtube.com/watch?v=rxlJJpu3O_g
How to sterilize equipment such as petri dishes - MIcrobehunter microscopy - https://www.youtube.com/watch?v=MVtEBtxkhGk
mycelium inoculation in the lab - southwest mushrooms - https://www.youtube.com/watch?v=Ng_Wq9PnEVI&t=560s
Mushroom Cultivation, how it should and shouldnt look: https://www.shroomery.org/forums/showflat.php/Number/17231150
Recognizing and dealing with contamination: https://www.shroomery.org/forums/showflat.php/Number/23130868
Sterilization vs Pasturization - http://www.differencebetween.net/science/difference-between-sterilization-and-pasteurization/
Mushrooms, Molds and Mycorrhizae: A Fungal Immersion Course Part 1 - https://www.youtube.com/watch?v=dD1IL2dBLQ8
Mushrooms, Molds and Mycorrhizae: A Fungal Immersion Course Part 2 - https://www.youtube.com/watch?v=_bam3tF_a7M
Mushrooms, Molds and Mycorrhizae: A Fungal Immersion Course Part 3 - https://www.youtube.com/watch?v=AiIUGGKjuwU
Mushrooms, Molds and Mycorrhizae: A Fungal Immersion Course Part 4 - https://www.youtube.com/watch?v=KLfwruf2xVA
Guide to Oysters, Gourmet, Freshcap - https://www.youtube.com/watch?v=EZAjz6bZjpg
Cooking Oyster mushrooms, TGS - https://www.youtube.com/watch?v=7qb2KF6kvhA
5 gallon bucket tek - oyster mushrooms - gourmet - https://www.youtube.com/watch?v=45b2t7fqhjA&t=60s
Mycophilia YT 'All About Aborts' discussion: https://www.youtube.com/watch?v=9C8x_32Saxg
Bacterial colony morphology - https://www.youtube.com/watch?v=4JZAFUPckUg
Mycelium morphology : how to select healthy mycelium when breeding mushrooms -- https://www.youtube.com/watch?v=leUpfsonVxc&t=1s
mycotrophic - agar xfers/sectoring off healthiest growth - https://www.youtube.com/watch?v=XMxGwkj9Wn4
DayTrippers Microscopy library of contam and healthy mycelium examples: https://www.reddit.com/r/ContamFam/comments/nnquol/microscopy_of_healthy_mycelium_and_contamination/
PH trich conversation pt 2 DT: https://www.reddit.ccom/r/ContamFam/comments/jldtuw/my_garden_of_contam_free_grow_it_is_all_about_the/
DayTrippers Trip Tips - apply PH casing layer to prevent trich: https://www.reddit.com/r/ContamFam/comments/m3unbr/daytrippers_trip_tips_video_tutorial_on_how_to/
Trich contamfam library: https://www.reddit.com/r/ContamFam/comments/115gyj2/trichoderma_the_green_monster/?utm_source=share&utm_medium=web2x&context=3
If FAE is a problem: not pinning, getting Cobweb, Stroma Overlay! “READ THIS”: https://www.reddit.com/r/ContamFam/comments/jur5ar/daytrippers_trip_tip_why_cant_i_get_this_if_fae/
DayTripper’s Trip Tips: Two cultivation tricks to solve common problems of insufficient Fresh Air Exchange and Overlay growth in monotubs: https://www.reddit.com/r/ContamFam/comments/10w1yxm/daytrippers_trip_tips_two_cultivation_tricks_to/?utm_source=share&utm_medium=web2x&context=3
#mycology#lgbtqia2s#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s+#myc#enby#myco#fungi#fungusamongus#fungus#funthey#funthem#nb#non binary#psychedlia
13 notes
·
View notes
Text
This is why I do what I do:

patreon.com/mycochaotix
youtube.com/myco_chaotix
Instagram: @myco_chaotix
www.linktr.ee/mycochaotix
#mcx#mycology#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s#lgbtqia2s+#myc#enby#queer#trans#non binary#funthey#funthem#fungi#fungus#mycojourney#mycoeducation#mycopal
8 notes
·
View notes
Text
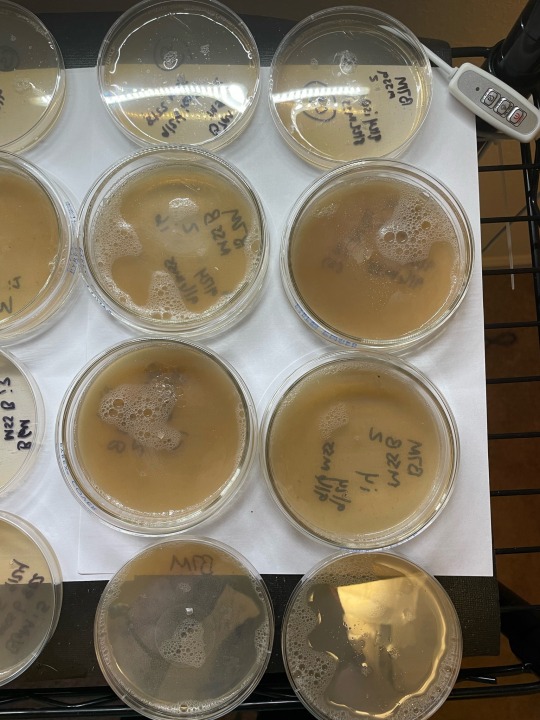
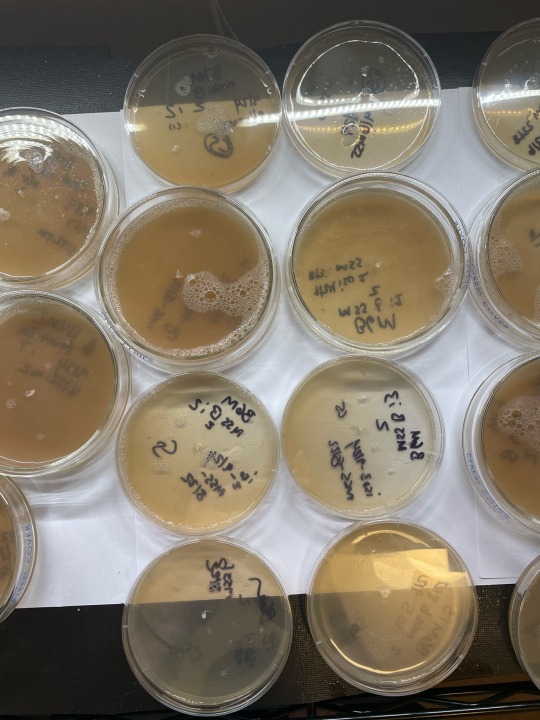
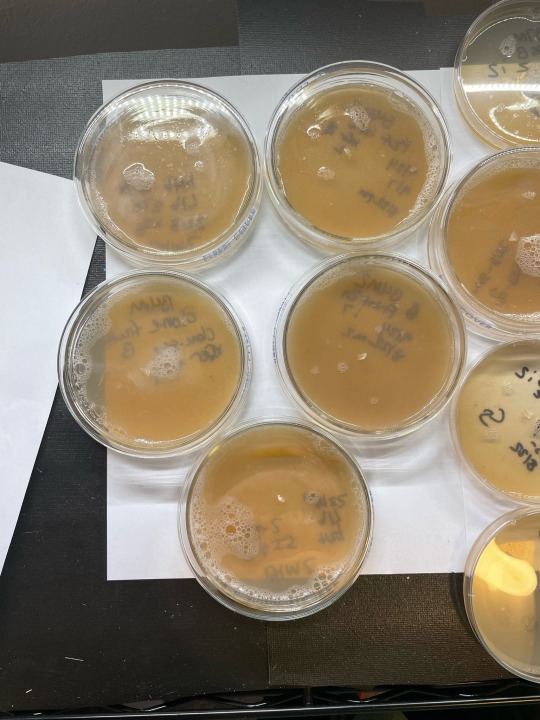


Just finished some lab work this AM. Got a new round of plates for daily update pics :) still workin the agar. No grain jars or tubs running atm. I always consider this period ‘downtime’. I also use my research closet in different ways during this time so that its a little warmer and less fae than usual, because I do not generally seal my plates. Pics show: these are (1st) pic is huatuala strain, 2nd pic is penisenvy, and third is b4m (umbo), that will be order pics r posted moving forward daily. Last 2 pics show umbo and huat/PE mss plates i took the xfers from. Only took one of the unbo clone plates to another xfer… rest were weak or literally showing signs of metabolites and in agar stage thats never good xD ——
Linktr.ee/mycochaotix
#mycology#lgbtqia2s#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s+#myc#enby#fungi#fungus#funthey#funthem#gay#nb#nonbinary#agar#lab#spore
12 notes
·
View notes