#methanol
Link
The need for technology that can capture, remove and repurpose carbon dioxide grows stronger with every CO2 molecule that reaches Earth's atmosphere. To meet that need, scientists at the Department of Energy's Pacific Northwest National Laboratory have cleared a new milestone in their efforts to make carbon capture more affordable and widespread. They have created a new system that efficiently captures CO2 -- the least costly to date -- and converts it into one of the world's most widely used chemicals: methanol.
Snaring CO2 before it floats into the atmosphere is a key component in slowing global warming. Creating incentives for the largest emitters to adopt carbon capture technology, however, is an important precursor. The high cost of commercial capture technology is a longstanding barrier to its widespread use.
PNNL scientists believe methanol can provide that incentive. It holds many uses as a fuel, solvent, and an important ingredient in plastics, paint, construction materials and car parts. Converting CO2 into useful substances like methanol offers a path for industrial entities to capture and repurpose their carbon.
Read more.
91 notes
·
View notes
Link
An international team of researchers, led by scientists at the University of Manchester, has developed a fast and economical method of converting methane, or natural gas, into liquid methanol at ambient temperature and pressure. The method takes place under continuous flow over a photo-catalytic material using visible light to drive the conversion.
[...]
Naturally occurring methane is an abundant and valuable fuel, used for ovens, furnaces, water heaters, kilns, automobiles and turbines. However, methane can also be dangerous due to the difficulty of extracting, transporting and storing it.
Methane gas is also harmful to the environment when it is released or leaks into the atmosphere, where it is a potent greenhouse gas. Leading sources of atmospheric methane include fossil fuel production and use, rotting or burning biomass such as forest fires, agricultural waste products, landfills and melting permafrost.
Excess methane is commonly burned off, or flared, to reduce its environmental impact. However, this combustion process produces carbon dioxide, which itself is a greenhouse gas.
Industry has long sought an economical and efficient way to convert methane into methanol, a highly marketable and versatile feedstock used to make a variety of consumer and industrial products. This would not only help reduce methane emissions, but it would also provide an economic incentive to do so.
Continue Reading.
353 notes
·
View notes
Text
methanol <- telo pi lukin ala.
8 notes
·
View notes
Text
Table 19.5 gives the boiling points of three low-molar-mass thiols.
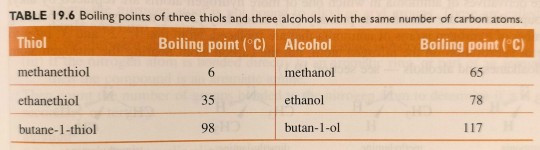
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#thiol#alcohol#methanethiol#ethanethiol#butanethiol#methanol#ethanol#butanol#boiling point
4 notes
·
View notes
Text
Figure 19.1 shows a Lewis structure and a ball-and-stick model of methanol, CH3OH, the simplest alcohol.
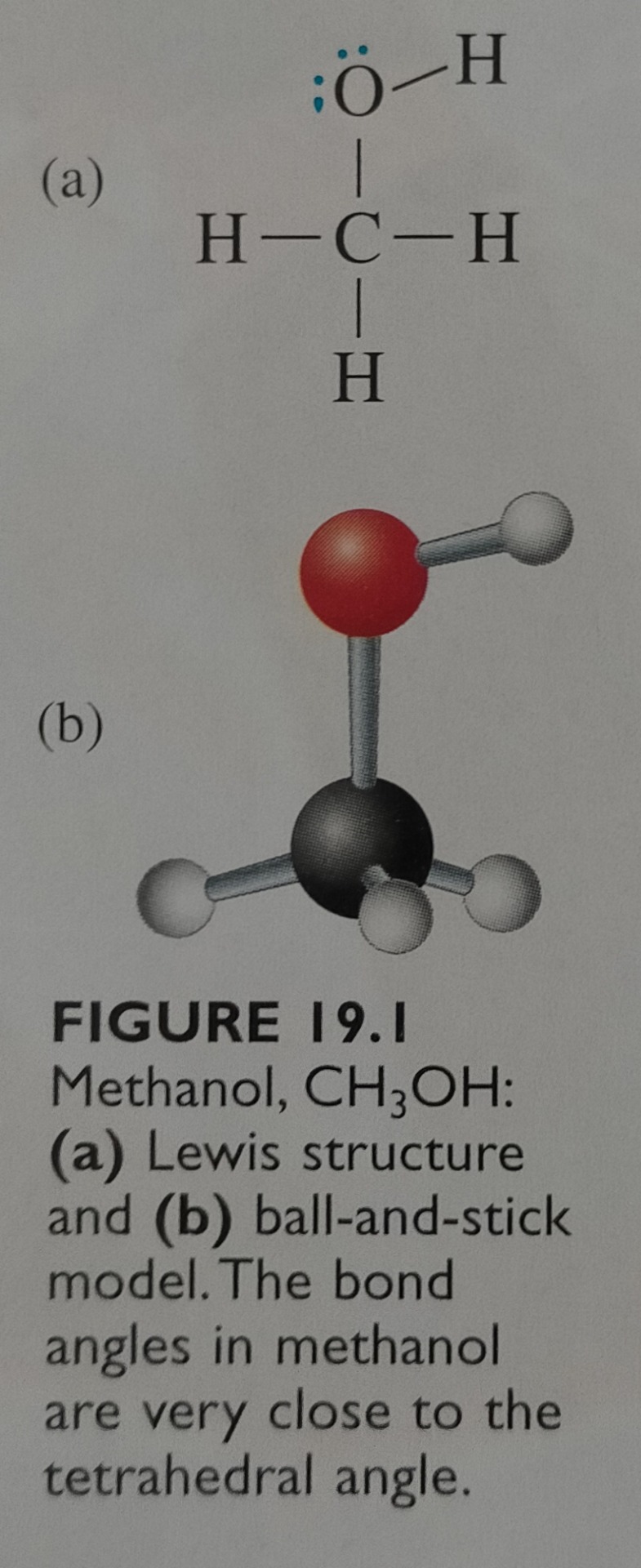
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
5 notes
·
View notes
Link
“Community members and environmental groups declared victory in a near decade-long fight to stop South Louisiana Methanol from constructing a petrochemical complex in St. James Parish, a predominantly Black community already overburdened with industrial pollution.
The Louisiana Department of Environmental Quality (LDEQ) released a letter stating it has withdrawn its review of South Louisiana Methanol’s application to modify the air permits for its planned methanol complex. LDEQ had given South Louisiana Methanol until August 19, 2022 to confirm whether it intended to go forward with its development plans before formally withdrawing its review of the pending application. The company failed to respond, therefore confirming the end of South Louisiana Methanol’s project.
“South Louisiana Methanol finally threw in the towel having learned that our community will not back down in the fight to protect our health and wellbeing from more industrial pollution,” said Sharon Lavigne, founder of RISE St. James. “Today is a tremendous victory, but we will never stop fighting against polluters who think our health is less important than their dirty profits.”
The methanol complex site was located between two historic Black neighborhoods, including Freetown, a Reconstruction Era community established by people who had been formerly enslaved at area sugar plantations. It would have also wrapped around a public park which houses a playground, ball fields, community gathering shelter, walking path and senior center.” -via Earthjustice, 9/9/22
#louisiana#united states#petrochemical#environmental racism#pollution#environment#methanol#good news#hope
18 notes
·
View notes
Text
10 notes
·
View notes
Photo

Went to the speedway for the first time last night. Amazingly talented riders at a well run event. Great way to spend a Saturday evening. Might have to get a mullet though. #pinjarspeedway #pinjarpark #speedway #flattrack #methanol #mulletcity #motorcycle (at Pinjar Park Speedway) https://www.instagram.com/p/Cje94Mmh8au/?igshid=NGJjMDIxMWI=
14 notes
·
View notes
Text
“In real life, we end up using the poor’s Fomepizole, which is, pisco.”
About methanol poisoning.
You can, of course, also use vodka, if you are feeling more elegant.
#medicine#med school#medblr#emergency medicine#toxicology#alcohol#poisoning#methanol#fomepizole#tw drinking#tw alcohol#pisco#vodka
4 notes
·
View notes
Text
Ansasol to produce one million tonnes of renewable methanol by 2029
A German-Spanish solar and renewable hydrogen developer has unveiled plans to build a massive 800MW hydrogen methanol plant in southern Spain that will produce one million tonnes of green methanol a year by 2029.
Ansasol plans that the first phase of the MetGreenPort plant in the port of Huelva will produce 150,000 tonnes of green methanol from renewable hydrogen – and carbon dioxide from nearby plants – by 2027.
Within two years, the company plans to more than six times the size of MetGreenPort, taking it to one million tonnes by 2029 with a total electrolysis capacity of 800MW, making it the largest green methanol project in Europe.
The methanol will be produced using renewable hydrogen produced at the plant – the estimated size of the electrolyser in the first phase will be around 120MW.
However, the company has not disclosed how much it will cost to build both phases of the plant, how it will be financed and where it intends to market the methanol.
Read more HERE

#world news#world politics#news#europe#european news#european union#eu news#eu politics#germany#german economy#spain#spanish economy#ansasol#energy efficiency#solar energy#energy#energy update#empowerment#growth#renewableenergy#renewable power#renewable resources#green energy#methanol
0 notes
Text

Scientists make methanol at room temperature
A more sustainable method of creating methanol—a key component of fuels, plastics, and medicines—has been developed by Cardiff University scientists and an international team of collaborators.
The process, which uses a highly active catalyst, converts oxygen and the natural gas methane into methanol at room temperature without the need for external energy sources such as light or electricity.
The breakthrough builds on the Cardiff team's efforts to move away from expensive and energy-intensive processes by developing new catalytic methods with industry and promoting the use of catalysis as a sustainable 21st century technology.
Their findings, published in an article titled "H2-reduced phosphomolybdate promotes room-temperature aerobic oxidation of methane to methanol" in Nature Catalysis, represent a significant step toward cleaner, greener methanol production which, with further development, could be used in industrial processes worldwide.
Read more.
17 notes
·
View notes
Photo

Turning Sunlight into Green Fuel: A Breakthrough in CO₂ Conversion | CeBoz.com
Researchers have successfully transformed CO₂ into methanol by shining sunlight on single atoms of copper deposited on a light-activated material, a discovery that paves the way for creating new green fuels.
0 notes
Text
Methanol: Fueling the Future with a Green Revolution
In the quest for sustainable and clean energy solutions, methanol emerges as a versatile and promising player in the global energy landscape. This comprehensive exploration delves into the multifaceted world of methanol, unraveling its origins, production processes, applications across various industries, and its pivotal role in driving the transition towards a greener and more sustainable future. From its historical roots to cutting-edge innovations, the journey of methanol unfolds as a narrative of energy transformation, environmental stewardship, and its potential to reshape the way we power our world.
Unveiling the Essence of Methanol
The Birth of Methanol: Historical Roots and Industrial Evolution
We begin by tracing the historical roots of methanol, from its discovery in the 17th century to the industrial revolution that propelled its mass production. Exploring its journey from an industrial solvent to a critical component in modern energy solutions sets the stage for understanding the diverse applications and transformative potential of methanol.
Defining Methanol: A Molecule with Many Facets
At its core, methanol is a simple yet powerful molecule. We delve into the chemical composition of methanol, its properties, and the versatile ways in which it can be harnessed across various industries, including energy, transportation, chemicals, and beyond.
Grab Sample PDF
Methanol as a Green Energy Source
Green Methanol Production: From Carbon Capture to Renewable Resources
Methanol's green credentials lie in its potential to be produced sustainably. We explore innovative production methods, including carbon capture utilization and renewable resources such as biomass, emphasizing the shift towards eco-friendly processes that minimize the environmental footprint of methanol production.
Powering the Future: Methanol as a Clean-Burning Fuel
Methanol's role as a clean-burning fuel takes center stage. We discuss how methanol's combustion produces fewer emissions compared to traditional fuels, contributing to improved air quality and reduced greenhouse gas emissions, making it a viable alternative for powering a range of applications from transportation to electricity generation.
Methanol Fuel Cells: Unleashing the Potential of Direct Methanol Fuel Cell (DMFC) Technology
The advancements in direct methanol fuel cell (DMFC) technology open new frontiers in sustainable energy solutions. We explore how methanol fuel cells are driving innovation in portable electronics, vehicles, and stationary power systems, offering efficient and environmentally friendly alternatives to conventional energy sources.
Methanol in Transportation: Revolutionizing the Way We Move
Methanol-Powered Vehicles: Navigating the Road to Sustainable Mobility
Methanol's potential as a transportation fuel is highlighted. We delve into the development of methanol-powered vehicles, exploring their performance, efficiency, and the infrastructure needed to support a widespread transition to methanol as a clean and convenient fuel for cars, buses, and other modes of transportation.
Shipping Industry: Sailing Towards Green Horizons with Methanol
Methanol's entry into the maritime sector transforms the shipping industry's environmental impact. We discuss how methanol-powered ships are emerging as a green alternative, addressing concerns related to emissions and contributing to the decarbonization of the global shipping fleet.
Aviation Sector: Soaring to New Heights with Methanol
The aviation industry's quest for sustainable aviation fuels brings methanol into focus. We explore how methanol, as a potential aviation fuel, offers a path towards reducing carbon emissions in the aviation sector, aligning with the industry's goals for sustainable and eco-friendly air travel.
Methanol's Role in Chemical Manufacturing
Methanol as a Feedstock: Catalyst for Sustainable Chemical Processes
Methanol's significance as a feedstock in chemical manufacturing is explored. We discuss how methanol serves as a versatile building block for a myriad of chemicals, fostering sustainable and innovative approaches in the production of plastics, solvents, and other essential materials.
Green Chemistry: Methanol's Contribution to Sustainable Chemical Practices
Methanol plays a pivotal role in the principles of green chemistry. We delve into how the adoption of methanol in chemical processes contributes to reduced environmental impact, improved energy efficiency, and the development of more sustainable manufacturing practices across diverse industries.
Methanol and Energy Storage: Unlocking Renewable Potential
Methanol as an Energy Carrier: Storing Renewable Energy for the Future
Methanol's versatility extends to energy storage. We explore how methanol can serve as an energy carrier, storing excess energy generated from renewable sources such as solar and wind, addressing the intermittent nature of these energy forms and providing a reliable solution for clean and efficient energy storage.
Power-to-Methanol: Harnessing Renewable Hydrogen for Sustainable Methanol Production
The concept of power-to-methanol represents a key innovation. We discuss how renewable hydrogen, produced through electrolysis, can be utilized in the synthesis of methanol, creating a closed-loop system that transforms renewable energy into a storable and transportable fuel for various applications.
Challenges and Considerations in Methanol Implementation
Carbon Footprint: Evaluating the Net Environmental Impact
While methanol offers environmental benefits, its overall carbon footprint is a consideration. We discuss the importance of evaluating the net environmental impact of methanol production and use, considering factors such as feedstock sources, production methods, and end-use applications.
Infrastructure Development: Paving the Way for a Methanol-Powered Future
The widespread adoption of methanol as a fuel requires substantial infrastructure development. We explore the challenges and considerations in establishing the necessary refueling and distribution infrastructure to support the seamless integration of methanol into our energy and transportation systems.
Regulatory Landscape: Navigating Policies and Standards
The regulatory landscape plays a crucial role in shaping the future of methanol. We discuss the challenges and opportunities associated with navigating policies, regulations, and international standards, emphasizing the need for a supportive regulatory framework that encourages the sustainable growth of the methanol industry.
Future Trends and Innovations: Shaping the Trajectory of Methanol
Green Methanol Innovations: Advancements in Sustainable Production
The future of methanol involves continuous advancements in sustainable production methods. We explore emerging technologies and innovations that aim to further reduce the carbon footprint of methanol, ensuring that it remains a leading contender in the portfolio of green energy solutions.
Methanol Economy: A Vision for Integrated and Sustainable Energy Systems
The concept of a methanol economy envisions an integrated and sustainable energy system. We discuss how the methanol economy could potentially reshape the global energy landscape, offering a decentralized and flexible approach that harnesses the power of methanol for various energy needs.
Global Collaboration: Uniting Efforts for a Methanol-Powered World
Global collaboration is key to realizing the full potential of methanol. We explore how international cooperation, research initiatives, and industry partnerships can accelerate the adoption of methanol as a sustainable energy carrier, fostering a collaborative approach to address global energy and environmental challenges.
Other Report:
Biochar: A Deep Dive into Sustainable Agriculture and Environmental Solutions
1 note
·
View note
Text
In the following oxidation-reduction reaction, Na is oxidised to Na+ and H+ is reduced to H2 (figure 19.6).


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#redox reaction#oxidation#reduction#chemical reactions#methanol#sodium methoxide#sodium#hydrogen
3 notes
·
View notes
Text
Step 2: Proton transfer from the carbocation intermediate to methanol (which in this instance is both the solvent and reactant) gives the alkene:
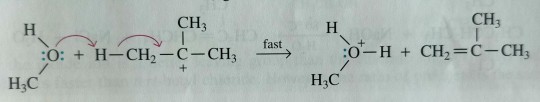
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#proton#chemical reactions#carbocation#methanol#solvent#reactant#alkene
2 notes
·
View notes