#aluminum oxide powder
Text
Napoleon's Marshals and their Birthstones Part 1
This is a three-part series where I'll be listing the birthstones of all 26 marshals. Part one will cover months January-April, the next post will cover March-August and the last post will cover the remaining 4 months. I'll mainly be focusing on the gemstone's physical and chemical properties as well as writing "short" facts about each gemstone. Before starting the list, I'll provide the definitions of some terms that will be used through the post.
_________________________________________________
Mineral: A naturally occurring solid which contains a crystalline structure that is made up of a single native element or multiple chemical compounds.
Mohs Scale: A scale system used to measure the scratch resistance of a mineral ranging from 1 (softest) to 10 (hardest). This is done by scratching a mineral with another mineral or with another object like a penny or nail.
Cleavage: The way in which a mineral breaks along the softest plane. Classification of a cleavage ranges from perfect, good, poor, indistinct, to none. A mineral can have a basal, prismatic, cubic, rhomboherdal, octahedral, or dodecahedral cleavage.
Fracture: The texture or shape of a mineral's surface. Some types of fractures are described as conchoidal (ripples), earthy (resembles broken soil), hackly (jagged fractures), uneven, and splintery (resembles splinters).
Luster: The way which light reflects off of a mineral. Minerals can have vitreous (glassy), dull (earthy), adamantine (shiny), greasy, silky, metallic, non-metallic, pearly, resinous, or waxy lusters.
Streak: The color of the powder left behind by a mineral when it is scratched on a piece of unglazed porcelain. The color of the powder is usually different from the mineral's color.
Now, onto the list!!
-------------------------------------------------------------
Garnet (January)
Marshals-Ney and Bernadotte
Type: Mineral
Group: Silicate (SiO₄)₃
Color: red, orange, pink, green, yellow blue (rare)
Cleavage: Indistict
Fracture: Conchoidal to uneven
Mohs Scale: 6.5-7.5
Luster: Vitreous
Streak: White
Fun Fact: Garnet is its own family that contains six main species divided into two groups: pyrope, almandine, and spessartine species, which are part of the aluminum group (aluminum is present in its structure). Colors in the Aluminum group range from red to pink; these are the garnet species people think of when looking for jewelry. When pyrope is mixed with almandine, it creates rhodolite, and when mixed with spessartine, it creates malaya. Grossular, uvarovite, and andradite species are part of the calcium group (Calcium is present in structure) and are composed of green to yellow garnet. Uvarovite is the rarest of the calcium group because it grows in small chunks, making it hard to work with when making it into a gemstone.
Amethyst (February)
Marshals- Mortier
Type: Mineral
Group: Silicate (SiO₂)
Color: Purple to Violet
Cleavage: Indistict to none
Fracture: Conchoidal
Mohs Scale: 7
Luster: Vitreous
Streak: White
Fun Fact: Amethyst is part of the quartz family and it used to be part of the cardinal or most valuable gemstones, along with diamonds, rubies, and sapphires, because it was available in small amounts. Its value dropped after large deposits were discovered in Brazil during the 18th century, making it one of the more affordable gemstones.
Aquamarine (March)
Marshals- Brune, Murat, Soult, Suchet
Type: Mineral
Group: Beryl (Be₃Al₂Si₆O₁₈)
Color: Pale blue, light green, bluish-green, sometimes yellow
Cleavage: Indisticnt to none
Fracture: Conchoidal to uneven
Mohs Scale: 7.5-8
Luster: Vitreous
Streak: White
Fun Fact: Aquamarine got its name because its color resembles the sea. It contains small traces of iron, which (depending on the oxidation state) can change its bluish color to green or yellow. These oxidation states are ferrous iron, which gives Aquamarine its blue color, and ferric iron, which gives it a greenish/yellowish color. Heating the mineral removes the greenish color to restore its blue color[1]. Aquamarine also has weak to moderate flourescent properties under UV light [2].
Diamond (April)
Marshals- Jourdan, Lannes, Oudinot, and Saint-Cyr
Type: Mineral
Group: Native mineral (Carbon (C))
Color: Yellow, brown, gray, white, colorless,
Cleavage: Octahedral, Perfect on all sides
Fracture: Uneven
Mohs Scale: 10
Luster: Adamantine
Steak: Colorless
Fun Facts: Diamonds are formed within the Earth's mantle when carbon-rich materials or carbon dioxide are subjected to extreme temperatures and pressure. It reaches the surface via volcanic eruption and gets trapped inside igneous rocks after the magma cools off. The formation of diamonds takes thousands of years, contributing to their high value [3]. Diamonds seen as potential gemstones have little to no impurities or foreign objects within their structure. In contrast, diamonds with high impurities, irregular shapes, and defects are used in commercial industries due to their durability and hardness. Diamonds are the hardest minerals, and are very difficult to scratch or break, but it's not impossible to do so. They also has a high dispersion of white light that creates a rainbow-like effects, also known as 'fire.[4]'
--------------------------------------------------------------
Sources
Garnet: King, H. M. (n.d.). Garnet. geology. https://geology.com/minerals/garnet.shtml
Amethyst: Geary, T.F.; Whalen, D. (2008). The Illustrated Bead Bible: Terms, Tips & Techniques. Sterling Pub. p. 69.
Aquamarine: [1]King, H. M. (n.d.). Aquamarine. geology. https://geology.com/gemstones/aquamarine/
[2]MAT, M. (2023, June 3). Aquamarine: Properties, formation, occurrence " Gemstone. Geology Science. https://geologyscience.com/gemstone/aquamarine/?amp
Diamond: [4]King, H. M. (n.d.). Diamond. geology. https://geology.com/minerals/diamond.shtml
[3]MAT, M. (2023, September 25). Diamond: Properties, formation, occurrence, deposits. Geology Science. https://geologyscience.com/minerals/diamond/
#napoleonic era#napoleon bonaparte#french history#napoleon's mashals#rocks and minerals#gemstone#birthstones#napoleonic wars#sorry for this VERY long post but I had to share my new project of cataloging all of the marshal's birthstones 🥲#I'm dedicating part of my winter break to research minerals and crystals just because :)
26 notes
·
View notes
Text


Semiconductors: Zinc oxide
An inorganic compound used in a wide variety of applications, zinc oxide (ZnO) also finds applications as a semiconductor. Pure ZnO is a white powder, though the naturally occurring mineral (zincite) often contains impurities that have it varying in color from yellow to red. It has two main crystal structures, hexagonal and cubic, and in addition to being a semiconductor is also piezoelectric and thermochromic.
As a semiconductor, ZnO is considered a wide band gap semiconductor (typically n-type) with a value of around 3.3-3.4eV at room temperature. As such, it is often used in higher temperature and higher power applications. Tuning of ZnO's bandgap is usually done by alloying the materials with magnesium or cadmium oxides. Dopant atoms typically include group III elements (such as aluminum) to substitute with zinc or group VII elements (such as chlorine) to substitute with oxygen. Doping with transition metal or rare earth elements can provide interesting optical properties as compared to pure ZnO and as such applications also often include optoelectronics.
One advantage ZnO has over other some other semiconducting materials (such as gallium nitride) is that it can be grown in bulk as single crystals. However, ZnO typically displays n-type superconductivity and doping to produce a p-type semiconductor has proven to be very challenging, which limits the current applications of the material. Primarily, as a semiconductor, it is used in LEDs and lasers as well as transparent contacts.
Sources/Further Reading: (Images source - Wikipedia) (2022 article) (2014 article) (2009 article)
16 notes
·
View notes
Text
Chapter 222 Trivia (Part 1)
Fun fact: this chapter is from issue #2 of WSJ's 2022 collection!
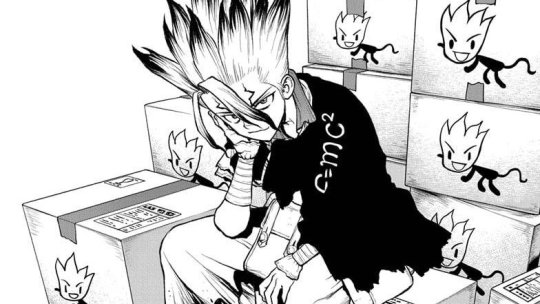
The Apollo mission had spacesuits tailor-made to each astronaut, but these days it's easier to use interchangeable parts and switch them out according to the size of the wearer, rather than having the whole suit fitted.
However, the gloves are always custom-sized for dexterity.


Vinyl fabric doesn't seem to have ever been used as part of a spacesuit, however spandex and nylon have, especially in the inner layers.
Outer layers include Teflon, Kevlar, and aluminized Mylar.
It's possible that rather than being used for the fabric, the vinyl is used for the suit's interior cooling tube system, and the aluminum is used for the Mylar rather than for the exterior metal parts, as pure aluminum is easily scratched.


You probably recognize this panel from the end of chapter 219. The only difference is Ryusui's head has been swapped with Stanley's.


Japanese doesn't have a "V" sound, which is why Chrome says "by" rather than "vi" or "vy".
Generally English words used in Japanese make this switch, for example "violin" becoming "baiorin" due to the lacking of "V" and "L" sounds.

This building may be where they're assembling the SENKU 11 rocket, however in this first panel it appears completed, but in later ones it's still under construction.


The PS5 was first announced in April 2019, and released November 2020. First images of the console were revealed on June 11th 2020.
The first global petrification happened in June 2019, so this person would know about the console but not known what it was meant to look like.


The robot maid request is most likely a reference to "Me and Roboco", another manga currently being published in Weekly Shonen Jump alongside Dr. Stone. It's a comedy series that follows a powerful-but-clumsy maid robot in a grade schooler's service.


(Later, Me and Roboco came out with a Dr. Stone parody for the 15th volume cover)

The vacuum tubes are back in the form of cavity magnetrons. These produce the microwaves that bounce around the microwaves' interior body.
The cooking effect was first discovered in 1945 when Percy Spencer noticed a candy bar had melted in his pocket after testing magnetrons.


Plastic wrap is vinyl that has been flattened to between 8-12 μm thick, (approximately 0.001 cm). For context, this is about as thick as a spider's web or the size of a droplet of water in fog.


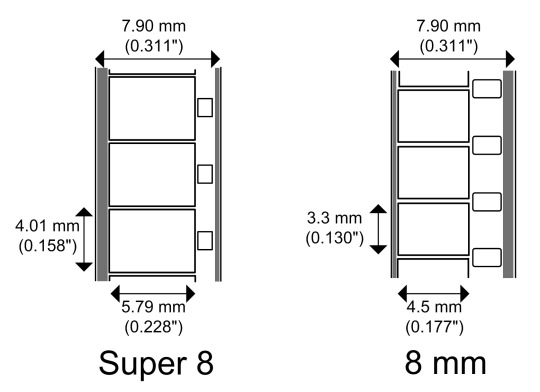
The film Senku makes here is cellulose triacetate film, which is less flammable than earlier celluloid film, earning it the nickname "safety film".
The 8 mm part is the width of the film strip.


Unlike reusable hand-warmers that use supersaturated sodium acetate, these are one-time use and rely on oxidation to create heat. Once the packaging is opened, air penetrates the bag, oxidizing the iron. Vermiculite is added to remove moisture & salt is added as a catalyst.


Chrome's design wouldn't work properly because he uses iron sand rather than iron powder. Iron sand is mostly magnetite, which is already an iron oxide and thus won't have the oxidation reaction or create heat.

The fridge (or maybe mini wine cellar/fridge?) design is a parody of Smeg, a kitchen appliance brand.
You can also see the Senku-brand PlayStation, robot maid, and protein powder.


(Next part)
18 notes
·
View notes
Note
Mixing this aluminum powder and ferric oxide, with a magnesium ignition strip, on a cold winters day, to provide light and heat. Alas, I cannot see, as I've caused retina burns.
huhwhosaidthat?
6 notes
·
View notes
Note
Fun Fact: You can make thermite outa just two simple ingredients! Mix three parts Iron Oxide (rust) with one part powdered aluminum, and there ya go 😀🔥
(Beetlejuice)
compared to the other fun facts. sure. Ill take this fun little fact. thx bj
Back to fruits magazine beloved I go. cuz u knooowww Imma dress u in some dumbass punk shit cuz I love dumbass punk shit.





Besides the usual punk ass clownery lemme get u smth more fancy as well.



I think punk and more fancy type shit do go together quite nicely actually
4 notes
·
View notes
Text
Weather Modification Patents
(Conspiracy Theory, except it’s not)
YEAR - PATENT NUMBER - PATENT NAME
1891 – US462795A – method of producing rain-fall
1914 – US1103490A – rain maker (balloon images)
1917 – US1225521A – protection from poisonous gas in warfare
1920 – US1338343A – process and apparatus for the production of intense artificial clouds, fogs, or mists
1924 – US1512783A – composition for dispelling fogs
1927 – US1619183A – process of producing smoke clouds from moving aircraft
1928 – US1665267A – process of producting artificial fogs
1932 – US1892132A – atomizing attachment for airplane engine exhausts
1933 – US1928963A – electrical system and method (for spraying chemtrails)
1934 – US1957075A – airplane spray equipment
1936 – US2045865A – skywriting apparatus
1936 – US2052626A – method of dispelling fog (mit)
1937 – US2068987A – process of dissipating fog
1939 – US2160900A – method for vapor clearing
1941 – US2232728A – method and composition for dispelling vapors
1941 – US2257360A – desensitized pentaerythritol tetranitrate explosive
1946 – US2395827A – airplane spray unit (us. dept. of agriculture)
1946 – US2409201A – smoke-producing mixture
1949 – US2476171A – smoke screen generator
1949 – US2480967A – aerial discharge device
1950 – US2527230A – method of crystal formation and precipitation
1951 – US2550324A – process for controlling weather
1951 – US2570867A – method of crystal formation and precipitation (general electric)
1952 – US2582678A – material disseminating apparatus for airplanes
1952 – US2591988A – production of tio2 pigments (dupont)
1952 – US2614083A – metal chloride screening smoke mixture
1953 – US2633455A – smoke generator
1954 – US2688069A – steam generator
1955 – US2721495A – method and apparatus for detecting minute crystal forming particles suspended in a gaseous atmosphere (general electric)
1956 – US2730402A – controllable dispersal device
1957 – US2801322A – decomposition chamber for monopropellant fuel
1958 – US2835530A – process for the condensation of atmospheric humidity and dissolution of fog
1959 – US2881335A – generation of electrical fields (haarp – for re-charging clouds!)
1959 – US2903188A – control of tropical cyclone formation
1959 – US2908442A – method for dispersing natural atmospheric fogs and clouds
1960 – US2962450A – fog dispelling composition (see references)
1960 – US2963975A – cloud seeding carbon dioxide bullet
1961 – US2986360A – aerial insecticide dusting device
1962 – US3044911A – propellant system
1962 – US3056556A – method of artificially influencing the weather
1964 – US3120459A – composite incendiary powder containing metal coated oxidizing salts
1964 – US3126155A – silver iodide cloud seeding generator (main commercial ingredient)
1964 – US3127107A – generation of ice-nucleating crystals
1964 – US3131131A – electrostatic mixing in microbial conversions
1965 – US3174150A – self-focusing antenna system (haarp)
1966 – US3257801A – pyrotechnic composition comprising solid oxidizer, boron and aluminum additive and binder
1966 – US3234357A – electrically heated smoke producing device
1966 – US3274035A – metallic composition for production of hydroscopic smoke
1967 – US3300721A – means for communication through a layer of ionized gases (haarp)
1967 – US3313487A – cloud seeding apparatus
1967 – US3338476A – heating device for use with aerosol containers
1968 – US3410489A – automatically adjustable airfoil spray system with pump
1969 – US3429507A – rainmaker
1969 – US3430533A – aircraft dispensor pod having self-sealing ejection tubes
1969 – US3432208A – fluidized particle dispenser (us air force)
1969 – US3437502A – titanium dioxide pigment coated with silica and aluminum (dupont)
1969 – US3441214A – method and apparatus for seeding clouds
2001 -US20030085296A1 - Hurricane and tornado control device
#pay attention#educate yourself#educate yourselves#reeducate yourself#knowledge is power#reeducate yourselves#think for yourself#think for yourselves#think about it#do your homework#do your research#do your own research#question everything#ask yourself questions#ask yourselves#look things up
31 notes
·
View notes
Note
hey bah! here goes a very romantic warmup prompt for hangster
(thanks for putting it on my dash btw 😉)
Hangster + Red Threads
THERMITE
noun
a mixture of finely powdered aluminum and iron oxide that produces a very high temperature on combustion, used in welding and for incendiary bombs'
"thermite grenade"
Let's get together on the sharp edge of a knife
I'm gonna let love tear me down
The first time they lay hands on each other turns out that they want to get under each other's skin like animals claw through fur and flesh. Bradley Bradshaw has grown weary of Jake Seresin running his mouth like he runs a three ring circus. A showman of his own demise, Hangman commands attention and says things he means because he always means something; like he's a poet of terrible preambles.
Bradley understand he does so after spending enough time around the man, that every word comes chock full of reasoning behind, even if at first you can't catch it. But the first time Rooster's hand come in contact with Hangman's too-warm skin isn't because of desire; it's because of white hot rage.
Burning like thermite, bursting anger at words that the blond had thrown his way, he wraps one hand around his arm and another around his throat with eyes burning wet. If this were a cartoon scene, Rooster would be steaming out the ears as he pushes him against a wall. He can hear the way Hangman's breath is knocked out of his lungs and his eyes cross a little as his head hits the wooden wall. In fact, all he can hear and feel is Hangman under his hands, skin too warm, muscles too firm, eyes too bright.
People's shouting around them are all muted, Bradley's ears are roaring as Jake laughs. He laughs in his face even as he's pinned against a wall; laughter that burns back like mustard gas, blistering his lungs as he breathes it in. He may have one hand around Hangman's throat, but it feels like he is the one having the life choked out of himself.
He pulls his hand from the man's throat and closes a pulled fist back but allows his knuckles to connect with the wood right by Hangman's right ear instead. His heart racing like he's ran up a mountain, his own breathing so loud in his ears. Whoever is pulling on Hangman's noose like strings, making him dance to this song of cruelty like the unreal boy he is, has no mercy for Rooster. Splinters from the wood etch themselves into his skin but all he sees are green eyes digging back into his.
When they're finally pulled apart from each other, Rooster realizes there's no coming back from this; whatever this is. Tangled into each other forever, when he tries to fall asleep that night, Hangman's — no, Jake Seresin's rapacious eyes made of verdigris patina burn into his memory and keep him awake, tossing and turning.
Bradley can still hear Hangman's cruel, fluttering laughter loud and clear, making his heart skip enough beats to play another song.
#abt: my writing#sereshaw#hangster#bradley rooster bradshaw#jake hangman seresin#rooster x hangman#top gun fanfic#writing warmups#//IDK I THINK I POPPED OFF WITH THIS ONE#long post#//guess I'm inspired today
27 notes
·
View notes
Text
The invention is a powder that almost instantly kills thousands of waterborne bacteria when simply mixed with water and exposed to normal sunlight for a few seconds. It consists of nano-sized flakes of aluminum oxide, molybdenum sulfide, copper, and iron oxide, which combine with sunlight to form hydrogen peroxide and hydroxyl radicals, Phys.org reported.
These newly formed chemical byproducts work quickly to kill off any bacteria and then dissipate just as quickly.
The powder has several advantages over existing methods of cleaning drinking water. It does not use any chemicals that create lasting toxic byproducts, and it does not require ultraviolet light, which takes a long time and requires electricity, according to Phys.org.
In addition, the powder is recyclable. It can be removed from the now-clean water with a magnet, and researchers were able to reuse the same powder 30 times.
3 notes
·
View notes
Note
Addition of a small amount of glycerine to a pile of iron (III) oxide, aluminum powder, and potassium permanganate produces a flame, sparks, and molten iron.

2 notes
·
View notes
Text
Dimethyl ether, carbon tetrachloride, sodium thiohydrate, pyridine, hydrogen bromide, barium hydroxide, barium sulfide, phenol, hydrochloric acid, dibromomethane, sodium hydroxide, n-butylene ether, 3-methylpyridine, bromoethane, aluminum trichloride solution, benzene, ethanethiol, octadecyl acetamide, acetonitrile, N N-diisopropylethylamine, hydrogen fluoride [anhydrous], potassium antimony tartrate, n-butylacetate, ethylene oxide, cyclohexane, potassium hydroxide, aluminum trichloride [anhydrous], 2-nitroanisole, 1, 2-dichloropropene, n-butanol, magnesium, O O ≤-diethylthiophosphoyl chloride, phenol solution, N-(phenylethyl-4-piperidine) propionamide citrate, ethyl acetate, 1,4-xylene, 2-aminopropane, isophthaloyl chloride, 2-chlorotoluene, cyclopentene, propionic acid, hydrofluoric acid, 2-butenaldehyde, 2-methylpentane, ethylamine, bromine, coal tar pitch, ethyl formate, ammonia solution [containing ammonia > 10%] 1-aminohydrin, 4-ethoxyphenylamine, diisopropylamine, sodium ethanolate, nitrifying asphalt, hydrazide hydrate [containing hydrazide ≤ 64%], dimethyl sulfate, acetic acid [content > 80%], acetaldehyde, 2-butylketone, aluminum borohydrate, phenylethanolnitrile, 2-chlorobenzoyl chloride, sodium hypochlorite solution [containing available chlorine > 5%], 2-aminophenol, chloroplatinic acid, barium chloride, tert-butylbenzene, tribromide, methyl sulfide, Diphosphate pentasulfide, diethylamine, chlorobenzene, n-butylbenzene, 1,3-xylene, hydrogen peroxide solution [content > 8%], terephthaloyl chloride, red phosphorus, tetramethyl ammonium hydroxide, methanol, propionaldehyde, 2-methoxyphenylamine, bleach powder, triethyl propropionate, 1-bromobutene, cyclohexanone, di-(tert-butylperoxy) phthalate [paste Content ≤ 52%], tetrahydrofuran, trichloroethylene, magnesium aluminum powder, formic acid, sodium ethanol ethanol solution, isopropyl ether, acetic acid solution [10% < content ≤ 80%], 2-methyl-1-propanol, diethyl carbonate, sodium aluminum hydroxide, 2-methylpyridine, n-butylamine, toluene, thiourea, magnesium alloy [flake, banded or striped Containing magnesium > 50%], methyl benzoate, hydrobromide, 4-methylpyridine, iodine monochloride, sodium sulfide, 3-bromo-1-propene, 2-propanol, potassium borohydroxide, triethylamine, ammonia, 4-nitro-2-aminophenol, 1, 2-epichlorohydrin, 1-propanol, cyclopentane chloride, n-propyl acetate, bromoacetic acid, zinc chloride solution, trichloromethane, 1-bromopropane, monoamine [anhydrous], perchloric anhydride acetic anhydride solution, 1-bromopropane Potassium hydroxide solution [content ≥ 30%], boric acid, sodium borohydrate, hydroacetic acid bromide solution, acrylic acid [stable], cyclopentane chloride, ammonium hydrogen sulfate, calcium hydroxide, 2-ethoxyaniline, dimethyl carbonate, sodium nitroso, monomethylamine solution, zinc chloride, hydrogen sulfide, trimethyl acetate, iodine trichloride, nitric acid, sodium hydroxide solution [content ≥ 30%], trimethyl orthoformate, hydrogen chloride [anhydrous], 4-methoxyaniline, sulfur, succinile, acetic anhydride, dipropylamine, methyl acetate, isopropylbenzene, propionyl chloride, ethyl formate, phosphorus pentoxide, formaldehyde solution, nitrogen trifluoride, acetone, ethanol [anhydrous], white phosphorus, 1, 2-xylene, 1, 3-dichloropropene, 1, 1, 1-dichloroethane, N N-diethylethanolamine, sulfuric acid, N, N-dimethyl formamide, methyl mercaptan, 4-chlorotoluene, 1, 2-dichloroethane, dichloromethane, succinyl chloride, 2, 3-dichloropropene, xylene isomer mixture, tartrate nicotine, cyclopentane, petroleum ether, bromocyclopentane Potassium perchlorate, potassium chlorate, aluminum powder, chromic acid, iron chloride, lead nitrate, magnesium powder, nickel chloride, nickel sulfate, perchloroethylene, phosphate, potassium dichromate, sodium dichromate, zinc nitrate
2 notes
·
View notes
Text
Aluminum Market: Products, Applications & Beyond
Aluminum is a versatile element with several beneficial properties, such as a high strength-to-weight ratio, corrosion resistance, recyclability, electrical & thermal conductivity, longer lifecycle, and non-toxic nature. As a result, it witnesses high demand from industries like automotive & transportation, electronics, building & construction, foil & packaging, and others. The high applicability of the metal is expected to drive the global aluminum market at a CAGR of 5.24% in the forecast period from 2023 to 2030.

Aluminum – Mining Into Key Products:
Triton Market Research’s report covers bauxite, alumina, primary aluminum, and other products as part of its segment analysis.
Bauxite is anticipated to grow with a CAGR of 5.67% in the product segment over the forecast years.
Bauxite is the primary ore of aluminum. It is a sedimentary rock composed of aluminum-bearing minerals, and is usually mined by surface mining techniques. It is found in several locations across the world, including India, Brazil, Australia, Russia, and China, among others. Australia is the world’s largest bauxite-producing nation, with a production value of over 100 million metric tons in 2022.
Moreover, leading market players Rio Tinto and Alcoa Corporation operate their bauxite mines in the country. These factors are expected to propel Australia’s growth in the Asia-Pacific aluminum market, with an anticipated CAGR of 4.38% over the projected period.
Alumina is expected to grow with a CAGR of 5.42% in the product segment during 2023-2030.
Alumina or aluminum oxide is obtained by chemically processing the bauxite ore using the Bayer process. It possesses excellent dielectric properties, high stiffness & strength, thermal conductivity, wear resistance, and other such favorable characteristics, making it a preferable material for a range of applications.
Hydrolysis of aluminum oxide results in the production of high-purity alumina, a uniform fine powder characterized by a minimum purity level of 99.99%. Its chemical stability, low-temperature sensitivity, and high electrical insulation make HPA an ideal choice for manufacturing LED lights and electric vehicles. The growth of these industries is expected to contribute to the progress of the global HPA market.
EVs Spike Sustainability Trend
As per the estimates from the International Energy Agency, nearly 2 million electric vehicles were sold globally in the first quarter of 2022, with a whopping 75% increase from the preceding year. Aluminum has emerged as the preferred choice for auto manufacturers in this new era of electromobility. Automotive & transportation leads the industry vertical segment in the studied market, garnering $40792.89 million in 2022.
In May 2021, RusAl collaborated with leading rolled aluminum products manufacturer Gränges AB to develop alloys for automotive applications. Automakers are increasingly substituting stainless steel with aluminum in their products owing to the latter’s low weight, higher impact absorption capacity, and better driving range.
Also, electric vehicles have a considerably lower carbon footprint compared to their traditional counterparts. With the growing need for lowering emissions and raising awareness of energy conservation, governments worldwide are encouraging the use of EVs, which is expected to propel the demand for aluminum over the forecast period.
The Netherlands is one of the leading countries in Europe in terms of EV adoption. The Dutch government has set an ambitious goal that only zero-emission passenger cars (such as battery-operated EVs, hydrogen FCEVs, and plug-in hybrid EVs) will be sold in the nation by 2030. Further, according to the Canadian government, the country’s aluminum producers have some of the lowest CO2 footprints in the world.
Alcoa Corporation and Rio Tinto partnered to form ELYSIS, headquartered in Montréal, Canada. In 2021, it successfully produced carbon-free aluminum at its Industrial Research and Development Center in Saguenay. The company is heralding the beginning of a new era for the global aluminum market with its ELYSIS™ technology, which eliminates all direct GHG emissions from the smelting process, and is the first technology ever to emit oxygen as a byproduct.
Wrapping Up
Aluminum is among the most widely used metals in the world today, and is anticipated to underpin the global transition to a low-carbon economy. Moreover, it is 100% recyclable and can retain its properties & quality post the recycling process.
Reprocessing the metal is a more energy-efficient option compared to extracting the element from an ore, causing less environmental damage. As a result, the demand for aluminum in the sustainable energy sector has thus increased. The efforts to combat climate change are thus expected to bolster the aluminum market’s growth over the forecast period.
#Aluminum Market#aluminum#chemicals and materials#specialty chemicals#market research#market research reports#triton market research
4 notes
·
View notes
Note
hehe funny evil
Give me iron oxide, powdered aluminum, and barium nitrate I will be responsible with those I promise :3
6 notes
·
View notes
Text
Manufacturing Process of Aluminum Foil
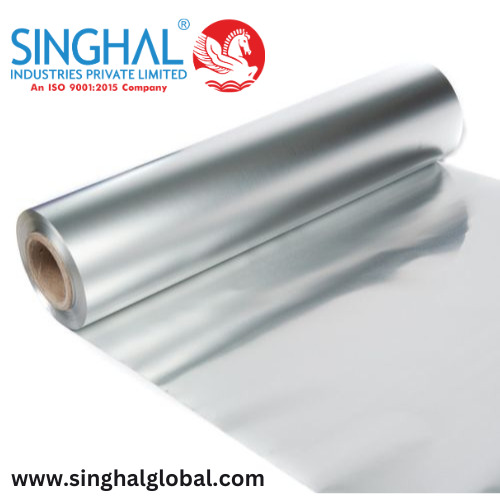
Introduction
Aluminum foil is an essential item in kitchens around the world, but its uses extend far beyond household applications. It's used in packaging, insulation, and even in industrial processes. Understanding the manufacturing process of aluminum foil provides insight into the complexities and advancements in modern production techniques. This article will explore the step-by-step process of creating aluminum foil sheets and address some frequently asked questions. Keywords such as "Aluminium foil sheet," "aluminum foil sheet," and "aluminium foil price" will be included to ensure a comprehensive understanding.
The Manufacturing Process
Mining and Refining:
The journey of aluminum foil starts with the extraction of bauxite ore, which is rich in aluminum oxide. The bauxite is then refined into alumina through the Bayer process. This involves crushing the bauxite and mixing it with sodium hydroxide, which dissolves the alumina. The solution is then cooled and the alumina precipitates out, which is filtered and heated to remove water, resulting in pure alumina powder.
Smelting:
The next step is to convert alumina into aluminum through the Hall-Héroult process. This involves dissolving alumina in molten cryolite and then applying a direct current to produce aluminum metal at the cathode and oxygen gas at the anode. The aluminum collected at the bottom of the electrolytic cell is then siphoned off.
Casting and Rolling:
The molten aluminum is cast into large slabs called ingots or billets. These ingots are then preheated and passed through a series of rollers to be flattened into thin sheets. This process is known as rolling. Initially, the aluminum sheet is thick and is gradually rolled thinner and thinner until it reaches the desired thickness for aluminum foil.
Annealing:
To ensure the aluminum foil is flexible and can be easily wrapped or folded, it undergoes an annealing process. This involves heating the rolled aluminum sheets in a furnace to soften the material. Annealing adjusts the mechanical properties of the aluminum, making it more ductile and easier to work with.
Final Rolling and Slitting:
After annealing, the aluminum sheet is rolled again to achieve the final thickness. This can be as thin as 0.006 mm. The sheets are then slit into the desired width and length, creating Aluminum foil sheet ready for packaging and distribution.
Packaging:
The finished aluminum foil sheets are packaged for shipment. They are wound into large rolls for industrial use or cut into smaller rolls for consumer use. Packaging is done in a way to protect the foil from damage and contamination.
Conclusion
The manufacturing process of aluminum foil sheets is a sophisticated and multi-step procedure that transforms raw bauxite into thin, flexible sheets of aluminum. This process involves refining, smelting, rolling, annealing, and packaging, ensuring that the final product meets various standards of quality and usability. Understanding this process, along with the factors affecting the Aluminium foil price, provides valuable insight into this ubiquitous material. Whether referred to as "aluminium foil sheet" or "aluminum foil sheet," this versatile product plays an essential role in numerous applications, from everyday kitchen use to high-tech industrial functions.
Frequently Asked Questions
Q: What is the difference between "aluminium foil sheet" and "aluminum foil sheet"?
A: "Aluminium" is the spelling used in British English, while "aluminum" is the spelling used in American English. Both terms refer to the same product, aluminum foil sheets, used for wrapping, cooking, and various industrial applications.
Q: How is the thickness of aluminum foil measured?
A: The thickness of aluminum foil is measured in micrometers (µm) or mils (thousandths of an inch). Household aluminum foil typically has a thickness ranging from 0.016 mm (16 µm) to 0.024 mm (24 µm), while heavy-duty aluminum foil can be up to 0.05 mm (50 µm) thick.
Q: What affects the aluminium foil price?
A: Several factors influence the price of aluminum foil, including the cost of raw materials (bauxite), energy costs for refining and smelting, labor costs, and market demand. Additionally, fluctuations in global aluminum prices and economic conditions can impact the aluminium foil price.
Q: What are the environmental impacts of aluminum foil production?
A: The production of aluminum foil involves significant energy consumption, especially during the smelting process. This can lead to greenhouse gas emissions if non-renewable energy sources are used. However, aluminum is highly recyclable, and recycling aluminum foil requires only 5% of the energy needed to produce new aluminum, significantly reducing its environmental footprint.
0 notes
Text
Remove Powder Coating from Metal: Easy Techniques

To remove powder coating from metal, use acetone as an effective solvent. Acetone can help break down the coating for easy removal, making it a suitable option for stripping powder coating from metal surfaces.
When it comes to removing powder coating, acetone is a mild solvent that can efficiently dissolve the coating, aiding in its removal process. This method is commonly used in preparing surfaces for powder coating applications or stripping existing coatings. Acetone's versatility and effectiveness make it a preferred choice for removing powder coating from metal surfaces.
Chemical Stripping
Chemical stripping is a highly effective method for removing powder coating from metal surfaces. This process involves using chemical solvents to break down and dissolve the powder coating, making it easier to remove. Chemical stripping is often preferred for its ability to remove coating from intricate or hard-to-reach areas.
Using Acetone
Acetone is a widely used solvent for removing powder coating from metal. It is a mild yet effective solvent that can break down the coating without causing damage to the metal surface. When using acetone, it's important to apply it to the coated surface and allow it to sit for a few minutes before gently scrubbing or wiping off the softened coating. Acetone is readily available and is a popular choice for DIY enthusiasts.
Other Effective Solvents
Aside from acetone, there are other solvents that can effectively remove powder coating from metal. Methylene chloride is another commonly used solvent for this purpose. It can break down the coating, making it easier to remove. However, it's important to handle these solvents with care and follow safety guidelines when using them.
Thermal Stripping
Thermal stripping is a method used to remove powder coating from metal surfaces by utilizing heat. This process involves exposing the coated metal to high temperatures to break down and strip away the powder coating effectively.
High-temperature Burning
In high-temperature burning, the powder coating is subjected to extreme heat to burn off the coating layer. This method is efficient for removing thick or stubborn coatings from metal surfaces.
Heat Guns
Heat guns are a common tool used in thermal stripping processes. By directing intense heat onto the powder-coated surface, the coating softens and can be easily peeled or scraped off the metal.
https://www.youtube.com/watch?v=tQkzpCBbXfM
Abrasive Stripping
Abrasive stripping is a highly effective method for removing powder coating from metal surfaces. It involves using abrasive materials to physically strip away the coating, leaving the metal clean and ready for refinishing.
Sandblasting
Sandblasting, also known as abrasive blasting, is a common technique for removing powder coating. It involves propelling fine abrasive particles at high velocity onto the surface to remove the coating.
Media Blasting
Media blasting is another form of abrasive stripping that uses various types of media such as glass beads, aluminum oxide, or walnut shells to remove the powder coating from metal surfaces.
Popular Techniques Explained
Dmax909's Powder Coating Strip In Minutes
DMAX909's method allows for quick and efficient removal of powder coating from metal surfaces. By using a specially formulated stripping compound, they demonstrate how to achieve results in a matter of minutes. From spraying the stripping compound to the final step of removing the remaining coating, this technique offers a rapid solution for powder coat removal.
Cycle Fab's Best Powder Coat Stripper From Your Local Store
Cycle Fab presents a method using readily available tools from your local store to strip powder coating effectively. This technique emphasizes the tools needed, the process of cleaning up, and provides a comprehensive guide on how to use the best powder coat stripper for efficient results.
Alexltdlx's The Easiest Way To Remove Powder Coat!
AlexLTDLX showcases an easy method to remove powder coating, highlighting the process, materials needed, and the final results. This technique offers a straightforward approach to effectively remove powder coating from metal surfaces, making it an accessible option for many.
What Solvent Breaks Down Powder Coating?

Acetone, a mild solvent, is effective for removing powder coating from metal surfaces. Other methods include chemical stripping, thermal stripping, abrasive, and laser removal techniques. Methylene chloride is also known to break down powder coating effectively.
When it comes to removing powder coating from metal, there are several options available. One of the most effective ways is to use a solvent that can break down the coating. But what solvent should you use? Let's explore two options: acetone and methylene chloride.
Acetone:
Acetone is a mild solvent that is effective as a general cleaner. It can be used to prepare surfaces for powder coating. However, it can also be used to remove powder coatings as required. Acetone is readily available and affordable, making it a popular choice for DIY enthusiasts.
Methylene Chloride:
Methylene chloride is a stronger solvent than acetone. It is generally effective at removing powder coating. However, it is important to use it with caution as it is a hazardous substance that should only be used in a well-ventilated area. It can also be expensive compared to other solvents.
In conclusion, both acetone and methylene chloride are effective at removing powder coating from metal. Acetone is a milder solvent that is readily available and affordable. Methylene chloride is a stronger solvent but should be used with caution due to its hazardous nature. Regardless of which solvent you choose, always follow the manufacturer's instructions and take proper safety precautions.
Tips And Tricks
If you need to remove powder coating from metal, it's essential to have the right tips and tricks up your sleeve. Here are some valuable insights to help you through the process.
Protective Equipment And Clothing
When undertaking the removal of powder coating from metal, it's crucial to prioritize safety. Here's a rundown of the protective equipment and clothing you'll need:
- Chemical-resistant gloves
- Safety goggles or a face shield
- Respirator mask
- Protective clothing to cover skin
Cleaning And Prepping The Surface
Prior to starting the removal process, proper cleaning and surface preparation are essential. Follow these steps to ensure the surface is ready for treatment:
- Remove any dirt, grease, or debris from the metal surface.
- Use a degreaser to thoroughly clean the area.
- Rinse the surface with water and allow it to dry completely.
Choosing The Right Technique For Your Project
There are various techniques for removing powder coating from metal, and selecting the right one for your project is crucial. Here are the options to consider:
- Chemical stripping: Utilizing a chemical solvent to break down the coating.
- Thermal stripping: Applying heat to the metal to burn off the powder coating.
- Abrasive methods: Using sandblasting or media blasting to remove the coating.
- Laser removal: Employing laser technology to strip the powder coating.

Frequently Asked Questions
What Chemical Will Remove Powder Coating?
Acetone is a mild solvent effective for removing powder coating and preparing surfaces for powder coating application.
How Do You Get Powder Coat Off Metal?
To remove powder coat from metal, utilize chemical stripping, thermal stripping, abrasive methods, or laser removal for effective results.
What Breaks Down Powder Coating?
Powder coating breaks down through chemical stripping, thermal stripping, abrasive methods, and laser removal.
What Is The Solvent For Powder Coating?
The solvent for powder coating is acetone. It effectively removes powder coating and can also be used to prepare surfaces for powder coating.
Conclusion
Removing powder coating from metal can be achieved through various methods such as chemical stripping, thermal stripping, abrasive blasting, and laser removal. Acetone and methylene chloride are effective solvents for this purpose. It's essential to carefully consider safety measures and environmental impact when choosing a removal method.
With the right approach, powder coating removal can be done efficiently and safely.
Read the full article
0 notes
Text
Exothermic powder supplier
The Marvels of Exothermic Powder: A Deep Dive into Its Applications and Benefits
In the realm of chemistry and industrial applications, exothermic powder stands out as a fascinating and highly useful substance. Known for its ability to release heat when reacting, this type of powder has a multitude of applications across various fields. From welding to metalwork, and even emergency fire-starting kits, the versatility and efficiency of exothermic powder make it an indispensable tool. In this blog post, we'll explore what exothermic powder is, its primary uses, and the benefits it offers.
What is Exothermic Powder?
Exothermic powder is a chemical compound designed to produce a significant amount of heat through an exothermic reaction. This reaction occurs when the powder is ignited or subjected to certain conditions, causing it to oxidize and release energy in the form of heat. The composition of exothermic powder can vary, but it typically includes a mix of metal powders and oxidizers. One of the most common formulations includes aluminum powder and iron oxide, known as thermite.
Key Applications of Exothermic Powder
1. Welding and Metal Joining
One of the primary uses of exothermic powder is in welding and metal joining processes. In thermite welding, the powder is used to create molten metal that fuses pieces of metal together. This method is particularly useful for joining railroad tracks, where strong and durable bonds are essential. The high temperatures generated by the exothermic reaction ensure a complete and robust weld, making it a preferred choice in heavy-duty applications.
2. Metal Cutting
Exothermic powder is also employed in metal cutting, particularly in scenarios where conventional cutting methods are impractical. The intense heat produced by the powder can cut through thick metal plates with ease, making it invaluable in demolition and emergency rescue operations. For example, firefighters and rescue teams use exothermic cutting torches to quickly cut through metal barriers and gain access during critical situations.
3. Chemical Production
In the chemical industry, exothermic powder is used to initiate specific reactions that require high temperatures. These reactions can produce various chemicals and compounds essential for different industrial processes.
0 notes
Text
Calcined Aluminum Oxide Powder: A Versatile Material for Various Applications
Calcined aluminum oxide powder, also known as calcined alumina, is a high-purity material with a wide range of applications. In this article, we’ll explore its properties, uses, and benefits.Get more news about Calcined Aluminum Oxide Powder,you can vist our website!
What Is Calcined Alumina?
Calcined alumina is produced by heating hydrated alumina (Al2O3) at high temperatures (typically 1200°C to 1300°C). This process converts it into alpha-alumina, which is the most stable form of aluminum oxide. The resulting powder exhibits exceptional purity, making it ideal for various industrial and technical purposes.
Applications of Calcined Alumina:
Advanced Technical Ceramics:
Calcined alumina serves as a crucial component in the production of advanced ceramics. Its high purity and excellent thermal stability make it suitable for ceramic substrates, insulators, and other electronic components.
Surface Finishing:
The fine particle size and hardness of calcined alumina make it an effective abrasive material for surface finishing. It is commonly used in polishing applications, where precision and consistency are essential.
Lapping and Polishing:
Industries such as optics, semiconductor manufacturing, and precision engineering rely on calcined alumina for lapping and polishing processes. Its uniform particle size distribution ensures smooth and precise surface finishes.
Wear-Resistant Additives:
Calcined alumina can be incorporated into resin systems as wear-resistant additives. Its hardness and color (similar to white aluminum oxide) contribute to improved durability in various materials.
Benefits:
High Purity: Calcined alumina typically has a purity level exceeding 99.0%, ensuring minimal impurities in the final product.
Stability: Alpha-alumina remains stable even at high temperatures, making it suitable for extreme environments.
Consistent Particle Size: The fine particle size distribution allows for precise control in various applications.
Conclusion:
Calcined aluminum oxide powder plays a vital role in modern industries, from ceramics to surface finishing. Its versatility and reliability make it a valuable material for engineers, manufacturers, and researchers alike.
0 notes