#coco3
Text
RECEITA MARAVILHOSA DE PAÇOCA LOWCARB
Hoje vamos trazer uma maravilhosa receita lowcarb de paçoca. Você pode fazer todos vão amar essa delícia de receita
Fonte da receita: Revista Café

Ingredientes250g de amendoim torrado sem casca e sem sal100g de farinha de amêndoas ou de coco3 colheres (sopa) manteiga (sem sal) amolecida ou então óleo de coco4 colheres (sopa) adoçante low-carb (xilitol ou eritritol)1 pitada de salModo de preparoPrimeiramente vamos colocar todos os ingredientes no processador. Claro que você pode colocar em um liquidificador mais potente.Então, é só ligar e bater até ficar uma farofa fina. Em seguida, coloque essa farofa numa forma ou em várias forminhas (formato opcional). Aperte bem a massa contra a forma e se estiver na forma grande, pode cortar em cubinhos ou como preferir.Por fim leve na geladeira por cerca de 30 minutos. Retire da geladeira e então é só servir essa deliciosa paçoquinha caseira low carb.
0 notes
Text





Pfffft!
Well, some vitamins are scam tbh, so there’s some basis for Miguel’s skepticism.
#casual liveblog10#missfinefeather liveblogs#casual liveblogging#movie night#coco3#cocoliveblog#liveblog#coco#pixar#blacklist Missfinefeather
51 notes
·
View notes
Note
Hey coco3, long time fan, how much for you hunt me and down, break into my house and beat me to death with a blunt instrument in the small hours of the morning?
I’ve heard that falling coconuts have killed more people than shark attacks have, but not all coconuts are violent, bloodthirsty brutes. This kind of stereotyping has got to stop!
24 notes
·
View notes
Text
Notes from Emmanuel Cooper on glazing.
All glazes have 3 mutual components; fluxes (make the glaze melt), amphoterics or stabilisers (give body) and acidic oxides (glass forming or bone part of the glaze)
fritz are a way of adding water soluble materials to a glaze, and act as a powerful flux. Potassium, sodium and borax are readily available in fritted form.
Glaze from local clays:
clay should be dried out completely and broken into smaller lumps and these should be dropped into warm water.
Once they have slaked down, the clay slip should be mixed thoroughly and poured through a 80s mesh sieve.
it will take around 2-3 days to settle, then the slip should be put out to dry (or kept in container to paint with)
most local clays, particularly those rich in iron (approx 8%) make great glaze materials. They will melt on their own at 1250 to form a dark coloured (usually brown or black) shiny glaze.
if the local clay isn’t as rich in iron or doesn’t flux well due to particle size being too large, it can be ground down in a pestle and mortar.
colouring glazes:
many potters will experiment with a handful of good workable glazes rather than try out different recipes. e.g. a reliable clear shiny glaze can be easily opacified by the addition of tin oxide or zironium silicate. Such a glaze can also be coloured adding metal oxides, underglaze colour and stains.
Keep to one clay body when figuring out glaze- need to keep a constant.
white glazes:
Tin Oxide (SnO2) will make most shiny glazes opaque- an addition of 8-12% will give a clear, blue white. Zincronium Silicate (ZrSiO4) (zircon) is a less refined form of zincronium oxide and is used as an opacifier- 6-15% will give a neutral or cream white.
coloured glazes (oxides):
Chromium Oxide (Cr2O3) in most glazes gives an opaque green if added 0.5-2%.
Cobalt Carbonate (CoCO3) produces a blue glaze varying from pink mauve (in a dolomite glaze), and a vivid blue in an alkaline glaze, to midnight blue in a feldspathic glaze- additions will vary from 0.25-3%, and results are not affected strongly by reduction or oxidation atmospheres.
Cobalt Oxide (CoO) gives similar results cobalt carbonate but it is, weight for weight, more powerful. Tends to distribute itself less evenly in the glaze and can cause blue specks.
Copper Carbonate (CuCO3) produces colours that range from pink (in dolomite glazes) and red (0.5% in reduction), green (4%- strong in lead glazes) to turquoise (2% alkaline in oxidation)
Copper Oxide (CuO) is similar to copper carbonate but is weight for weight more powerful. (note: both copper carbonate and copper oxide encourage the release of lead during the glaze firing making the lead soluble in acid solutions- therefore, it is not food safe and should not be present on the inside of functional ware.
Llmenite (FeO2TiO2); a naturally occuring ore containing iron and titanium, can cause interesting brown speckles in bodies and glazes
Iron Oxide: Black (FeO) or Red (Fe2O3): Black iron oxide is stronger than the red, but better results are obtained using the synthetic red iron oxide. Depending on the amount added (1-15%) and the firing atmosphere, the colour will range from pale blue green to brown black red (reduction), pale honey to olive brown to black red (oxidation) in feldspathic glazes. In dolomite glazes, the colour tends to be more muddy and muted. Iron oxide is a flux, and will cause glazes to run more.
Maganese carbonate (MnCo3) gives pink mauve colours in alkaline and dolomite glazes, and browns in feldspathic glazes using 1-8%.
maganese dioxide (MnO2) gives similar results to MnCo3 but is weight for weight more powerful.
Nickel Oxide: (NiO) will give colours ranging from ice blue (with zinc oxide glazes), yellow (with zinc oxide and titanium dioxide), pink mauve (with barium carbonate and zinc oxide) to muted greens and greys in most glazes. amounts added range from 1-3%
Rutile (FeTiO3) sometimes called the rutile sand, is an ore containing titanium with iron oxide. It gives buff or brown colours in oxidation glazes, which can be mottled or crystalline, and it opacifies the glaze. In reduction rich blue grey colours can be achieved. Amounts added may be 2-15%.
Titanium Dioxide (TiO2) gives glazes a matt creamy white colour in oxidation, and is often used in crystalline glazes, in reduction it gives rich blue mottled effect. add 2-10%
Vanadium Pentoxide (V2O5) gives colours ranging from yellow to brown and tends to break up the glaze- added in amounts of 3-8%
Yellow ochre (Fe2O3) natural form of iron oxide containing clay, gives similar effects in the glaze.
0 notes
Photo






✿ Icons Suho;; ✿
━ Follow it and make your requests. ღ
━ like or reblog if you save.
━ With more quality: http://imgur.com/a/Coco3
¸.•*¨*•.¸♪¸.•*¨*•.¸♥¸.•*¨*•.¸ Have a nice day¸ .•*¨*•.¸♥¸.•*¨*•.¸♪¸.•*¨*•.¸
20 notes
·
View notes
Text
Puding de chía y fresa
Ingredientes:
250 gramos de yogurt de coco3 cucharadas de chíaErititrol al gusto,en este caso 50 g4 fresas3 onzas de chocolate derretido
Preparación:
Mezclar el yogurt con el erititrol y la chía y dejar reposar en la nevera 3-4 horas o toda la noche.
Cortar las fresas en láminas y decorar y por encima añadir el chocolate derretido.
Y ya está listo,es así de sencillo sin más complicaciones,a…

View On WordPress
0 notes
Photo

Fashion Quotes : coco3 https://ift.tt/2D6OQ8v
0 notes
Text
Inorganic Compounds and Their Uses
Common Names and Formulas of Important Chemical Compounds for SSC Exams
If you keep an eye on the latest trend followed by SSC for General Awareness questions, you would notice that Science facts are covering major portions of it. Questions belonging to physics, chemistry & biology are frequently asked in most of the competitive exams including SSC CGL, SSC CHSL, SSC MTS, SSC CPO, SCC JEE, SSC LDC, IBPS PO, IBPS Clerk, IBPS SO, IPPB Sc. I, LIC AAO etc. For students who had science in 10+2, answering such questions is not hard but for those who did not, it becomes quite difficult. Nowadays a lot of question about Common names of chemical compounds are being included in SSC CPO exams 2017. To help you quickly take a look at important Chemical compounds and their common names, we are providing you with a list of chemical compounds and their common names. Before you take a look at the list of Chemical Compounds and formula SSC, let’s understand some basic definitions below.
Generally questions from the topic Common Names and Formulas of Important Chemical Compounds are asked in every competitive exam. So, here are short notes on Common Names and Formulas of Important Chemical Compounds which will be useful for upcoming SSC as well as other competitive exams as well.
Comprehensive Study List of Chemical Compounds and Formula
Common Name
Formula
Chemical Name
Used In
Alum
KAl(SO4)2·12H2O
Potassium Aluminum Sulphate
Purification Of Water To Remove Dirt.
Baking Soda
NaHCO3
Sodium Bicarbonate
Fire Extinguisher, Cooking, Antacid Etc.
Bleaching Powder
Ca(ClO)2
Calcium Oxychloride
Used As Bleaching Agent And Disinfectant.
Blue Vitriol
CuSO4·5H2O
Copper Sulphate
Bordeaux Mixture.
Borax
Na2B4O7·10H2O
Sodium Tetraborate
Used As A Flux In Optical Gas, In Match, Stick To Prevent After Glow, As A Preservative.
Bordeaux Mixture Or Bordo Mix
CuSO4 & Ca(OH)2
Mixture Of Copper Sulphate And Milk Of Lime
Used As A Fungicide.
Caustic Soda
NaOH
Sodium Hydroxide
Manufacture Of Soap, Paper, Rayon, Etc.
Common Salt
NaCl
Sodium Chloride
Food Preservative.
Corrosive Sublimate
HgCl2
Mercuric Chloride
Batteries.
Dry Ice
CO2
Solid Carbon Dioxide
Used To Induce Artificial Rain, Cinema Locations Etc.
Epsom Salt
MgSO4
Magnesium Sulphate (Hepta Hydrate)
Used As Laxative.
Glauber's Salt
Na2SO4
Sodium Sulphate
Manufacture Of Window Glass, Brown Paper, As Detergent Additive.
Gypsum or Plaster Of Paris
CaSO4·2H2O
Calcium Sulphate
Cement, Production Of (Dihydrate) Plaster Of Paris Etc.
Heavy Spar
BaSO4
Barium Sulphate
Used As A Barium Meal For Contrast Dye X
Hypo
Na2S2O3
Sodium Thiosulphate
Photography For Fixing Or Washing.
Indian Salt Peter
KNO3
Potassium Nitrate
Gun Powder Which Is A 6:1:1 Mixture Of Potassium, Charcoal, And Sulphur.
Lime Stone, Marble
CaCO3
Calcium Carbonate
Cement, Glass Mortar, White Washing Spar Etc.
Lunar Caustic
AgNO3
Silver Nitrate
Silver Mirror, Marking Ink For Identification Of Person In Elections Etc.
Lime Water
Ca(OH)2
Calcium Hydroxide
Cement, Glass, Mortar, White Washing Etc.
Oil Of Vitriol
H2SO4
Sulphuric Acid
King Of Chemicals, Most Industries.
Pearl Ash
K2CO3
Potassium Carbonate
Soft Soap, Washing Wool Etc.
Philosopher's Wool
ZnO
Zinc Oxide
Paints, As A Filler In Rubber Etc.
Quick Lime
CaO
Calcium Oxide
Cement, Glass, Mortar, White Washing Etc.
Sal Ammoniac
NH4Cl
Ammonium Chloride
Used For Soldering, In Dry Cell Etc.
Washing Soda
Na2CO3
Sodium Carbonate
Manufacture Of Glass, Softening Of Water For Washing Cloths Etc.
Water Glass
Na2O3Si
Sodium Silicate
Used As A Filler In Soap, Fire Proofing Timber And Textiles Etc.
White Vitriol
ZnSO₄
Zinc Sulphate
Used To Produce White Paint By Mixing With With Barium Sulphate.
Common Names of Chemical Compounds and Formula SSC - GK Notes
Chemical name starts from “A”:
Aluminium antimonide – AlSb
Aluminium arsenide – AlAs
Aluminium nitride – AlN
Aluminium oxide – Al2O3
Aluminium phosphide – AlP
Aluminium chloride – AlCl3
Aluminium fluoride – AlF3
Aluminium hydroxide – Al(OH)3
Aluminium nitrate – Al(NO3)3
Aluminium sulfate – Al2(SO4)3
Ammonia – NH3
Ammonium azide – NH4N3
Ammonium bicarbonate – NH4HCO3
Ammonium chromate – (NH4)2CrO4
Ammonium cerium(IV) nitrate – (NH4)2Ce(NO3)6
Ammonium chloride – NH4Cl
Ammonium chlorate – NH4ClO3
Ammonium cyanide – NH4CN
Ammonium dichromate – (NH4)2Cr2O7
Ammonium hydroxide – NH4OH
Ammonium hexachloroplatinate – (NH4)2(PtCl6)
Ammonium nitrate – NH4NO3
Ammonium sulfide – (NH4)2S4
Ammonium sulfite – (NH4)2SO3
Ammonium sulfate – (NH4)2SO4
Ammonium persulfate – (NH4)2S2O8
Ammonium perchlorate – NH4ClO4
Ammonium tetrathiocyanatodiamminechromate(III) – NH4
Antimony hydride – SbH3
Antimony pentachloride – SbCl5
Antimony pentafluoride – SbF5
Antimony trioxide – Sb2O3
Arsine – AsH3
Arsenic trioxide (Arsenic(III) oxide) – As2O3
Arsenous acid – As(OH)3
Chemical name starts from “B”:
Barium azide – Ba(N3)3
Barium chloride – BaCl2
Barium chromate – BaCrO4
Barium chlorate – BaClO3
Barium carbonate – BaCO3
Barium hydroxide – Ba(OH)2
Barium iodide – BaI2
Barium nitrate – Ba(NO3)2
Barium sulfate – BaSO4
Barium fluoride – BaF2
Barium ferrite – BaFe2O4
Barium ferrate – BaFeO4
Barium titanate – BaTiO3
Barium oxide – BaO
Barium peroxide – BaO2
Beryllium bromide – BeBr2
Beryllium carbonate – BeCO3
Beryllium chloride – BeCl2
Beryllium fluoride – BeF2
Beryllium hydride – BeH2
Beryllium hydroxide – Be(OH)2
Beryllium iodide – BeI2
Beryllium nitrate – Be(NO3)2
Beryllium nitride – Be3N2
Beryllium oxide – BeO
Beryllium sulfate – BeSO4
Beryllium sulfite – BeSO3
Beryllium borohydride – Be(BH4)2
Beryllium telluride – BeTe
Bismuth(III) oxide – Bi2O3
Bismuth(III) telluride – Bi2Te3
Borane – Diborane: B2H6, Pentaborane: B5H9 Decaborane: B10H14
Borax – Na2B4O7·10H2O
Boric acid – H3BO3
Boron carbide – B4C
Boron nitride – BN
Boron oxide – B2O3
Boron suboxide – B6O
Boron trichloride – BCl3
Boron trifluoride – BF3
Bromine pentafluoride – BrF5
Bromine trifluoride – BrF3
Bromine monochloride – BrCl
Chemical name starts from “C”:
Cacodylic acid – (CH3)2AsO2H
Cadmium arsenide – Cd3As2
Cadmium bromide – CdBr2
Cadmium chloride – CdCl2
Cadmium fluoride – CdF2
Cadmium iodide – CdI2
Cadmium nitrate – Cd(NO3)2
Cadmium selenide – CdSe (of quantum dot fame)
Cadmium sulfate – CdSO4
Cadmium telluride – CdTe
Caesium bicarbonate – CsHCO3
Caesium carbonate – Cs2CO3
Caesium chromate – Cs2CrO4
Caesium chloride – CsCl
Caesium fluoride – CsF
Caesium hydride – CsH
Calcium carbide – CaC2
Calcium chlorate – Ca(ClO3)2
Calcium chloride – CaCl2
Calcium chromate – CaCrO4
Calcium cyanamide – CaCN2
Calcium fluoride – CaF2
Calcium hydride – CaH2
Calcium hydroxide – Ca(OH)2
Calcium sulfate (Gypsum) – CaSO4
Carbon dioxide – CO2
Carbon disulfide – CS2
Carbon monoxide – CO
Carbonic acid – H2CO3
Carbon tetrabromide – CBr4
Carbon tetrachloride – CCl4
Carbon tetraiodide – CI4
Carbonyl fluoride – COF2
Carbonyl sulfide – COS
Carboplatin – C6H12N2O4Pt
carborundum SiC
Cerium(III) chloride – CeCl3
Cerium(III) bromide – CeBr3
Cerium(IV) sulfate – Ce(SO4)2
Cerium magnesium – CeMg
Cerium aluminium – CeAl
Cerium zinc – CeZn
Cerium silver – CeAg
Cerium cadmium – CeCd
Cerium mercury – CeHg
Cerium thallium – CeTl
Chloric acid – HClO3
Chlorine – Cl2
Chlorine monoxide – ClO
Chlorine dioxide – ClO2
Chlorine trioxide – ClO3
Chlorine tetroxide, the peroxide – O3ClOOClO3
Chromic acid – CrO3
Chromium(III) chloride – CrCl3
Chromium(II) chloride – CrCl2 (also chromous chloride)
Chromium(III) oxide – Cr2O3
Chromium(IV) oxide – CrO2
Chromium(II) sulfate – CrSO4
Chromium trioxide (Chromic acid) – CrO3
Chromyl chloride – CrO2Cl2
Cisplatin (cis-platinum(II) chloride diammine)– PtCl2(NH3)2
Cobalt(II) bromide – CoBr2
Cobalt(II) chloride – CoCl2
Cobalt(II) carbonate – CoCO3
Cobalt(II) sulfate – CoSO4
Columbite – Fe2+Nb2O6
Copper(II) azide – Cu(N3)2
Copper(II) carbonate – CuCO3
Copper(I) chloride – CuCl
Copper(II) chloride – CuCl2
Copper(II) hydroxide – Cu(OH)2
Copper(II) nitrate – Cu(NO3)2
Copper(I) oxide – Cu2O
Copper(II) oxide – CuO
Copper(II) sulfate – CuSO4
Copper(I) sulfide – Cu2S
Copper(II) sulfide – CuS
Cyanogen – (CN)2
Cyanogen chloride – CNCl
Cyanuric chloride – C3Cl3N3
Cyanogen bromide – CNBr
Cyanogen iodide – ICN
Chrome-alum; K2SO4Cr2(SO4)3.24H2O
Chemical name starts from “D”:
Dichlorine monoxide – Cl2O
Dichlorine dioxide – Cl2O2
Dichlorine trioxide – Cl2O3
Dichlorine tetroxide,also known as chlorine perchlorate – ClOClO3
Dichlorine hexoxide – Cl2O6
Dichlorine heptoxide – Cl2O7
Decaborane (Diborane) – B10H14
Diammonium phosphate – (NH4)2HPO4
Diborane – B2H6
Dichlorosilane – SiH2Cl2
Digallane – Ga2H6
Dinitrogen pentoxide (nitronium nitrate) – N2O5
Dinitrogen tetroxide – N2O4
Disilane – Si2H6
Disulfur dichloride S2Cl2
Dysprosium(III) chloride – DyCl3
Dysprosium oxide – Dy2O3
Dysprosium titanate – Dy2Ti2O7
Chemical name starts from “E”:
Erbium(III) chloride – ErCl3
Europium(III) chloride – EuCl3
Erbium-copper – ErCu
Erbium-gold – ErAu
Erbium-silver – ErAg
Erbium-Iridium – ErIr
Chemical name starts from “G”:
Gadolinium(III) chloride – GdCl3
Gadolinium(III) oxide – Gd2O3
Gallium antimonide – GaSb
Gallium arsenide – GaAs
Gallium trichloride – GaCl3
Gallium nitride – GaN
Gallium phosphide – GaP
Germanium(IV) hydride (Germane)– GeH4
Germanium(III) hydride – Ge2H6
Germanium(II) fluoride – GeF2
Germanium(IV) fluoride – GeF4
Germanium(II) chloride – GeCl2
Germanium(IV) chloride – GeCl4
Germanium(II) bromide – GeBr2
Germanium(IV) bromide – GeBr4
Germanium(II) iodide – GeI2
Germanium(IV) iodide – GeI4
Germanium(II) oxide – GeO
Germanium(IV) oxide – GeO2
Germanium(II) sulfide – GeS
Germanium(IV) sulfide – GeS2
Germanium(II) selenide – GeSe
Germanium(IV) selenide – GeSe2
Germanium telluride – GeTe
Germanium(IV) nitride – Ge3N4
Gold(I) chloride – AuCl
Gold(III) chloride – AuCl3
Gold(I,III) chloride – Au4Cl8
Gold(III) chloride – (AuCl3)2
Gold(III) fluoride – AuF3
Gold(V) fluoride – AuF5
Gold(I) bromide – AuBr
Gold(III) bromide – (AuBr3)2
Gold(I) iodide – AuI
Gold(III) iodide – AuI3
Gold(III) oxide – Au2O3
Gold(I) sulfide – Au2S
Gold(III) sulfide – Au2S3
Gold(III) selenide – AuSe
Gold(III) selenide – Au2Se3
Gold ditelluride – AuTe2
Chemical name starts from “H”:
Hafnium tetrafluoride – HfF4
Hafnium tetrachloride – HfCl4
Hexadecacarbonylhexarhodium – Rh6(CO)16
Hydrazine – N2H4
Hydrazoic acid – HN3
Hydrobromic acid – HBr
Hydrochloric acid – HCl
Hydroiodic acid – HI
Hydrogen bromide – HBr
Hydrogen chloride – HCl
Hydrogen fluoride – HF
Hydrogen peroxide – H2O2
Hydrogen selenide – H2Se
Hydrogen sulfide – H2S
Hydrogen telluride – H2Te
Hydroxylamine – NH2OH
Hypochlorous acid – HClO
Hypophosphorous acid – H3PO2
Chemical name starts from “I”:
Indium antimonide – InSb
Indium arsenide – InAs
Indium(I) chloride – InCl
Indium nitride –InN
Indium phosphide – InP
Iodic acid – HIO3
Iodine heptafluoride – IF7
Iodine pentafluoride – IF5
Iodine monochloride – ICl
Iodine trichloride – ICl3
Iridium(IV) chloride – IrCl4
Iron(II) chloride – FeCl2 including hydrate
Iron(III) chloride – FeCl3
Iron Ferrocyanide – Fe7(CN)18
Iron(II) oxide – FeO
Iron(III) nitrate – Fe(NO3)3(H2O)9
Iron(II,III) oxide – Fe3O4
Iron(III) oxide – Fe2O3
Iron-sulfur cluster
Iron(III) thiocyanate – Fe(SCN)3
Chemical name starts from “K”
Krypton difluoride – KrF2
Chemical name starts from “L”:
Lanthanum carbonate – La2(CO3)3
Lanthanum magnesium – LaMg
Lanthanum aluminium – LaAl
Lanthanum zinc – LaZn
Lanthanum silver – LaAg
Lanthanum cadmium – LaCd
Lanthanum mercury – LaHg
Lanthanum tallium – LaTl
Lead(II) carbonate – Pb(CO3)
Lead(II) chloride – PbCl2
Lead(II) iodide – PbI2
Lead(II) nitrate – Pb(NO3)2
Lead hydrogen arsenate – PbHAsO4
Lead(II) oxide – PbO
Lead(IV) oxide – PbO2
Lead(II) phosphate – Pb3(PO4)2
Lead(II) sulfate – Pb(SO4)
Lead(II) selenide – PbSe
Lead(II) sulfide – PbS
Lead(II) telluride – PbTe
Lead zirconate titanate – PbO3 (e.g., x = 0.52 is Lead zirconium titanate)
Lithium aluminium hydride – LiAlH4
Lithium bromide – LiBr
Lithium borohydride – LiBH4
Lithium carbonate (Lithium salt) – Li2CO3
Lithium chloride – LiCl
Lithium hypochlorite – LiClO
Lithium chlorate – LiClO3
Lithium perchlorate – LiClO4
Lithium cobalt oxide – LiCoO2
Lithium peroxide – Li2O2
Lithium hydride – LiH
Lithium hydroxide – LiOH
Lithium iodide – LiI
Lithium iron phosphate – FeLiO4P
Lithium nitrate – LiNO3
Lithium sulfide – Li2S
Lithium sulfite – HLiO3S
Lithium sulfate – Li2SO4
Lithium superoxide – LiO2
Chemical name starts from “M”:
Magnesium antimonide – MgSb
Magnesium carbonate – MgCO3
Magnesium chloride – MgCl2
Magnesium oxide – MgO
Magnesium phosphate – Mg3(PO4)2
Magnesium sulfate – MgSO4
Manganese(IV) oxide (manganese dioxide) – MnO2
Manganese(II) sulfate monohydrate – MnSO4.H2O
Manganese(II) chloride – MnCl2
Manganese(III) chloride – MnCl3
Manganese(IV) fluoride – MnF4
Manganese(II) phosphate – Mn3(PO4)2
Mercury(I) chloride – Hg2Cl2
Mercury(II) chloride – HgCl2
Mercury fulminate – Hg(ONC)2
Mercury(II) selenide – HgSe
Mercury(I) sulfate – Hg2SO4
Mercury(II) sulfate – HgSO4
Mercury(II) sulfide – HgS
Mercury(II) telluride – HgTe
Metaphosphoric acid – HPO3
Molybdate orange
Molybdenum trioxide – MoO3
Molybdenum disulfide – MoS2
Molybdenum hexacarbonyl – C6O6Mo
Molybdic acid – H2MoO4
Chemical name starts from “N”:
Neodymium(III) chloride – NdCl3
Nessler’s reagent –K2
Nickel(II) carbonate – NiCO3
Nickel(II) chloride – NiCl2 and hexahydrate
Nickel(II) hydroxide – Ni(OH)2
Nickel(II) nitrate – Ni(NO3)2
Nickel(II) oxide – NiO
Niobium oxychloride – NbOCl3
Niobium pentachloride – NbCl5
Nitric acid – HNO3
Nitrogen monoxide – NO
Nitrogen dioxide – NO2
Nitrosylsulfuric acid – NOHSO4
Chemical name starts from “O”:
Osmium tetroxide (osmium(VIII) oxide) – OsO4
Osmium trioxide (osmium(VI) oxide) – OsO3
Oxybis(tributyltin) – C24H54OSn2
Oxygen difluoride – OF2
Ozone – O3
Chemical name starts from “P”:
Palladium(II) chloride – PdCl2
Palladium(II) nitrate – Pd(NO3)2
Pentaborane – B5H9
Pentasulfide antimony – Sb2S5
Perchloric acid – HClO4
Perchloryl fluoride – ClFO3
Persulfuric acid (Caro’s acid) – H2SO5
Perxenic acid – H4XeO6
Phenylarsine oxide – (C6H5)AsO
Phenylphosphine – C6H7P
Phosgene – COCl2
Phosphine – PH3
Phosphite – HPO32-
Phosphomolybdic acid – H3PMo12O40
Phosphoric acid – H3PO4
Phosphorous acid (Phosphoric(III) acid) – H3PO3
Phosphorus pentabromide – PBr5
Phosphorus pentafluoride – PF5
Phosphorus pentasulfide – P4S10
Phosphorus pentoxide – P2O5
Phosphorus sesquisulfide – P4S3
Phosphorus tribromide – PBr3
Phosphorus trichloride – PCl3
Phosphorus trifluoride – PF3
Phosphorus triiodide – PI3
Phosphotungstic acid – H3PW12O40
Platinum(II) chloride – PtCl2
Platinum(IV) chloride – PtCl4
Plutonium(III) chloride – PuCl3
Plutonium dioxide (Plutonium(IV) oxide) – PuO2
Potash Alum– K2SO4.Al2(SO4)3·24H2O
Potassium aluminium fluoride – KAlF4
Potassium borate – K2B4O7•4H2O
Potassium bromide – KBr
Potassium calcium chloride – KCaCl3
Potassium carbonate – K2CO3
Potassium chlorate – KClO3
Potassium chloride – KCl
Potassium cyanide – KCN
Potassium ferrioxalate – K3
Potassium hydrogencarbonate – KHCO3
Potassium hydrogen fluoride – HF2K
Potassium hydroxide – KOH
Potassium iodide – KI
Potassium iodidate – KIO3
Potassium monopersulfate – K2SO4·KHSO4·2KHSO5
Potassium nitrate – KNO3
Potassium perbromate – KBrO4
Potassium perchlorate – KClO4
Potassium permanganate – KMnO4
Potassium sulfate – K2SO4
Potassium sulfide – K2S
Potassium titanyl phosphate – KTiOPO4
Potassium vanadate – KVO3
Praseodymium(III) chloride – PrCl3
Protonated molecular hydrogen – H3+
Prussian blue (Iron(III) hexacyanoferrate(II)) – Fe43
Pyrosulfuric acid – H2S2O7
Chemical name starts from “R:
Radium chloride – RaCl2
Radon difluoride – RnF2
Rhodium(III) chloride – RhCl3
Rubidium bromide – RbBr
Rubidium chloride – RbCl
Rubidium fluoride – RbF
Rubidium hydroxide – RbOH
Rubidium iodide – RbI
Rubidium nitrate – RbNO3
Rubidium oxide – Rb2O
Rubidium telluride – Rb2Te
Ruthenium(VIII) oxide – RuO4
Chemical name starts from “S”:
Samarium(II) iodide – SmI2
Samarium(III) chloride – SmCl3
Scandium(III) triflate – Sc(OSO2CF3)3
Scandium(III) chloride – ScCl3 and hydrate
Scandium(III) fluoride – ScF3
Scandium(III) nitrate – Sc(NO3)3
Scandium(III) oxide – Sc2O3
Selenic acid – H2SeO4
Selenious acid – H2SeO3
Selenium trioxide – SeO3
Selenium tetrafluoride – SeF4
Selenium hexafluoride – SeF6
Selenium hexasulfide – Se2S6
Selenium tetrachloride – SeCl4
Selenium dioxide – SeO2
Selenium disulfide – SeS2
Selenium oxydichloride – SeOCl2
Selenium oxybromide – SeOBr2
Selenoyl fluoride – SeO2F2
Samarium(III) chloride – SmCl3
Scandium(III) triflate – Sc(OSO2CF3)3
Scandium(III) chloride – ScCl3 and hydrate
Scandium(III) fluoride – ScF3
Scandium(III) nitrate – Sc(NO3)3
Scandium(III) oxide – Sc2O3
Silane – SiH4
Silica gel – SiO2·nH2O
Silicic acid – n
Silicon tetrabromide – SiBr4
Silicon carbide – SiC
Silicochloroform, Trichlorosilane – Cl3HSi
Silicofluoric acid – H2SiF6
Silicon dioxide – SiO2
Silicon tetrachloride – SiCl4
Silicon monoxide – SiO
Silicon nitride – Si3N4
Silver azide – AgN3
Silver bromate – AgBrO3
Silver bromide – AgBr
Silver chloride – AgCl
Silver chlorate – AgClO3
Silver chromate – Ag2CrO4
Silver(I) fluoride – AgF
Silver(II) fluoride – AgF2
Silver subfluoride – Ag2F
Silver fluoroborate – AgBF4
Silver fulminate – AgCNO
Silver hydroxide – AgOH
Silver iodide – AgI
Silver nitrate – AgNO3
Silver nitride – Ag3N
Silver oxide – Ag2O
Silver orthophosphate – Ag3PO4
Silver perchlorate – AgClO4
Silver sulfide – Ag2S
Silver sulfate – Ag2SO4
Silver tio sulfate – Ag…
Soda lime –
Sodamide – NaNH2
Sodium aluminate – NaAlO2
Sodium azide – NaN3
Sodium borohydride – NaBH4
Sodium bromide – NaBr
Sodium bromite – NaBrO2
Sodium bromate – NaBrO3
Sodium perbromate – NaBrO4
Sodium hypobromite – NaBrO
Sodium borate – Na2B4O7
Sodium perborate – NaBO3.nH2O
Sodium carbonate – Na2CO3
Sodium carbide – Na2C2
Sodium chloride – NaCl
Sodium chlorite – NaClO2
Sodium chlorate – NaClO3
Sodium perchlorate – NaClO4
Sodium cyanide – NaCN
Sodium cyanate – NaCNO
Sodium dioxide – NaO2
Sodium ferrocyanide – Na4Fe(CN)6
Sodium hydride – NaH
Sodium hydrogen carbonate (Sodium bicarbonate) – NaHCO3
Sodium hydrosulfide – NaSH
Sodium hydroxide – NaOH
Sodium hypochlorite – NaOCl
Sodium iodide – NaI
Sodium iodate – NaIO3
Sodium periodate – NaIO4
Sodium hypoiodite – NaIO
Sodium monofluorophosphate (MFP) – Na2PFO3
Sodium molybdate – Na2MoO4
Sodium manganate – Na2MnO4
Sodium nitrate – NaNO3
Sodium nitrite – NaNO2
Sodium oxide – Na2O
Sodium percarbonate – 2Na2CO3.3H2O2
Sodium phosphate; see Trisodium phosphate – Na3PO4
Sodium hypophosphite – NaPO2H2
Sodium nitroprusside – Na2.2H2O
Sodium persulfate – Na2S2O8
Sodium peroxide – Na2O2
Sodium perrhenate – NaReO4
Sodium permanganate – NaMnO4
Sodium persulfate – Na2S2O8
Sodium selenite – Na2SeO3
Sodium selenate – Na2O4Se
Sodium selenide – Na2Se
Sodium biselenide – NaHSe
Sodium silicate – Na2SiO3
Sodium sulfate – Na2SO4
Sodium sulfide – Na2S
Sodium sulfite – Na2SO3
Sodium tellurite – Na2TeO3
Sodium tungstate – Na2WO4
Sodium thioantimoniate – Na3(SbS4).9H2O
Sodium thiocyanate – NaSCN
Sodium thiocyanate – Na2S2O3
Sodium uranate – Na2O7U2
Stannous chloride (tin(II) chloride) – SnCl2
Stibine – SbH3
Strontium carbonate – SrCO3
Strontium chloride – SrCl2
Strontium hydroxide – Sr(OH)2
Strontium nitrate – Sr(NO3)2
Strontium oxide – SrO
Strontium titanate – SrTiO3
Sulfamic acid – H3NO3S
Sulfane – H2S
Sulfur dioxide – SO2
Sulfur tetrafluoride – SF4
Sulfur hexafluoride – SF6
Disulfur decafluoride – S2F10
Sulfuric acid – H2SO4
Sulfurous acid – H2SO3
Sulfuryl chloride – SO2Cl2
Chemical name starts from “T”:
Tantalum carbide – TaC
Tantalum(V) oxide – Ta2O5
Telluric acid – H6TeO6
Tellurium dioxide – TeO2
Tellurium tetrachloride – TeCl4
Tellurous acid – H2TeO3
Terbium(III) chloride – TbCl3
Tetraborane(10) – B4H10
Tetrachloroauric acid – AuCl3
Tetrafluorohydrazine – N2F4
Tetramminecopper(II) sulfate – SO4
Tetrasulfur tetranitride – S4N4
Thallium(I) carbonate – Tl2CO3
Thallium(I) fluoride – TlF
Thallium(III) oxide – Tl2O3
Thallium(III) sulfate – Tl2(SO4)2
Thionyl chloride – SOCl2
Thiophosgene – CSCl2
Thiophosphoryl chloride – Cl3PS
Thorium dioxide – ThO2
Thortveitite – (Sc,Y)2Si2O7
Thulium(III) chloride – TmCl3
Tin(II) chloride – SnCl2
Tin(II) fluoride – SnF2
Tin(IV) chloride – SnCl4
Titanium boride – TiB2
Titanium carbide – TiC
Titanium dioxide (titanium(IV) oxide) – TiO2
Titanium dioxide (B) (titanium(IV) oxide) – TiO2
Titanium nitride – TiN
Titanium(IV) bromide (titanium tetrabromide) – TiBr4
Titanium(IV) chloride (titanium tetrachloride) – TiCl4
Titanium(III) chloride – TiCl3
Titanium(II) chloride – TiCl2
Titanium(IV) iodide (titanium tetraiodide) – TiI4
Trifluoromethylisocyanide – C2NF3
Trifluoromethanesulfonic acid – CF3SO3H
Trimethylphosphine – C3H9P
Trioxidane – H2O3
Tripotassium phosphate – K3PO4
Trisodium phosphate – Na3PO4
Triuranium octaoxide (pitchblende or yellowcake) – U3O8
Tungsten carbide – WC
Tungsten(VI) chloride – WCl6
Tungsten(VI) Fluoride – WF6
Tungstic acid – H2WO4
Tungsten hexacarbonyl – W(CO)6
Chemical name starts from “U”:
Uranium hexafluoride – UF6
Uranium pentafluoride – UF5
Uranium tetrachloride – UCl4
Uranium tetrafluoride – UF4
Uranyl carbonate – UO2CO3
Uranyl chloride – UO2Cl2
Uranyl fluoride – UO2F2
Uranyl hydroxide – UO2(OH)2
Uranyl hydroxide – (UO2)2(OH)4
Uranyl nitrate – UO2(NO3)2
Uranyl sulfate – UO2SO4
Chemical name starts from “V”:
Vanadium carbide – VC
Vanadium oxytrichloride (Vanadium(V) oxide trichloride) – VOCl3
Vanadium(IV) chloride – VCl4
Vanadium(II) chloride – VCl2
Vanadium(II) oxide – VO
Vanadium(III) nitride – VN
Vanadium(III) bromide – VBr3
Vanadium(III) chloride – VCl3
Vanadium(III) fluoride – VF3
Vanadium(IV) fluoride – VF4
Vanadium(III) oxide – V2O3
Vanadium(IV) oxide – VO2
Vanadium(IV) sulfate – VOSO4
Vanadium(V) oxide – V2O5
Chemical name starts from “W”:
Water – H2O
Chemical name starts from “X”:
Xenon difluoride – XeF2
Xenon hexafluoroplatinate – Xe
Xenon tetrafluoride – XeF4
Xenon tetroxide – XeO4
Xenic acid – H2XeO4
Chemical name starts from “Y”:
Ytterbium(III) chloride – YbCl3
Ytterbium(III) oxide – Yb2O3
Yttrium(III) antimonide – YSb
Yttrium(III) arsenide – YAs
Yttrium(III) bromide – YBr3
Yttrium aluminium garnet – Y3Al5O12
Yttrium barium copper oxide – YBa2Cu3O7
Yttrium(III) fluoride – YF3
Yttrium iron garnet – Y3Fe5O12
Yttrium(III) oxide – Y2O3
Yttrium(III) sulfide – Y2S3
Yttrium copper – YCu
Yttrium silver – YAg
Yttrium gold – YAu
Yttrium rhodium – YRh
Yttrium iridium – YIr
Yttrium zinc – YZn
Yttrium cadmium – YCd
Yttrium magnesium – YMg
Chemical name starts from “Z”:
Zinc bromide – ZnBr2
Zinc carbonate – ZnCO3
Zinc chloride – ZnCl2
Zinc cyanide – Zn(CN)2
Zinc fluoride – ZnF2
Zinc iodide – ZnI2
Zinc oxide – ZnO
Zinc selenide – ZnSe
Zinc sulfate – ZnSO4
Zinc sulfide – ZnS
Zinc telluride – ZnTe
Zirconia hydrate – ZrO2·nH2O
Zirconium carbide – ZrC
Zirconium(IV) chloride – ZrCl4
Zirconium nitride – ZrN
Zirconium hydroxide – Zr(OH)4
Zirconium(IV) oxide – ZrO2
Zirconium orthosilicate – ZrSiO4
Zirconium tetrahydroxide – H4O4Zr
Zirconium tungstate – ZrW2O8
Read the full article
#ChemicalCompoundsUsedInDailyLife#ChemicalFormulaOfCompoundsNameAndFormulaPdf#CommonChemicalCompoundsList#CommonCompoundsList#CommonNamesofChemicalCompoundsandFormulaSSC-GKNotes#CommonNamesOfChemicalCompoundsAndFormulasPdf#ComprehensiveStudyListofChemicalCompounds&Formulas#InorganicCompoundsandTheirUses#ListOfChemicalCompoundsAndTheirCommonNamesAndFormulas#ListOfChemicalFormulasAndTheirCommonNames#ListOfChemicalNames#ListofimportantOrganicCompounds
0 notes
Text
Free Cell Phone Apps
As if you didn't know, free cell phone apps have come a long way, baby. In the old days, 1988, only those able to spend $2,000 or so for just the phone, could own one of those big black handsets. The ‘portable,' was a three watt brain eater that could easily have been a stand in for the field radio on the WWII 60's TV drama, Combat, Able, Baker, Charlie, Come in! It had a tiny LCD display which showed some modest numerical data.For training purpose, you can find mobile apps from iTunes.
Today's cell phones are no longer simply cell phones but have become smart. They are full fledged computers that can surf the web and handle real computing tasks on the fly with thousands of applications from which to choose.
There is a catch or two though, to these devices. Besides the health concerns and the jury is still out on that, they are like going back in time. In computing time that is, back ten or more years. Their actual computing power is akin to using a so-called ‘legacy' era device. This can be attributed to two main factors; one, it can be difficult for technologists to figure out how to make applications run effectively on the scaled down processing power, compared to modern desktop PC's or Notebook Computers and two, once they can accomplish that, they must further adapt their apps so that they do not burn out the finite battery life. One might conclude that they are succeeding more on the first point than on the latter. If you burn out your phone's charge by playing the myriad of free games and apps that are flooding the Internet, then when it comes time to make or take that important phone call, you have to call back later when you get to a pay phone or your hotel room to recharge. If you are looking for buy android reviews online or websites android and iOS apps you can contact by clicking on above link.
As if that weren't bad enough, all cell phones are not created equal. In some sense, it's another back in time affair, as you try to determine just which app will work on your smart phone. Again, in the old days, it was Windows or Mac or just DOS, with maybe, heaven forbid, Deskmate. Today it's Android, iOS, RIM or Windows Phone 7 and if you bought that $14.99 K-Mart special, none of the above. For that one, there are just a few modest apps that hearken even further on the way back machine to say, the Radio Shack TRS-80 (lovingly called the ‘Trash-80') or it's Color Computer 3 (CoCo3), which plugged into your analog TV.
That said, Disney has a useful mobile app that will help you connect with Mickey, Minnie or any of their other warm and fuzzy characters while visiting Disney's theme parks called ‘Mobile Magic.'
Technology Review has free apps available for the iOS and Android platforms. Do beware though as some of their content requires a subscription. This brings up another interesting point. Some free apps will contain advertising. A prominent cereal company is trying to muscle in on the popular kid's site, Habbo Hotel. If one is exposed to advertising, does that constitute a charge of some kind and thus render that app sort of not free?
On the Apple horizon is their iCloud software, which usurps Mobile Me. Luxuriate in the Clouds with 5GB of free storage. FindMyiPhone is a handy app when you have lost your precious appendage, it will display the gadget's whereabouts.
Blackberry Protect is an app that will back up your smart phones data in the Clouds and help you to find it when it's lost. Google Music is now available for the Android OS. WatchESPN is the app for sports fans. It works with Android and iOS. Pandora is the app to get for Internet Radio. It works with all of the above mentioned OS's.
That concludes the tip of the free cell phone app iceberg!
0 notes
Text

Oh my gog, the afterlife has TSA?
And it scans you to check if your photo is up somewhere!
Gog, I really love the design of all this!
...wow, so, the only person who went out of their way to remember this guy was his dentist? That sucks.
#casual liveblog10#missfinefeather liveblogs#casual liveblogging#movie night#coco3#cocoliveblog#liveblog#coco#pixar#blacklist Missfinefeather
47 notes
·
View notes
Photo

From the 1989 Tandy Computer Catalog, the Color Computer 3 certainly wasn't cutting-edge, but it was affordable! #retrotech #retrocomputing #computer #tandy #radioshack #colorcomputer3 #80s #coco #coco3 http://ift.tt/2k1IYnW
0 notes
Photo

TANDY TRS-80 Color Computer 3 128K
5 notes
·
View notes
Video
youtube
This is the most appalling port of any game I’ve ever seen and I have to wonder if all the effort the programmers went through was worth it. I feel bad for someone who had to own this over the far better DOS and Apple IIGS versions.
0 notes
Text
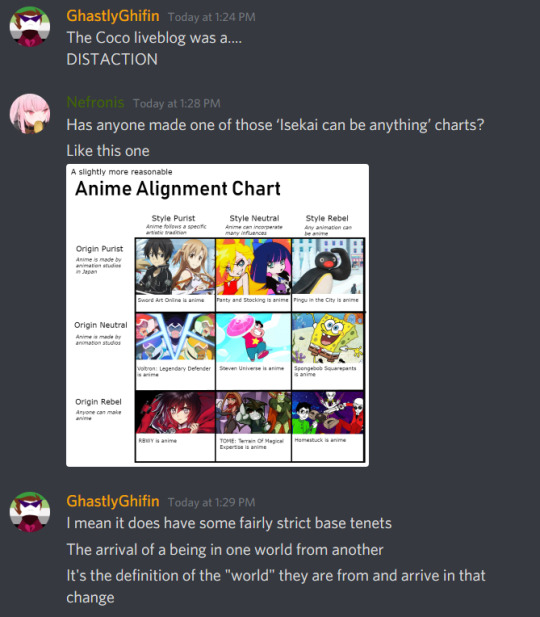
#casual liveblog10#missfinefeather liveblogs#casual liveblogging#movie night#coco3#cocoliveblog#liveblog#coco#pixar#blacklist Missfinefeather
22 notes
·
View notes
Text



PFFFFFT
There’s too many good jokes in this movie hahaahahaaha
#casual liveblog10#missfinefeather liveblogs#casual liveblogging#movie night#coco3#cocoliveblog#liveblog#coco#pixar#blacklist Missfinefeather
20 notes
·
View notes