#mhtt
Text
Dysregulated #miRNA and #mRNA Expression Affect Overlapping Pathways in a Huntington's Disease Model
Huntington's disease (HD) is a fatal neurodegenerative disorder caused by the expansion of a CAG trinucleotide repeat in the Huntingtin gene. Transcriptional dysregulation is one of the main cellular processes affected by mutant Huntingtin (mHtt). In this study, we investigate the alterations in #miRNA and #mRNA expression levels in a Drosophila model of HD by #RNA sequencing and assess the functional effects of misregulated #miRNAs in vivo. We found that in head samples of HD flies, the level of 32... https://pubmed.ncbi.nlm.nih.gov/37569316/?utm_source=dlvr.it&utm_medium=tumblr&utm_campaign=None&utm_content=1Zap-74u4XbiV7x0qz5lToBuxtoq00qwwHZUuXSRQOsim8UYds&fc=None&ff=20230816100626&v=2.17.9.post6%2086293ac
0 notes
Text
Well.... what to say?
Looked at the data on deaths concerning India….seems there almost 2000 people have died in the last 28 weeks while in Sweden about double the number have died…..now of course the death toll seems to be increasing in India…sad.. .. but also sad that during our winter we had half the number of deaths compared to India’s UHTT population. WE HAD 300 TIMES AS MANY DIED MHTT population IN A OVER HALF…
View On WordPress
0 notes
Text
GENERATION HD2-Clinical Trial Open to the Huntington's Disease Community
The HDSA RESEARCH WEBINAR: The Roche/Genentech GENERATION HD2 Study of Tominersen; a new clinical trial that is now open to folks with Huntington’s disease (HD) who meet the criteria. The webinar is worth watching.
Tominersen is an antisense therapy that acts by reducing the production of all forms of the huntingtin protein (HTT), including its mutated variant (mHTT), which is believed to be…

View On WordPress
0 notes
Text
media release: KOREA WELCOMES YOU! ~Korea Fest 2022 – Experience The Best of Korea~

In recent times, the appetite for all things Korean has only heightened and Korea Tourism Organization is ready to satiate that appetite with the return of Korea Fest 2022 that will take place from 4 to 6 November 2022 at LG Oval, 1 Utama Shopping Centre.
The festival promises a three-day immersive K-everything experience from K-beauty to K-Culture, and K-Performances to K-Food. Customers can enjoy attractive Korea Travel Deals through participating Travel Agency partners, Korean cultural activities and workshops, travel talk shows, innovative media art experiences and many more.
In addition, Korea Tourism Organization is proud and honoured to announce the appointment of South Korean actor, Lee Jae Wook as its Honourary Ambassador of Korea Tourism Organization. Lee Jae Wook made his acting debut in the science fiction thriller “Memories of the Alhambra (2018-2019)” and further cemented his status as the male lead with the notable role in the hit fantasy period drama “Alchemy of Souls (2022)”. As part of Korea Fest 2022, Lee Jae Wook will meet with Malaysian fans at the “Annyeong Kawan with Lee Jae Wook” meet and greet on 5 November 2022, 5:00 pm and officially receive this honourary appointment. Malaysian fans can stand a chance to meet actor Lee Jae Wook up close and personal by purchasing any Korea Travel Packages from participating Travel Agency and Airline Partners.
“We are honoured to have Lee Jae Wook participate in our event Korea Fest 2022 as the appointed Honourary Ambassador of Korea Tourism Organization. With this appointment, we hope to further elevate Korea as the top travel destination for Malaysians and create more opportunities for Malaysians to discover Korea with Lee Jae Wook”, Mr. Yang Kyung Soo, Managing Director of Korea Tourism Organization expressed.
Mr. Yang Kyung Soo added, “Together with Lee Jae Wook, we are excited to present South Korea's unique and attractive features through a series of videos on our social media channels and at the in-person meet and greet session. We expect a positive response from Malaysian fans, particularly for K-drama and K-pop fans.”

Korea Fest 2022 is organised by Korea Tourism Organization with the support of participating travel agency partners Apple Vacations, Airlines Booking Centre (ABC Holidays), Airlink Travel & Tour, Esplanad Holiday, Golden Tourworld Travel (GTT), Ice Holiday, Parlo Tours, Holiday Tours, Malaysian Harmony Tour & Travel (MHTT) and participating partners include CU Malaysia, Klook Malaysia, Malaysia Airlines and History of Whoo. The Official Venue Partner, 1 Utama Shopping Centre, is proudly ranked amongst the world’s largest malls and is recognized as “The Top Shopping Mall in Malaysia” with over 750 stores to shop-eat-play!
Visit Korea Fest 2022 at LG Oval, 1 Utama Shopping Centre from 4 to 6 November 2022 and www.koreafest2022.com to discover more.
Watch Annyeong Kawan with KTO's Honorary Ambassador Lee Jae Wook special message here!
youtube
*photos courtesy of Korea Tourism Organization
Don’t forget to like, follow and subscribe to MY K-POP WIRE for more K-Pop interview, debut, comeback and event updates!
Twitter: mykpopwire
Instagram: mykpopwire
Facebook: MY K-POP WIRE
YouTube: MY K-POP WIRE
0 notes
Text
Korea Fest 2022 — Experience The Best of Korea

KUALA LUMPUR, 26th October 2022 — In recent times, the appetite for all things Korean has only heightened and Korea Tourism Organization is ready to satiate that appetite with the return of Korea Fest 2022 that will take place from 4th to 6th November 2022 at LG Oval, 1 Utama Shopping Centre.
The festival promises a three-day immersive K-everything experience from K-beauty to K-Culture, and K-Performances to K-Food. Customers can enjoy attractive Korea Travel Deals through participating Travel Agency partners, Korean cultural activities and workshops, travel talk shows, innovative media art experiences and many more.

In addition, Korea Tourism Organization is proud and honoured to announce the appointment of South Korean actor, Lee Jae Wook as its Honourary Ambassador of Korea Tourism Organization. Lee Jae Wook made his acting debut in the science fiction thriller “Memories of the Alhambra (2018-2019) and further cemented his status as the male lead with the notable role in the hit fantasy period drama “Alchemy of Souls (2022)”. As part of Korea Fest 2022, Lee Jae Wook will meet with Malaysian fans at the “Annyeong Kawan with Lee Jae Wook” meet and greet on 5 November 2022, 5:00 pm and officially receive this honourary appointment. Malaysian fans can stand a chance to meet actor Lee Jae Wook up close and personal by purchasing any Korea Travel Packages from participating Travel Agency and Airline Partners.

“We are honoured to have Lee Jae Wook participate in our event Korea Fest 2022 as the appointed Honourary Ambassador of Korea Tourism Organization. With this appointment, we hope to further elevate Korea as the top travel destination for Malaysians and create more opportunities for Malaysians to discover Korea with Lee Jae Wook”, Mr. Yang Kyung Soo, Managing Director of Korea Tourism Organization expressed.
Mr. Yang Kyung Soo added, “Together with Lee Jae Wook, we are excited to present South Korea's unique and attractive features through a series of videos on our social media channels and at the in-person meet and greet session. We expect a positive response from Malaysian fans, particularly for K-Drama and K-pop fans.”
Korea Fest 2022 is organised by Korea Tourism Organization with the support of participating travel agency partners Apple Vacations, Airlines Booking Centre (ABC Holidays), Airlink Travel & Tour, Esplanad Holiday, Golden Tourworld Travel (GTT), Ice Holiday, Parlo Tours, Holiday Tours, Malaysian Harmony Tour & Travel (MHTT) and participating partners include CU Malaysia, Klook Malaysia, Malaysia Airlines and History of Whoo. The Official Venue Partner, 1 Utama Shopping Centre, is proudly ranked amongst the world’s largest malls and is recognized as “The Top Shopping Mall in Malaysia” with over 750 stores to shop-eat-play!
Visit Korea Fest 2022 at LG Oval, 1 Utama Shopping Centre from 4 to 6 November 2022 and www.koreafest2022.com to discover more.
Korea Fest 2022
Date: 4th – 6th November 2022
Time:
10AM – 10.30PM (4th & 5th November 2022)
10AM – 10PM (6th November 2022)
Location: LG Oval, 1 Utama Shopping Centre, Petaling Jaya.
Korea Fest 2022 - Annyeong Kawan with Lee Jae Wook
youtube
Date: 5th November 2022
Time: 5PM – 8.30PM
Location: LG Oval, 1 Utama Shopping Centre, Petaling Jaya.
Activities:
Korea travel information booths
Exhibitors consisting of regional tourism provinces will provide travel information
Gangwon Province
Jeju Special Self-Governing Province
Annyeong Kawan with Lee Jae Wook - Meet and Greet Session
Extreme Dance Comedy performance by Breakout - It’s physical theatre. It’s non-verbal. It’s dance. It’s comedy. It’s gymnastics. Break out! A prison fantasy combining extreme b-body dance styles and hilarious comedy.
Cultural Art Performance by Black Dot - Everything begins and ends with a dot. Black Dot, is a performance team that combines Korean Calligraphy and Taekwondo martial arts to express the true colors of Korea.
Official KTO mascot appearances and photo zone.
Complimentary Hanbok Experience.
VR Experience
Nice to CU Cafe
#AnnyeongKawan#TraveltoKoreaBeginsAgain#KoreaFest2022#LeeJaeWook#이재욱#LeeJaeWookinMY#pressrelease#Youtube
0 notes
Text
Những vì sao trên cao rất khó với tới. Chúng ta chỉ có thể ngắm nhìn thật lâu. Nhưng nhiều lúc tôi lo cho những ngôi sao ấy lại chẳng có cách nào nói được. Chỉ có thể lặng nhìn và cầu mong thôi. Hãy thật hạnh phúc nhé các ngôi sao của tôi
0 notes
Text
Huntington: trovata l'enzima-forbice che taglia la proteina malata
Huntington: trovata l’enzima-forbice che taglia la proteina malata
La malattia di Huntington è una malattia neurodegenerativa che provoca la morte delle cellule cerebrali, portando a movimenti incontrollati del corpo, perdita della parola e psicosi. Le mutazioni nel gene huntingtina (mHTT) causano la malattia, determinando l’aggregazione tossica di questa proteina. L’accumulo di questi aggregati provoca la neurodegenerazione e di solito porta alla morte del…
View On WordPress
#cellule staminali#enzima#farmaco#fosfatasi#gene#inibitore#malattia di Huntington#malattia genica#mutazione#neurodegenerazione#neuroni#protena#sclerosi laterale amiotrofica
0 notes
Text
IJMS, Vol. 23, Pages 12412: Truncated Analogues of a G-Quadruplex-Forming #aptamer Targeting Mutant Huntingtin: Shorter Is Better!
Two analogues of the MS3 #aptamer, which was previously shown to have an exquisite capability to selectively bind and modulate the activity of mutant huntingtin (mHTT), have been here designed and evaluated in their physicochemical and biological properties. Featured by a distinctive propensity to form complex G-quadruplex structures, including large multimeric aggregates, the original 36-mer MS3 has been truncated to give a 33-mer (here named MS3-33) and a 17-mer (here named MS3-17). A combined use of different techniques (UV, CD, DSC, gel electrophoresis) allowed a detailed physicochemical characterization of these novel G-quadruplex-forming #aptamers, tested in vitro on SH-SY5Y cells and in vivo on a Drosophila Huntington’s disease model, in which these shorter MS3-derived oligonucleotides proved to have improved bioactivity in comparison with the parent #aptamer. https://www.mdpi.com/1422-0067/23/20/12412?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Roche stops dosing in trial of Huntington’s disease hopeful tominersen

Roche has stopped dosing in a phase 3 trial of tominersen, a potential Huntington’s disease (HD) drug developed with Ionis, after a pre-planned review by independent experts.
The companies had been working on tominersen since December 2017, when Roche licensed it in from Ionis.
There were high expectations for tominersen, which the companies hoped would be the first drug to treat the underlying cause of the disease, which leads to devastating neurological degeneration.
The Swiss pharma said the decision was based on a review by the GENERATION HD1 study’s Independent Data Monitoring Committee.
According to Roche the committee “made its recommendation based on the therapy’s potential risk/benefit profile for study participants”.
The company gave little else away but added that there were no new or emerging safety signals identified, meaning that the issue is likely to be that the drug simply wasn’t working in the placebo-controlled trial.
Roche said it plans to continue monitoring participants for safety and clinical outcomes, without dosing of tominersen or placebo.
Once full data from the study are available, the company said it will “share learnings and future plans” with the HD community.
Dosing will be paused in the open-label extension study (GEN-EXTEND) of tominersen while data are carefully analysed to inform next steps on this study.
Tominersen, previously IONIS-HTTRx or RG6042, is an investigational antisense therapy designed to reduce the production of all forms of the huntingtin protein (HTT), including its mutated variant, mHTT.
A phase 1/2 study involving 46 people with early-stage disease showed a significant dose-dependent reduction in mHTT in the cerebrospinal fluid of treated patients, plus a favourable safety and tolerability profile.
The study completed recruitment earlier this year, with 791 patients across around 100 sites signing up globally.
It was testing the efficacy and safety of tominersen treatment administered once every two months (eight weeks) or every four months (16 weeks) over a period of 25 months and was due to complete next year.
The post Roche stops dosing in trial of Huntington’s disease hopeful tominersen appeared first on .
from https://pharmaphorum.com/news/roche-stops-dosing-in-huntingtons-disease-trial/
0 notes
Text
New therapeutic target for Huntington’s treatment
New therapeutic target for Huntington’s treatment
New perspective on the role of sRNAs in the disease
Credit: UNIVERSITY OF BARCELONA
Huntington’s disease is caused by a mutation in the Huntingtin gene (HTT), which appears in adults and features motor, cognitive and psychiatric alterations. The origin of this disease has been associated with the anomalous functioning of the mutated protein: mHTT, but recent data showed the involvement of other…

View On WordPress
0 notes
Photo

New Post has been published on https://hajdu-horgasz.hu/2021/01/01/az-ev-hala-2021-ben-a-jaszkeszeg/
Az év hala 2021-ben a jászkeszeg
Jászkeszeg (Leuciscus idus)
A Magyar Haltani Társaság honlapján rendezett közönségszavazásra december 31-én délig 7683 szavazat érkezett be, ennek alapján alakult ki a végeredmény. A három jelölt közül az ősi vonásokat viselő és napjainkra erősen megfogyatkozott angolna a szavazatok 20 százalékával lett a harmadik. Második a főként hegy- és dombvidéki patakjainkban élő, mindössze 8–10 centit elérő fürge cselle. Erre a viszonylag ritka és védett halunkra a szavazók 31 százaléka voksolt. Az élen, 49 százalékos többséggel, a közepes és nagyobb folyóink alföldi szakaszait kedvelő jászkeszeg végzett.
A világos pikkelyei miatt Herman Ottó által még ónos jásznak nevezett jászkeszeg ezüstös színű pontyfélénk. Az alsó úszói többnyire rőtes-pirosas színűek, ezért gyakran összetévesztik a bodorkával vagy a vörösszárnyú keszeggel. A jászkeszeg pikkelyei azonban apróbbak ezért az oldalvonalán mindig 55-nél több számolható, míg a másik két fajnál 50-nél kevesebb.
Táplálékát zömmel az üledékben rejtőző gerinctelen állatok, férgek puhatestűek alkotják. Áramláskedvelő faj, de a gyakran felfrissülő tavakban is megtalálható, amilyen például a Tisza-tó, a Balatonban azonban ritkaság. Ívásuk késő tavasszal történik, ezért április 15-től május 31-ig a fogásuk tilos. Ikrájukat a sóderes mederfenékre vagy a vízi növényzetre rakják. Közepes termetű halunk, a kifogható legkisebb mérete 20 cm, de a 30 cm fölöttiek sem ritkák A húsa szálkás, de ízletes. A hazai horgászrekord 3,86 kg (1995).
Forrás: MHTT
0 notes
Text
Wave Life Sciences Reports Third Quarter 2020 Financial Results and Provides Business Update Nasdaq:WVE
Outcomes from all cohorts of PRECISION-HD1 and PRECISION-HD2 medical trials and preliminary OLE knowledge on monitor for 1Q 2021
Dosing in three new medical trials with novel compounds incorporating PN chemistry and concentrating on SNP3 in HD, C9orf72 in ALS / FTD and exon 53 skipping in DMD anticipated in 2021
Alpha-1 antitrypsin deficiency introduced as first ADAR enhancing program, with potential to handle each lung and liver manifestations of the illness by means of correction of single RNA base mutation
Strengthened stability sheet with fairness financing; money runway prolonged into 2Q 2023
Wave to host investor convention name and webcast at 8:30 a.m. ET right now
CAMBRIDGE, Mass., Nov. 09, 2020 (GLOBE NEWSWIRE) — Wave Life Sciences Ltd. (Nasdaq: WVE), a clinical-stage genetic medicines firm dedicated to delivering life-changing remedies for individuals battling devastating illnesses, right now introduced monetary outcomes for the third quarter ended September 30, 2020 and supplied a enterprise replace.
“The substantial progress made by Wave’s analysis and medical groups in the course of the third quarter has ushered in a brand new part for the corporate, throughout which we’re quickly advancing a number of new packages incorporating our novel PN spine chemistry modification. We’re on monitor to file medical trial purposes for WVE-003 for Huntington’s illness and WVE-004 for amyotrophic lateral sclerosis and frontotemporal dementia this quarter. As well as, we’re saying right now our plan to submit a medical trial software within the first quarter of 2021 for a 3rd PN backbone-containing molecule, WVE-N531 for sufferers with Duchenne muscular dystrophy who’ve mutations amenable to exon 53 skipping,” stated Paul Bolno, MD, MBA, President and Chief Govt Officer of Wave Life Sciences. “We’re additionally excited to announce our first ADAR enhancing goal, the SERPINA1 gene transcript, the place a single base mutation is usually the reason for alpha-1 antitrypsin deficiency. Utilizing our distinctive ADAR enhancing functionality to right the RNA transcript, we may have the chance to deal with each liver and lung manifestations of the illness.”
“The 32 mg cohorts within the PRECISION-HD1 and PRECISION-HD2 Section 1b/2a medical trials proceed to maneuver forward, and we sit up for sharing outcomes from all cohorts, in addition to preliminary knowledge from the continuing open-label extension trials, within the first quarter of 2021. We’re well-positioned to progress our deliberate and present packages with our present money stability.”
Latest Enterprise Highlights
PRECISION-HD packages for Huntington’s illness (HD): Wave is growing a novel portfolio of investigational stereopure oligonucleotides designed to selectively goal the mutant allele of the huntingtin (mHTT) gene, whereas leaving the wild-type (wtHTT) protein comparatively intact. Wave’s strategy to HD is guided by the popularity that, along with a achieve of perform of the mHTT protein, individuals with this illness have misplaced one copy of the wtHTT allele, leaving them with a smaller protecting reservoir of wholesome protein than unaffected people. A rising physique of scientific proof means that preserving as a lot of this important protein as attainable is vital for favorable well being outcomes over a lifetime with the illness.
PRECISION-HD and OLE trials of WVE-120101 and WVE-120102:
The PRECISION-HD1 and PRECISION-HD2 Section 1b/2a medical trials evaluating investigational WVE-120101 and WVE-120102, stereopure oligonucleotides designed to selectively goal the mHTT mRNA transcript that accommodates SNP rs362307 (SNP1) and rs362331 (SNP2), respectively, in sufferers with HD are ongoing.
Open-label extension (OLE) medical trials for sufferers exterior of the U.S. who participated within the Section 1b/2a PRECISION-HD trials are additionally ongoing.
Within the first quarter of 2021, Wave expects to report knowledge from all cohorts in each PRECISION-HD trials, together with the 32 mg dose cohorts, in addition to knowledge from sufferers who obtained a number of doses of 8 or 16 mg of WVE-120101 or WVE-120102 within the OLE trials.
HD SNP3 program (WVE-003):
Wave is growing a 3rd allele-selective HD candidate, WVE-003, which is designed to selectively goal an undisclosed SNP on the mHTT mRNA transcript (SNP3), whereas leaving wtHTT protein comparatively intact. Between its SNP1, SNP2 and SNP3-targeted molecules, Wave has the potential to supply allele-selective choices for as much as 80% of individuals with HD.
In preclinical research, WVE-003 confirmed selective discount of mHTT mRNA in vitro, and potent and sturdy knockdown of mHTT mRNA in vivo.
WVE-003 incorporates Wave’s novel PN chemistry into its design.
Wave expects to provoke medical growth of WVE-003 with the submission of a medical trial software (CTA) within the fourth quarter of 2020.
C9orf72 program (WVE-004) for amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD): WVE-004 is designed to selectively goal the transcript variants containing the hexanucleotide repeat growth (G4C2) within the C9orf72 gene. G4C2 expansions within the C9orf72 gene result in decreased expression of wholesome protein, accumulation of repeat-containing transcripts and RNA binding proteins into nuclear RNA foci, and aberrant expression of neurotoxic dipeptide repeat proteins (DPRs).
In preclinical research in transgenic mice following two intracerebroventricular administered doses, WVE-004 confirmed potent and sturdy knockdown of repeat-containing transcripts and DPRs whereas sparing wholesome C9orf72 protein over a interval of six months.
WVE-004 incorporates Wave’s novel PN chemistry into its design.
Wave expects to provoke medical growth of WVE-004 with the submission of a CTA within the fourth quarter of 2020.
Exon 53 program (WVE-N531) for Duchenne muscular dystrophy (DMD): Primarily based on compelling in vitro and in vivo preclinical outcomes from compounds incorporating Wave’s novel PN chemistry, Wave is advancing WVE-N531 to discover splicing in dystrophic muscle. Planning is underway for a medical trial to evaluate dystrophin manufacturing and preliminary security in sufferers with DMD amenable to exon 53 skipping.
WVE-N531 induced a dose-dependent improve in dystrophin manufacturing (as much as 71%) in vitro in DMD patient-derived myoblasts.
In an ongoing in vivo research of double knock-out mice (a mannequin missing dystrophin and utrophin protein with a extreme phenotype), an oligonucleotide with PN spine chemistry modifications appeared to considerably improve dystrophin manufacturing and considerably enhance muscle perform and survival.
WVE-N531 incorporates Wave’s novel PN chemistry into its design.
Wave expects to submit a CTA for WVE-N531 within the first quarter of 2021.
SERPINA1 program for alpha-1 antitrypsin deficiency (AATD) with ADAR-mediated RNA enhancing (ADAR enhancing): Wave introduced right now that it’s making use of its ADAR enhancing platform functionality to develop a possible novel remedy for AATD aimed toward addressing the lung and liver manifestations of the illness.
AATD is a uncommon, inherited genetic dysfunction that’s generally brought on by a single G-to-Some extent mutation within the SERPINA1 gene, referred to as the SERPINA1 Z allele. This mutation results in misfolding and aggregation of alpha-1 antitrypsin (AAT) protein in hepatocytes and an absence of practical AAT within the lungs. Folks with AATD usually exhibit progressive lung injury, liver injury or each, resulting in frequent hospitalizations and doubtlessly terminal lung illness and/or liver illness. The few authorised therapies for AATD modestly improve circulating ranges of AAT in these with the lung pathology; there aren’t any authorised therapies to deal with the liver pathology. Roughly 250,000 individuals worldwide are homozygous for the Z allele, which is probably the most extreme type of the illness.
Wave’s novel RNA enhancing platform functionality makes use of endogenous ADAR (adenosine deaminases performing on RNA) enzymes by way of free uptake (non-viral, no nanoparticles) of A-to-I (G) RNA enhancing oligonucleotides, making this a doubtlessly best-in-class modality for correcting the G-to-A disease-causing mutation in mRNA coded by the SERPINA1 Z allele.
By correcting the one RNA base mutation, ADAR enhancing might present an excellent strategy for rising circulating ranges of wild-type AAT protein and decreasing aggregation within the liver, thus concurrently addressing each the lung and liver manifestations of the illness.
In a major hepatocyte SERPINA1 Z cell mannequin, enhancing the Z transcript again to wild-type prevented protein misfolding and elevated secretion from hepatocytes.
Wave is growing a proprietary in vivo mannequin system, which makes use of human ADAR and human goal transcript, to help the continuing growth of its ADAR enhancing platform. Information from its humanized SERPINA1/ADAR mannequin are anticipated within the first half of 2021.
Wave’s SERPINA1 program and ADAR enhancing platform functionality incorporate the corporate’s novel PN chemistry.
Central nervous system (CNS) packages in collaboration with Takeda: Wave is leveraging learnings from PRISMTM, the corporate’s propriety discovery and drug growth platform, to design further stereopure oligonucleotides with optimized profiles for CNS indications, together with in Alzheimer’s illness, Parkinson’s illness and others, as a part of its ongoing collaboration with Takeda.
Wave is using PN spine chemistry modifications to provide compelling in vivo knowledge and actively progress as much as six preclinical targets.
In an in vivo research in non-human primates (NHPs) for probably the most superior therapeutic program within the collaboration, roughly 90% knockdown of the goal mRNA was noticed one month after a single 12 mg intrathecal dose. The therapeutic candidate distributed broadly throughout a number of related CNS tissues.
Novel PN chemistry introduced at Analyst & Investor Analysis Webcast: On August 25, 2020, Wave held an Analyst and Investor Analysis Webcast to spotlight latest developments to PRISM, together with the growth of its repertoire of spine modifications with the introduction of PN spine chemistry.
PN chemistry is a spine modification that includes changing a non-bridging Oxygen atom with a Nitrogen-containing moiety.
In preclinical experiments, even handed use of PN spine chemistry modifications in stereopure oligonucleotides have typically elevated efficiency, publicity and sturdiness throughout Wave’s silencing, splicing and enhancing modalities.
Wave’s present preclinical and discovery-stage packages incorporate PN spine chemistry modifications.
Third Quarter 2020 Monetary Outcomes and Monetary Steering
As of September 30, 2020, Wave had $216.4 million in money and money equivalents as in comparison with $147.2 million as of December 31, 2019. In the course of the third quarter of 2020, Wave considerably prolonged its money runway by elevating $93.7 million in web proceeds from its September 2020 public providing and $48 million in web proceeds from its at-the-market fairness program, and receiving $16.8 million in refundable tax credit.
Wave reported a web lack of $33.1 million within the third quarter of 2020 as in comparison with $50.7 million in the identical interval in 2019.
Analysis and growth bills have been $28.3 million within the third quarter of 2020 as in comparison with $44.6 million in the identical interval in 2019. The lower in analysis and growth bills within the third quarter was primarily as a consequence of decreased exterior bills associated to suvodirsen, as a consequence of Wave’s December 2019 determination to discontinue this system, in addition to decreased headcount and different exterior bills pushed by Wave’s February 2020 value discount plan, partially offset by elevated exterior bills associated to Wave’s medical and preclinical actions associated to its HD packages and its C9orf72 program for ALS and FTD.
Basic and administrative bills have been $9.6 million within the third quarter of 2020 as in comparison with $12.5 million in the identical interval in 2019. The lower typically and administrative bills within the third quarter of 2020 was primarily as a result of February 2020 value discount plan, which included a workforce discount.
Wave expects that its present money and money equivalents, along with anticipated and dedicated money from its present collaboration, will allow the corporate to fund its working and capital expenditure necessities into the second quarter of 2023.
Investor Convention Name and Webcast
Wave administration will host an investor convention name right now at 8:30 a.m. ET to debate the corporate’s third quarter 2020 monetary outcomes and supply a enterprise replace. The convention name could also be accessed by dialing (866) 220-8068 (home) or (470) 495-9153 (worldwide) and getting into convention ID: 3563968. The reside webcast could also be accessed from the investor relations part of the Wave Life Sciences company web site at ir.wavelifesciences.com. Following the webcast, a replay can be out there on the web site.
About PRISM

PRISM is Wave Life Sciences’ proprietary discovery and drug growth platform that permits genetically outlined illnesses to be focused with stereopure oligonucleotides throughout a number of therapeutic modalities, together with silencing, splicing and enhancing. PRISM combines the corporate’s distinctive skill to assemble stereopure oligonucleotides with a deep understanding of how the interaction amongst oligonucleotide sequence, chemistry and spine stereochemistry impacts key pharmacological properties. By exploring these interactions by means of iterative evaluation of in vitro and in vivo outcomes and machine learning-driven predictive modeling, the corporate continues to outline design ideas which might be deployed throughout packages to quickly develop and manufacture medical candidates that meet pre-defined product profiles.
About Wave Life Sciences
Wave Life Sciences (Nasdaq: WVE) is a clinical-stage genetic medicines firm dedicated to delivering life-changing remedies for individuals battling devastating illnesses. Wave aspires to develop best-in-class medicines throughout a number of therapeutic modalities utilizing PRISM, the corporate’s proprietary discovery and drug growth platform that permits the exact design, optimization and manufacturing of stereopure oligonucleotides. Pushed by a resolute sense of urgency, the Wave group is concentrating on a broad vary of genetically outlined illnesses in order that sufferers and households might understand a brighter future.
Ahead-Wanting Statements
This press launch accommodates forward-looking statements regarding our targets, beliefs, expectations, methods, goals and plans, and different statements that aren’t essentially primarily based on historic information, together with statements concerning the next, amongst others: the anticipated graduation, affected person enrollment, knowledge readouts and completion of our medical trials, and the announcement of such occasions; the protocol, design and endpoints of our ongoing and deliberate medical trials; the long run efficiency and outcomes of our packages in medical trials; future preclinical actions and packages; regulatory submissions; the progress and potential advantages of our collaborations with companions; the potential of our in vitro and in vivo preclinical knowledge to foretell the conduct of our compounds in people; our identification of future candidates and their therapeutic potential; the anticipated therapeutic advantages of our potential therapies in comparison with others; our skill to design compounds utilizing a number of modalities and the anticipated advantages of that mannequin; the anticipated advantages of our proprietary manufacturing processes and our inner manufacturing capabilities; the potential advantages of PRISM and our stereopure oligonucleotides in contrast with stereorandom oligonucleotides; the advantage of nucleic acid therapeutics typically; the power of our mental property; the anticipated length of our money runway; and our expectations concerning the influence of the COVID-19 pandemic on our enterprise. Precise outcomes might differ materially from these indicated by these forward-looking statements because of varied vital elements, together with the next: our skill to finance our drug discovery and growth efforts and to lift further capital when wanted; the severity and length of the COVID-19 pandemic and its doubtlessly detrimental influence on the conduct of, and the timing of enrollment, completion and reporting with respect to, our medical trials; every other impacts on our enterprise because of or associated to the COVID-19 pandemic; the flexibility of our preclinical packages to provide knowledge enough to help our medical trial purposes and the timing thereof; our skill to take care of the corporate infrastructure and personnel wanted to attain our targets; the medical outcomes of our packages, which can not help additional growth of product candidates; actions of regulatory businesses, which can have an effect on the initiation, timing and progress of medical trials; our effectiveness in managing future medical trials and regulatory interactions; the effectiveness of PRISM, together with PN spine chemistry; the effectiveness of our novel ADAR-mediated RNA enhancing platform functionality; the continued growth and acceptance of oligonucleotides as a category of medicines; our skill to reveal the therapeutic advantages of our candidates in medical trials, together with our skill to develop candidates throughout a number of therapeutic modalities; our dependence on third events, together with contract analysis organizations, contract manufacturing organizations, collaborators and companions; our skill to fabricate or contract with third events to fabricate drug materials to help our packages and progress; our skill to acquire, preserve and defend our mental property; our skill to implement our patents towards infringers and defend our patent portfolio towards challenges from third events; and competitors from others growing therapies for comparable indications, in addition to the data below the caption “Threat Elements” contained in our most up-to-date Annual Report on Kind 10-Okay filed with the Securities and Alternate Fee (SEC) and in different filings we make with the SEC now and again. We undertake no obligation to replace the data contained on this press launch to mirror subsequently occurring occasions or circumstances.
WAVE LIFE SCIENCES LTD.
UNAUDITED CONSOLIDATED BALANCE SHEETS
(In 1000’s, besides share quantities)
September 30, 2020 December 31, 2019 Belongings Present property: Money and money equivalents $ 216,363 $ 147,161 Present portion of accounts receivable 30,000 20,000 Pay as you go bills 7,966 9,626 Different present property 4,101 8,689 Complete present property 258,430 185,476 Lengthy-term property: Accounts receivable, web of present portion — 30,000 Property and gear, web 31,116 36,368 Working lease right-of-use property 16,725 18,101 Restricted money 3,650 3,647 Different property 140 10,658 Complete long-term property 51,631 98,774 Complete property $ 310,061 $ 284,250 Liabilities, Collection A most popular shares and shareholders’ fairness Present liabilities: Accounts payable $ 9,699 $ 9,073 Accrued bills and different present liabilities 10,182 16,185 Present portion of deferred income 86,192 89,652 Present portion of working lease legal responsibility 3,591 3,243 Complete present liabilities 109,664 118,153 Lengthy-term liabilities: Deferred income, web of present portion 56,288 63,466 Working lease legal responsibility, web of present portion 26,574 29,304 Different liabilities 1,420 1,721 Complete long-term liabilities $ 84,282 $ 94,491 Complete liabilities $ 193,946 $ 212,644 Collection A most popular shares, par worth; shares issued and
excellent at September 30, 2020 and December 31, 2019 $ 7,874 $ 7,874 Shareholders’ fairness: Unusual shares, par worth; 48,769,049 and 34,340,690 shares issued
and excellent at September 30, 2020 and December 31, 2019, respectively $ 694,066 $ 539,547 Further paid-in capital 68,354 57,277 Accrued different complete earnings 301 267 Accrued deficit (654,480 ) (533,359 ) Complete shareholders’ fairness $ 108,241 $ 63,732 Complete liabilities, Collection A most popular shares and shareholders’ fairness $ 310,061 $ 284,250
WAVE LIFE SCIENCES LTD.
UNAUDITED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In 1000’s, besides share and per share quantities)
Three Months Ended
September 30, 9 Months Ended
September 30, 2020 2019 2020 2019 Income $ 3,450 $ 2,929 $ 10,638 $ 13,583 Working bills: Analysis and growth 28,275 44,585 100,911 126,303 Basic and administrative 9,590 12,523 32,791 35,064 Complete working bills 37,865 57,108 133,702 161,367 Loss from operations (34,415 ) (54,179 ) (123,064 ) (147,784 ) Different earnings (expense), web: Dividend earnings 40 1,208 560 4,176 Curiosity earnings (expense), web (17 ) 6 (16 ) 25 Different earnings, web 1,292 2,239 1,399 6,715 Complete different earnings, web 1,315 3,453 1,943 10,916 Loss earlier than earnings taxes (33,100 ) (50,726 ) (121,121 ) (136,868 ) Earnings tax provision — — — — Web loss $ (33,100 ) $ (50,726 ) $ (121,121 ) $ (136,868 ) Web loss per share attributable to atypical
shareholders—primary and diluted $ (0.86 ) $ (1.48 ) $ (3.36 ) $ (4.06 ) Weighted-average atypical shares utilized in
computing web loss per share attributable to
atypical shareholders—primary and diluted 38,364,224 34,281,203 36,021,256 33,719,055 Different complete earnings (loss): Web loss $ (33,100 ) $ (50,726 ) $ (121,121 ) $ (136,868 ) International forex translation 23 2 34 129 Complete loss $ (33,077 ) $ (50,724 ) $ (121,087 ) $ (136,739 )
Investor Contact:
Kate Rausch
617-949-4827
[email protected]
Graham Morrell
781-686-9600
[email protected]
Media Contact:
Alicia Suter
617-949-4817
[email protected]
Source link
from Diaspora9ja https://diaspora9ja.com/wave-life-sciences-reports-third-quarter-2020-financial-results-and-provides-business-update-nasdaqwve/?utm_source=rss&utm_medium=rss&utm_campaign=wave-life-sciences-reports-third-quarter-2020-financial-results-and-provides-business-update-nasdaqwve
0 notes
Photo

The doesn't look like a hair from his head . #anime #animeforlife #animefans #animes #animelove #animelover #animepage #cuteanime #animecute #animefans #otaku #otakus #animeswallpapers #animewallpapers #animeart #animecomics #animewallpaperhd #wallpaperanime #animewallpaper #animeartwork #animepics #mhtt #animecouple #cuteanimecouple #kawai #myheroacademia #mha #bokunoheroacademia #bnha (at India) https://www.instagram.com/p/CFNFKbTlla-/?igshid=seifquj61jjm
#anime#animeforlife#animefans#animes#animelove#animelover#animepage#cuteanime#animecute#otaku#otakus#animeswallpapers#animewallpapers#animeart#animecomics#animewallpaperhd#wallpaperanime#animewallpaper#animeartwork#animepics#mhtt#animecouple#cuteanimecouple#kawai#myheroacademia#mha#bokunoheroacademia#bnha
0 notes
Text

罹患亨丁頓氏舞蹈症的病人,變異的亨丁頓蛋白帶有過長的聚麩醯胺酸鏈(polyglutamine),隨著聚麩醯胺酸鏈的增加,亨丁頓蛋白容易堆積在腦部,破壞神經元。
亨丁頓氏舞蹈症(Huntington’s Disease)是一種神經退化疾病,因突變基因讓亨丁頓蛋白(mHtt)異常聚集,造成腦部神經持續性退化,患者發病後手腳不聽使喚,像在跳舞一樣,末期急速退化,平均存活約15至20年,目前仍無法完全治癒。
中央研究院化學研究所黃人則副研究員與前生醫所杜邦憲助研究員組成的研究團隊,設計出「雙極性胜肽分子」,運用至罹病小鼠後,發現其運動與認知功能失調明顯得到改善。本研究已於今(2020)年1月刊登於《先進科學》(Advanced Science)。
雙極性胜肽分子 可讓變異「壞蛋白」分散
黃人則指出,罹患亨丁頓氏舞蹈症的病人,變異的亨丁頓蛋白帶有過長的聚麩醯胺酸鏈(polyglutamine),隨著聚麩醯胺酸鏈的增加,亨丁頓蛋白容易堆積在腦部,破壞神經元。
研究團隊設計出雙極性胜肽分子,分子的其中一端可以辨識變異的亨丁頓蛋白並附著上去,分子的另一端帶正電,可以利用電荷排斥力,把聚集的變異蛋白分散開來,讓變異的亨丁頓壞蛋白「被分手」,減少對神經細胞的毒性及損傷。
另外,將雙極性胜肽分子透過鼻腔給藥至罹病小鼠,可以使小鼠在滾筒跑步機上維持平衡,運動功能獲得顯著改善,並且在T型迷宮實驗中,展現較好的認知功能。
新突破! 從活細胞追蹤變異蛋白的動態結構變化
過去有關亨丁頓氏舞蹈症的研究,不易從生物體內觀察變異蛋白聚集化的過程,黃人則表示,此研究另一突破是建立生物光電影像平台,透過螢光影像顯微系統,觀測亨丁頓蛋白在活細胞中聚集與分散的過程。
研究團隊在小鼠神經細胞實驗中,把變異的亨丁頓蛋白標示上兩種不同的螢光蛋白,此時,若變異的亨丁頓蛋白互相靠近,將改變螢光蛋白的放光特性,藉此觀察變異蛋白是否聚集,以及雙極性胜肽分子的抑制效果。目前已獲得和此研究相關的臺灣專利,其他國家專利則正申請中。
加入【健康醫療網】,天天關注您健康!LINE@ ID:@healthnews
訂閱【健康愛樂活】影音頻道,閱讀健康知識更輕鬆
健康資訊由熱新聞提供
原文連結: 手腳不聽使喚,像在跳舞一樣... 中研院有望逆轉「亨丁頓氏舞蹈症」
更多相關內容

化療前先凍卵 癌女順利生子圓夢

健康醫療網
健康醫療網是以健康新聞、治療新知為主的全方位健康媒體平台,致力於提供最專業、最即時、最樂活的多元化資訊。
0 notes
Quote
Top 5 loại máy phi thuyền “sang chảnh” được các spa sử dụng nhiều nhất hiện nay
Tắm trắng, giảm cân bằng máy phi thuyền là một trong những dịch vụ làm đẹp “hot” tại các spa, thẩm mỹ viện cao cấp hiện nay. Vậy sử dụng loại máy phi thuyền để tăng đạt được hiệu quả tốt nhất, mang đến sự hài lòng tuyệt đối cho khách hàng?
Theo đánh giá của các chuyên gia trong lĩnh vực làm đẹp thì máy phi thuyền mang lại hiệu quả cho liệu trình tắm trắng, giảm béo hơn gấp nhiều lần so với các công nghệ khác. Đó là nhờ vào trường năng lượng phát ra từ máy giúp dưỡng chất trong mỹ phẩm làm trắng thẩm thấu tốt hơn.
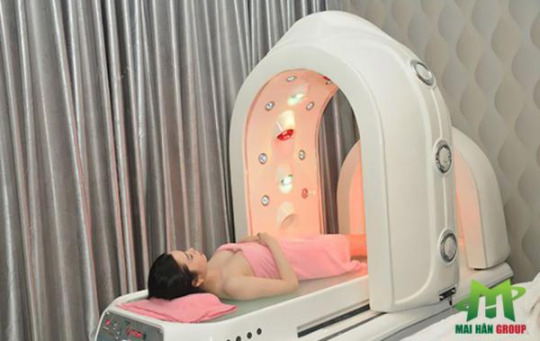
Giảm cân tắm trắng bằng phi thuyền là công nghệ cần phải có ở các spa cao cấp
Một ưu điểm nổi bật nữa khi dùng máy phi thuyền đó là không tốn nhiều thời gian, kết quả điều trị cao. Một lần sử dụng dịch vụ chỉ mất khoảng 1 tiếng, liệu trình từ 3 – 6 buổi. Đặc biệt, hiệu quả làm trắng – giảm cân sẽ duy trì lâu dài, không mất thời gian nghỉ dưỡng hay bảo vệ da sau quá trình điều trị.
Hiện nay, có rất nhiều dòng máy phi thuyền khác nhau được bày bán trên thị trường. Nhưng nổi bật nhất vẫn là 3 loại máy phi thuyền “sang chảnh” được các spa sử dụng nhiều nhất dưới đây:

Top 1: Máy phi thuyền Hoàng Gia DM - 5018A trắng mịn da ngay từ lần đầu tiên
Phi thuyền tắm trắng Hoàng Gia ứng dụng công nghệ ánh sáng hồng ngoại tác động vào da làm giãn nở lỗ chân lông, giúp dưỡng chất hấp thụ tốt hơn. Đồng thời ánh sáng quang phổ tăng cường đốt cháy chất béo và cải thiện sự trao đổi chất, ngăn chặn chất béo từ da và hồi phục trở lại, thắt chặt da, loại bỏ độc tố và thư giãn.
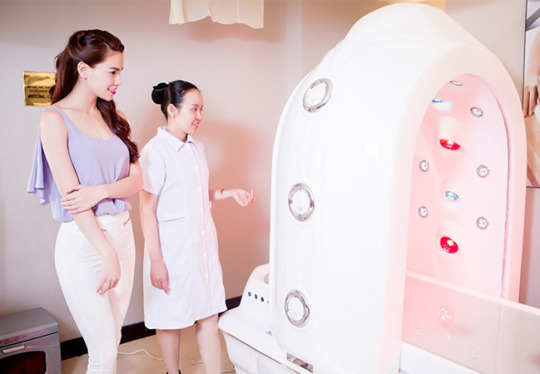
Ngay cả những người đẹp nổi tiếng cũng ưa chuộng việc tắm trắng bằng phi thuyền Hoàng Gia
Đặc biệt, khoang Photon là công nghệ đột phá có hiệu quả giúp nâng cao khả năng làm trắng da, giảm béo, đốt cháy mỡ toàn thân, tăng cường tuần hoàn máu, cải thiện hiện tượng phình huyết quản...
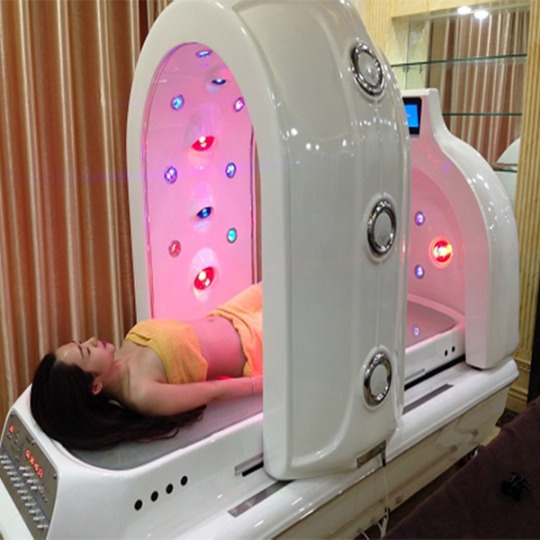
Công nghệ tắm trắng, giảm cân an toàn, hiệu quả
Không những thế với thiết kế màn LCD và kết hợp âm thanh nghe nhạc sẽ mang đến cho khách hàng cảm giác thoải mái. Mười hai cặp BIO miếng đệm điện sẽ làm tăng tính thư giãn khi các loại phi thuyền khác không có được cho rung động và nghỉ ngơi.
Một ưu điểm nổi bật nữa khiến phi thuyền hoàng gia MHTT - 5019A được các spa sử dụng nhiều nhất. Đó là sự an toàn và hiệu quả. Làn da của khách hàng sẽ trở nên trắng mịn hồng hào thấy được ngay lần đầu tiên sử dụng. Không gây vàng lông, không lột tẩy, không kích ứng da cũng như các tác dụng phụ khác…

Phi thuyền hoàng gia DM - 5018A đang được sử dụng tại Viện Thẩm Mỹ Thảo Tây – đơn vị được Mai Hân Spa setup – thiết kế - thi công và cung cấp trang thiết bị máy móc, nội thất
Với những tác dụng tuyệt vời như vậy nên phi thuyền hoàng gia DM - 5018A là lựa chọn lý tưởng cho mọi quy trình tắm trắng và giảm cân tại spa. Để tìm hiểu kỹ càng hơn về sản phẩm này hãy liên hệ sốt hotline 0287 3058 567, chuyên viên Mai Hân Spa sẽ tư vấn miễn phí cho bạn.
Xem chi tiết: Ưu điểm nổi bật của máy phi thuyền Hoàng Gia DM - 5018A
Top 2: Phi thuyền tắm trắng giảm cân 2 vòm D669 liệu pháp quang phổ

Đây là một trong những dòng máy phi thuyền sử dụng công nghệ đột phá đang được dân spa toàn thế giới ưa chuộng với những ưu điểm nổi trội:
- Sử dụng liệu pháp quang phổ an toàn và hiệu quả cao khi đốt cháy lượng mỡ thừa kết hợp làm trắng an toàn bằng cách khóa chặt melanin trên da.
- Quy trình dưỡng trắng da được thực hiện từ tác động bên trong và giải quyết tận gốc các nguyên nhân gây sạm, nám trong khi vẫn đảm bảo chất lượng an toàn và hiệu quả.

- Thiết kế 2 vòm dành riêng cho vùng thân trên, thân dưới hoàn toàn khác biệt với các loại máy phi thuyền khác tăng hiệu quả điều trị.
- Các dải ánh sáng được bố trí ở những vị trí hợp lí và có khả năng tác động lên toàn bộ cơ thể và mang đến sự tập trung cao nhất.

Khách hàng giảm cân với máy phi thuyền 2 vòm
- Kiểu dáng nhỏ gọn ít tốn diện tích và dễ sử dụng. Nên ngoài các spa thì một số gia đình có điều kiện kinh tế có thể trang bị thêm sản phẩm này bên cạnh phòng xông hơi, bồn jacuzzi để chăm sóc sức khỏe và làm đẹp ngay tại nhà.
Xem chi tiết: Thông số kỹ thuật, công dụng, cách sử dụng máy phi thuyền 2 vòm
Top 3: Phi thuyền tắm trắng giảm cân ánh sáng WS 5008 giảm từ 3 – 7 kg sau 4 buổi

Đây là sản phẩm đang được rất nhiều các spa lựa chọn bởi sự ứng dụng thông minh các bước sóng ánh sáng chuyên biệt hỗ trợ đẩy rerum dưỡng trắng vào tận sâu bên trong. Đồng thời khóa chặt hoạt động của hắc sắc tố và ngăn chặn sự hình thành hắc sắc tố mới trong cơ thể.
Thiết kế máy phi thuyền WS 5008 có thể di chuyển lên xuống theo các vùng cơ thể

Điểm nổi bật đầu tiên của WS 5008 đó là thiết kế phù hợp với những vùng cơ thể thường tập trung nhiều mỡ như eo, bắp tay, bắp chân. Các dải ánh sáng cũng được bố trí rất khoa học nên có thể tác động lên toàn bộ các vùng trên cơ thể, mang đến hiệu quả trị liệu tối đa
Đặc biệt, thiết bị này mang đến hiệu quả nhanh chóng chỉ sau 4 buổi trị liệu, khách hàng có thể giảm từ 3 đến 7 kg, da trắng hồng tự nhiên.

Hiệu quả thấy rõ chỉ sau một lần sử dụng
Thêm một ưu điểm nữa là những chi tiết của phi thuyền có thể tháo rời, gọn nhẹ vì vậy các spa có thể dễ dàng di chuyển phi thuyền tắm trắng giảm cân ánh sáng đến vị trí cần thiết.

Máy phi thuyền WS 5008 kết hợp giường massage đá nóng – một sản phẩm do Mai Hân Spa thiết kế, sản xuất, phân phối tăng cường hiệu quả trị liệu, thư giãn, chăm sóc sức khỏe
Với chiếc máy phi thuyền WS 5008 này các spa có thể đáp ứng được tất cả nhu cầu tắm trắng, giảm béo của khách hàng. Liên hệ ngay hotline 0287 3058 567 để mua sản phẩm mới mức giá tốt nhất.
Xem chi tiết: Ưu điểm nổi bật của Máy phi thuyền WS 5008
Top 4: Máy phi thuyền con sò

Đặc điểm nổi bật của dòng máy phi thuyền này là được thiết kế giống như hình dáng của một con sò.
Chính vì thế các ánh sáng màu sẽ tác động lên toàn bộ cơ thể, từ đó mang đến hiệu quả:
- Hỗ trợ da hấp thụ các dưỡng chất làm trắng, giảm béo tốt hơn từ đó đạt được hiệu quả làm đẹp tốt nhất.
- Thúc đẩy bài tiết mồ hôi để đào thải độc tố, kim loại nặng, bã nhờn.
- Thúc đẩy lưu thông tuần hoàn máu
- Thu nhỏ lỗ chân lông
- Xoa dịu cơ bắp và thần kinh
Xem chi tiết: Những ứng dụng của phi thuyền tắm trắng con sò
Top 5: Phi thuyền con sò hồng ngoại

Cũng được thiết kế dạng con sò với đầy đủ các tính năng, công dụng trên. Nhưng loại phi thuyền này được tích hơp thêm hệ thống đèn hồng ngoại cao cấp, mang lại hiệu quả tối ưu, thêm nhiều công dụng làm đẹp, chăm sóc sức khỏe.
Tia hồng ngoại cộng hưởng tác động lên các vùng mỡ thừa làm mềm chất béo và đào thải ra bên ngoài. Sau khoảng 20-30 phút sử dụng phi thuyền con sò hồng ngoại cơ thể có thể tiêu hao 600 calo năng lượng, tương đương như khi bạn chạy 100.000 mét.

Phi thuyền con sò hồng ngoại sử dụng trong spa
Nhiệt độ có thể lên đến 90 độ hỗ trợ cải thiện lưu thông hệ bạch huyết đào thảo độc tố giúp làn da và cơ thể thêm khỏe khoắn. Đồng thời liệu pháp Ion Oxy kết hợp các tia hồng ngoại sẽ giúp làm trắng và sáng da từ bên trong, trì hoãn quá trình lão hóa da.
Để nắm rõ hơn về thông số kỹ thuật, công năng và cách sử dụng máy phi thuyền con sò bạn hãy gọi đến số hotline 0287 3058 567 đội ngũ chuyên viên tư vấn chuyên nghiệp sẽ giải đáp mọi thắc mắc của bạn.
Xem chi tiết: Tính năng, công dụng nổi bật của phi thuyền con sò hồng ngoại
Mua máy phi thuyền tắm trắng giảm cân ở đâu đảm bảo hàng chính hãng, giá tốt nhất?
Với hơn 10 năm kinh nghiệm trong lĩnh vực setup – cung ứng các thiết bị công nghệ cao cho các spa, thẩm mỹ viện cao cấp như Thảo Tây, Hà Anh Spa… Đồng thời, luôn lấy khách hàng làm mục tiêu quan tâm hàng đầu, Mai Hân Spa chính là địa chỉ tin cậy để giúp spa của bạn sở hữu sản phẩm máy phi thuyền chất lượng với giá cả phải chăng nhất.

Nếu còn bất cứ thắc mắc nào liên quan đến các loại máy phi thuyền tắm trắng, giảm cân, bạn liên hệ ngay với chúng tôi theo thông tin bên dưới, chuyên viên Mai Hân Spa sẽ liên hệ và tư vấn cho bạn chi tiết nhất.
THÔNG TIN LIÊN HỆ
Mai Hân Spa
♦ Địa chỉ: 166 Đường D1, phường 25, Q.Bình Thạnh, TP.HCM
♦ HOTLINE: 0287.3058.567
♦ Website: https://maihanspa.com
♦ Email: [email protected]
0 notes
Text
復旦研究團隊為無法治癒的神經疾病帶來新曙光

和我們熟悉的阿爾茨海默病(AD)、帕金森病(PD)一樣,亨廷頓病(HD)是一種神經退行性疾病。這幾種疾病的發病機理都與蛋白質的錯誤折疊有關。儘管涉及的具體致病蛋白不盡相同,但它們都會堆積在神經細胞內,日積月累,最終將神經細胞毒死,進而導致患者出現運動、認知等多方面的功能障礙。
設法減少和消除細胞內的致病蛋白,是治療這類疾病的一條重要思路。為此,科學家們開始嘗試使用一些新興的生物工具,例如基因編輯CRISPR、反義RNA,靶向HD的致病蛋白——突變亨廷頓蛋白(mHTT)。

▲研究團隊主要成員(圖片來源:復旦大學,攝影:師源隆)
在這項研究中,魯伯塤教授、丁澦教授與費義艷研究員領銜的團隊提出了一種清除mHTT的新方法:利用細胞自身的蛋白降解系統——自噬作用(autophagy)。
提到細胞自噬,可能大家對它並不陌生。在2016年,日本科學家大隅良典因發現細胞自噬機製而獨得諾貝爾生理學或醫學獎。這種生理過程在真核生物中普遍存在,細胞利用溶酶體清除和降解自身的細胞結構、衰老的細胞器以及不再需要的生物大分子,可以說自噬作用就像是細胞內的“廢物回收站”。

▲利用自噬過程降解致病蛋白、同時保留野生型蛋白的示意圖(圖片來源:參考資料[2])
復旦研究團隊的思路就是把HD的致病蛋白丟進細胞裡的廢物回收站。為了精準靶向致病蛋白,同時又不誤傷“無辜”——尤其是承擔著神經保護功能的正常HTT蛋白,研究人員設想了一種“小分子綁定化合物”的策略,他們稱之為“小分子膠水”。
具體來說,就是利用特定的小分子,把自噬過程中的一個關鍵蛋白LC3和致病蛋白“粘”在一起,從而促進致病蛋白特異性地降解。
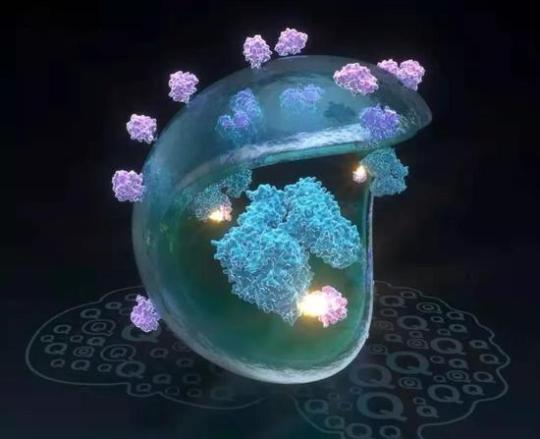
▲發光的小分子膠水連接了致病蛋白mHTT(藍色)和自噬小體蛋白LC3(紫色),將其帶入自噬小體進一步降解(圖片來源:復旦大學)
這支多學科團隊利用新型高通量藥物篩選平台,從近4000種小分子化合物中找到2種符合要求的小分子。接著,通過檢測具有類似結構的小分子,研究團隊一共獲得了4種理論上可行的“小分子膠水”。
那麼,這4種化合物的實戰效果如何呢?一系列實驗表明,在培養的小鼠神經元和HD患者的細胞,以及HD果蠅和小鼠模型中,這些化合物都能顯著降低mHTT水平,同時維持正常HTT水平不變。
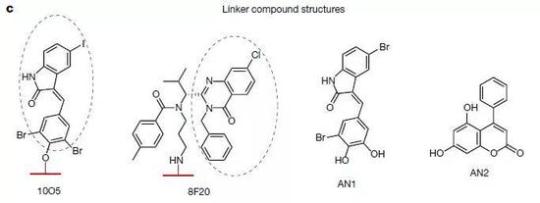
▲四種有治療潛力的小分子化合物(圖片來源:參考資料[1])
魯伯塤教授介紹,“這4種化合物中,有至少2種,可以跨過血腦屏障,並通過低劑量腹腔給藥直接降低HD小鼠的大腦皮層及紋狀體的變異亨廷頓蛋白水平,而不影響腦組織中的野生型亨廷頓蛋白水平,也改善了疾病相關的表型,為亨廷頓病口服或註射藥物的研發提供了切入點。”
的儘管目前在動物實驗中取得了可喜積極結果,神經退行性疾病領域的著名科學家Huda Zoghbi教授在專文評述中指出,下一步“必須先在小鼠身上開展長期臨床前試驗,以確定其在長期治療過程中具有持久穩定的療效”。
如果臨床上可以證明對HD有效,“自噬小體綁定化合物的藥物研發策略也有望應用於其他難以無法靶向的致病蛋白,甚至非蛋白的致病物質。”魯伯塤教授對這項成果的未來應用前景充滿期待。
我們祝賀科學家們在治療神經退行性疾病的攻堅戰中取得的這一成果,也期待後續的研究進展能早日造福患者。
.
from 復旦研究團隊為無法治癒的神經疾病帶來新曙光
via KKNEWS
0 notes