#phosphofructokinase
Text
Gemma Parker Takes Her First BBC
Huge cum on wife beautiful feet
lesbicas safadas
Embarazada prego fuck tits mom love wet pussy conxa
Really good dick sucking by horny brunette
Glory hole no shopping
Morena Sexo na WebCam
Let me bounce my tits for you while you jerk off JOI
SexyBBW Blowjob, Fucking and Creamy Squirt
twink monster cum
#nucle-#prescinding#unsee#drawled#railly#mediastino-pericarditis#isekai#smooth-podded#scaups#psychiatrists#fittier#tari#Samandura#columbotitanate#Minnesota#endosomes#Nullipennes#phosphofructokinase#quaintest#speech-bound
0 notes
Text
Zoey Monroe wet anal
Interracial Sex Scene Josephine Lorentzen
Amiga prostituta en me la cojo en el hotel
Hetero nerd reluctantly agrees to jerk off with another guy hidden cam
Cheating wife records New Years creampie with stranger
Esposa safada bem molhadinha
Dominican Thotty Bala Bunny
Tetona mendocina se chupa y se toca
tall thick girl twerking
Real, solo, gay amateur, voyeur, Luiggi, pasivo de closet, travesti, shemale, pene, potito, nalguitas, piernas, culo, sexo, reyna, lesviana, Ica
#scaups#psychiatrists#fittier#tari#Samandura#columbotitanate#Minnesota#endosomes#Nullipennes#phosphofructokinase#quaintest#speech-bound#Warthe#rebless#workbench's#pothousey#craftworker#Bayley#skolly#suspensation
0 notes
Text
I have discovered that phosphofructokinase is a thing that exists and that fructokinase is also a thing that exists and I will now be using that instead of fuck just to be an annoying little shit
0 notes
Text
Structure And Function Of Enzymes
What Is Enzyme Structure And What Is Function Of Enzymes?
What are enzymes and what do they do in our bodies? Enzymes are basically proteins that are produced by living organisms to bring about certain metabolic and biochemical reactions in the body. They are biological catalysts that speed up reactions inside the body.
What Is The Structure Of An Enzyme?
Enzymes, as mentioned above, are biological catalysts. While they hasten or speed up a process, they are actually providing an alternative pathway for the process. But, in the process, the structure or composition of the enzymes remain unaltered.
Enzymes are actually made up of thousands of amino acids that are linked in a specific way to form different enzymes. The enzyme chains fold over to form unique shapes and it is these shapes that provide the enzyme with its characteristic chemical potential. Most enzymes also contain a non-protein component known as the co-factor.
An enzyme’s function is intrinsically linked to its three-dimensional structure, determining how it performs substrate binding, catalysis, and regulation. X-ray crystallography has been the most important technique in the development of our understanding of enzyme structure and function. Nuclear magnetic resonance (NMR) has also been used successfully to study many structures, but crystallography remains the principle technique for structure elucidation. The first enzyme to be crystallised and have its structure successfully solved was chicken egg lysozyme in 1965. Importantly, as well as the structure of the free enzyme, it was possible to crystallise lysozyme with a substrate analogue bound in the active site. This structure, allowed the proposal of a chemical mechanism for the enzyme, based on positioning of groups around the site of substrate cleavage. The use of crystal structures with bound substrate and transition state analogues has helped to reveal the catalytic mechanisms of countless enzymes since.
Domains
Larger proteins tend to fold into a series of smaller domains, each of which forms a self-contained structural unit. These domains are often described as the units of evolution because they can often be swapped between proteins without disturbing the folding of other parts of the protein and thus novel functions can be created by novel combinations of domains within a single protein. In enzymes, certain functions are often contained within a domain. For instance, the nucleotide-binding Rossmann domain is found combined with a diverse range of separate catalytic domains, allowing each enzyme to bind similar nucleotide cofactors such as nicotinamide adenine dinucleotide (NADH), nicotinamide adenine dinucleotide phosphate (NADPH) and flavin mono-nucleotide (FMN), but perform quite different chemistry. Figure 1.9 shows two different Rossmann domain-containing enzymes: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR). Both enzymes contain the Rossmann domain with a common 3 parallel strand β sheet flanked by α helices. This sheet binds to the cofactor NAD in the case of GAPDH and NADP in the case of DXR. The remainder of the enzyme structure and functon is completely unrelated and contain quite different catalytic residues which allow them to catalyse their different reactions.
Active Sites And Clefts
Although enzymes are often large molecules comprising many hundreds of amino acids, the functional regions of an enzyme are generally restricted to clefts on the surface that comprise only a small part of the enzyme’s overall volume. The most important of these regions is the active site – the pocket or cleft in which the enzyme binds the substrate and in which the catalytic chemistry of the enzyme is performed. Analysis of enzyme structure and function have shown that active sites tend to be formed from the largest cleft on the surface of the protein.
Phosphofructokinase catalyses the phosphorylation of Dfructose 6-phosphate, converting ATP into ADP in the process. It is regulated by binding of ATP to an allosteric site, quite distinct from the active site, that inhibits the enzyme. These regulatory clefts as well as being able to bind regulatory molecules, also require the ability to transmit binding information from themselves to the active site, so that catalytic activity can be regulated.
How Do Enzymes Work?
For any reaction to occur in the universe, there is an energy requirement. In cases where there is no activation energy provided, a catalyst plays an important role to reduce the activation energy and carried forward the reaction. This works in animals and plants as well. Enzymes help reduce the activation energy of the complex molecules in the reaction. The following steps simplify how an enzyme works to speed up a reaction:
Step 1: Each enzyme has an ‘active site’ which is where one of the substrate molecules can bind to. Thus, an enzyme- substrate complex is formed.
Step 2: This enzyme-substrate molecule now reacts with the second substrate to form the product and the enzyme is liberated as the second product.
There are many theories that explain how enzymes work. But, there are two important theories that we will discuss here.
Theory 1: Lock and Key Hypothesis
This is the most accepted of the theories of enzyme action.
This theory states that the substrate fits exactly into the active site of the enzyme to form an enzyme-substrate complex. This model also describes why enzymes are so specific in their action because they are specific to the substrate molecules.
Theory 2: Induced Fit Hypothesis
This is similar to the lock and key hypothesis. It says that the shape of the enzyme molecule changes as it gets closer to the substrate molecule in such a way that the substrate molecule fits exactly into the active site of the enzyme.
What factors affect enzyme activity in the cell?
Concentration of Enzymes and Substrates: The rate of reaction increases with increasing substrate concentration up to a point, beyond which any further increase in substrate concentration produces no significant change in reaction rate. This occurs because after a certain concentration of the substrate, all the active sites on the enzyme are full and no further reaction can occur.
Temperature: With the increase in temperature, the enzyme activity increases because of the increase in kinetic energy of the molecules. There is an optimum level when the enzymes work at the best and maximum. This temperature is often the normal body temperature of the body. When the temperature increases beyond a certain limit, enzymes, which are actually made up of proteins, begin to disintegrate and the rate of reaction slows down.
pH: Enzymes are very sensitive to changes in the pH and work in a very small window of permissible pH levels. Below or above the optimum pH level, there is a risk of the enzymes disintegrating and thereby the reaction slows down.
Inhibitors: The presence of certain substances that inhibit the action of a particular enzyme occurs when the inhibiting substance attaches itself to the active site of the enzyme thereby preventing the substrate attachment and slows down the process.
0 notes
Text
What are the stages of Glycolysis?
All plants in an ecosystem produce food through the Photosynthesis process, however, in order to convert the produced food into energy the Glycolysis process plays a very important role. And here in the below article, we will help you understand all about the Glycolysis Cycle, and all the different stages involved in this process in much more detail.
The glycolysis process is defined as, a metabolic procedure that involves converting glucose into pyruvic acid. In other words, through this process, glucose is broken down to produce energy. Moreover, this process is commonly found in the cytoplasm of the cell, it generally results in the formation of pyruvate, water, NADH, and ATP. Besides, unlike other processes, this does not have any oxygen, and it is actively observed in aerobic as well as anaerobic organisms.
Stages of Glycolysis:
Stage 1
In this stage, the glucose is trapped inside a cell, by the addition of phosphate group to glucose, because of the enzyme hexokinase. Moreover, the addition of this phosphate group to glucose results in the formation of 6-phosphate.
Stage 2
Now in this stage, because of the enzyme phosphoglucomutase, Glucose 6-phosphate undergoes isomerization into forming fructose 6-phosphate.
Stage 3
The ATP Molecule inside the cell results in the transfer of phosphate molecule to fructose 6-phosphate, which results in the formation of fructose 1,6-biphosphate, because of the enzyme phosphofructokinase.
Stage 4
For this stage, the enzyme aldolase plays a major role in converting or forming isomers such as glyceraldehyde 3-phosphate, and dihydroxyacetone phosphate, by converting the fructose 1,6-biphosphate.
Stage 5
Triose-phosphate isomers play an active role and convert dihydroxyacetone phosphate into glyceraldehyde 3-phosphate.
Stage 6
Unlike other stages, this one involves two reactions,
The first reaction results in the formation of NADH +H+, by transferring one hydrogen molecule from glyceraldehyde phosphate to nicotinamide adenine dinucleotide. This process is possible because of the enzyme glyceraldehyde 3-phosphate.
Now the above-mentioned enzyme adds another phosphate to the oxidized glyceraldehyde phosphate, which will result in 1,3 biphosphoglycerate.
Stage 7
Next, in stage 7, the phosphate from 1,3 biphosphoglycerate to ADP to ATP, with the assistance of the enzyme, phosphoglycerokinase. Moreover, this stage will result in two molecules of ATP and Phosphoglycerate.
Stage 8
The two phosphoglycerate molecules will lose two phosphates from the third carbon to the second carbon, mainly by yielding two molecules of 2-phosphoglycerate with the help of the enzyme phosphoglyceromutase.
Stage 9
Now the water molecule, from the 2-phosphoglycerate, is removed, which will lead to the formation of phosphoenolpyruvate, with the enzyme enolase.
Stage 10
Through the enzyme pyruvate kinase, the phosphate from phosphoenolpyruvate gets transferred to ADP and will result in forming ATP and pyruvate.
Because of this, we suggest you join the online interactive classes offered by the Tutoroot platform. Through this online coaching program, the students can access various benefits, like Doubt Clearing Sessions, Best Educational Materials, Expert Staff Guidance, Cost Effective Prices, and a lot more.
1 note
·
View note
Text
The principal reactions associated with the classic glycolytic pathway in plants are almost identical to those in animal cells (Figure 12.3). (...) UDP-glucose pyrophosphorylase then converts UDP-glucose and pyrophosphate (PPi) into UTP and glucose 6-phosphate (see Figure 12.3). (...) These reactions also include two of the three essentially reversible reactions of the glycolytic pathway, which are catalyzed by hexokinase and phosphofructokinase (see Figure 12.3). (...) The phosphorylated carboxylic acid on carbon 1 of 1,3-bisphosphoglycerate (see Figure 12.3) has a large standard of free-energy change (∆G⁰') of hydrolysis (-49.3 kJ/mol). (...) Because the glycolytic reaction catalyzed by ATP-dependent phosphofructokinase is essentially irreversible (see Figure 12.3), an additional enzyme, fructose-1,6-bisphosphate phosphatase, converts fructose 1,6-bisphosphate irreversibly into fructose 6-phosphate and Pi during gluconeogenesis. (...) In plants, the interconversion of fructose 6-phosphate and fructose 1,6-bisphosphate is made more complex by the presence of an additional (cytosolic) enzyme, PPi-dependent phosphofructokinase (pyrophosphate: fructose 6-phosphate 1-phosphotransferase), which catalyzes the following reversible reaction (see Figure 12.3):
Fructose 6-P + PPi → fructose 1,6-bisphosphate + Pi
where -P represents a bound phosphate.


The oxaloacetate is then reduced to malate by the action of malate dehydrogenase, which uses NADH as a source of electrons (see Figure 12.3). (...) To overcome this limitation, plants and other organisms can further metabolize pyruvate by carrying out one or more forms of fermentation (see Figure 12.3). (...) Pyruvate kinase and PEP carboxylase, the enzymes that metabolize PEP in the last steps of glycolysis (see Figure 12.3), are in turn sensitive to feedback inhibition by citric acid cycle intermediates and their derivatives, including malate, citrate, 2-oxoglutarate, and glutamate. (...) As already described, malate can be synthesized from PEP in the cytosol via the enzymes PEP carboxylase and malate dehydrogenase (see Figure 12.3). (...) For example, during anaerobic stress caused by flooding, roots ferment pyruvate to lactate through the action of lactate dehydrogenase (see Figure 12.3). (...) These changes in enzyme activity quickly lead to a switch from lactate to ethanol production (see Figure 12.3).
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#glycolysis#plant cells#photorespiration#sugars#phosphorylation#carboxylic acid#phosphate#carbon#fructose#ethanol#pyruvate#lactate#acetaldehyde#sucrose#starch#energy production#organic chemistry
0 notes
Text
Sir Arthur Harden

was a British biochemist. He shared the Nobel Prize in Chemistry in 1929 with Hans Karl August Simon von Euler-Chelpin for their investigations into the fermentation of sugar and fermentative enzymes. He was a founding member of the Biochemical Society and editor of its journal for 25 years.
Arthur was born to Scottish Presbyterian businessman Albert Tyas Harden and Eliza Macalister. His early education was at a private school in Victoria Park run by Dr Ernest Adam. He went to study in 1877 at a Tettenhall College, Staffordshire, and entered Owens College in 1882, now the University of Manchester, in 1882, graduating in 1885. He studied chemistry under Professor Roscoe at Owens College and was influenced by J.B. Cohen.
Research
In 1886 Harden was awarded the Dalton Scholarship in Chemistry and spent a year working with Otto Fischer at Erlangen where he worked on the synthesis of β-nitroso-α-naphthylamine and studied its properties. After receiving a Ph.D. he returned to Manchester as a lecturer and demonstrator and taught along with Sir Philip Hartog. He researched the life and work of John Dalton during these years. In 1895 he wrote a textbook on Practical Organic Chemistryalong with F.C. Garrett. Harden continued to work at Manchester until 1897 when he was appointed chemist to the newly founded British Institute of Preventive Medicine, which later became the Lister Institute. He earned the degree Doctor of Science (D.Sc.) from the Victoria University(which included Owens College) in June 1902. Five years later, in 1907 he was appointed Head of the Biochemical Department, a position which he held until his retirement in 1930 (though he continued his scientific work at the Institute after his retirement).
At Manchester, Harden had studied the action of light on mixtures of carbon dioxide and chlorine, and when he entered the Institute he applied his methods to the investigation of biological phenomena such as the chemical action of bacteria and alcoholic fermentation. He studied the breakdown products of glucose and the chemistry of the yeast cell, and produced a series of papers on the antiscorbutic and anti-neuritic vitamins
Harden was knighted in 1926, and received several honorary doctorates. A Fellow of the Royal Society, he received the Davy Medal in 1935.
"Harden–Young ester"
Harden's work on glycolysis in yeast with William John Young led to the discovery of a phosphorylated ester that was known as Harden–Young ester until chemical analysis showed it to be fructose 1,6-bisphosphate. It is now known to be the product of phosphorylating fructose 6-phosphate by the action of phosphofructokinase; it is broken down into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate by the action of aldolase.
0 notes
Text
if I have to memorize one more ridiculously long enzyme name I am going to spontaneously combust!!
#bit of context: i'm taking an online biology course and the teacher talked a lot about how the ten steps of glycolysis#are the most important thing ever- and then forgot to actually tell us the ten steps of glycolysis on the web page#i guess something went wrong while he was embedding the diagram?#so i am left with a lot of notes on which part of which step to focus on#and nothing about what actually happens during each step- which is why i had to look it up online#and the only decent sources are INSANELY detailed and have the full names of everything which i don't even know if i should memorize#but the sources make it seem like knowing the names are the most important part and i can't even ask if they're on the test#because it's against the teacher's policy to tell us anything specific about the tests beyond Apply All You Have Learned In The Unit#and. there is one called hexokinase another called phosphoglucomutase which is entirely distinct from the one called phosphofructokinase#and i feel hell in this study session tonight#personal
4 notes
·
View notes
Text
WHAT THE FUCK IS A SLC3A1 or SLC7A9 GENE BITCH ILL KILL U
0 notes
Text
glucose and then hexokinase and then atp goes in and then its glucose 6 phosphate and then phosphoglucoisomerase and then fructose 6 ohosphatw and then phosphofructokinase and then atp goes in and then fructose 1,6 bisohosphatr and then aldolase and then dhap and then isomerase and then g3p and then triose phosphoate degydrogenase and then nadh comes out and then 1,3 bisphosphoglyceratr and then phosphoglycerokinase and then atp comes out and then 3 phosphoglycerate and then phosphoglyceromutase and then 2 phosphoglycerate and then enolade and then water comes out and then phosphoenolpyruvate and then pyruvate kinase and then atp comes out and then pyruvate.
pyruvate enters the mitochondria and then one carbon leaves as co2 and then nad+ becomes nadh so the molecule is charges and attracts a coenzyme so its acetyl coa.
oxaloacetate joins with acetyl coa and then coa leaves so its citrate and then h2o moves and its isocitrate and then nadh is generated and then co2 leaves so its alpha ketogluterate and then co2 leaves and nadh is generated and coa comes back ao its succinyl coa and then coa leaves again and atp is generated so its succinate and then fadh2 is generated and yhen its fumarate and then water joins and its malate and then nadh is generated and its oxaloacetate again
2 notes
·
View notes
Photo
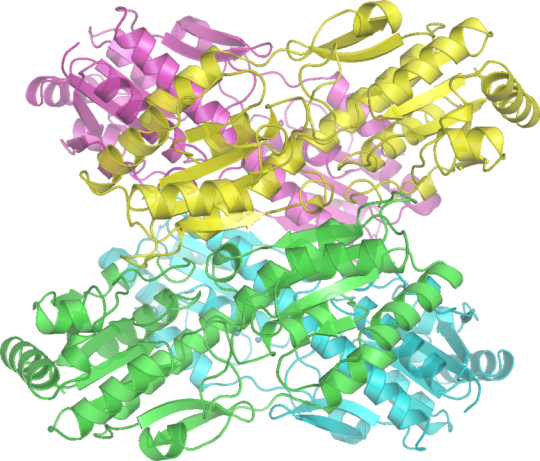
Q. Which Glycogen Storage Disease results from inability of cells to utilize carbohydrates for energy? (Hint: It's the only one to directly affect glycolysis!)
.
.
.
A. GSD VII (Tarui Disease) is due to defect in phosphofructokinase, the rate-limiting step in glycolysis and a gate-keeper in cellular metabolism of glucose. Lack of cellular energy leads to exercise-induced rhabdomyolysis, myoglobinuria, and hemolytic anemia that has infantile, classic, late-onset, and primarily hemolytic forms. Named for the Japanese physician who identified it in 1965, Seiichiro Tarui (1927- ).
Image credit Richard Wheeler, Wikipedia (2006).
13 notes
·
View notes
Note
There are 10 enzymes involved in the 10 steps of glycolysis. In order they are: Hexokinase, Phosphoglucoisomerase, Phosphofructokinase, Aldolase, Phosphotriose isomerase, Glyceraldehyde 3-phosphate dehydrogenase, Phosphoglycerate kinase, Phosphoglycerate mutase, Enolase, and Pyruvate kinase. During glycolysis, a single mole of 6-carbon glucose is broken down into two moles of 3-carbon pyruvate. Furthermore, two molecules of ADP are phosphorylated and converted to ATP.
I’m like 90% sure you made those words up but I appreciate the length of this message and your commitment to the bit, thank you for coming thru 🙏
#that was extremely boring very much appreciated#idk what to tag all these#reverse mike waters in MOPI: awake at inconvenient times
6 notes
·
View notes
Text
my prof: - so then, Fructose 2,6-Biphosphatase is dephosphorylated to form Fructose 6-Phosphatase, which causes Phosphofructokinase to be -
my brain: oh lET ME HAVE A RULE AND A SAW AND A BOARD AND I’LL CUT IT! I’LL CLIMB UP THE LADDER WITH A HAMMER AND A NAIL AND I’LL NAIL IT! WELL WE WORKED SO HA
24 notes
·
View notes
Text
Regulators
Hexokinase is regulated by its products: ADP and glucose-6-phosphate. Hexokinase is allosterically inhibited by glucose-6-phosphate.
Glucokinase is activated by un-phosphorylated phosphofructokinase 2 and fructose-2,6-bisphosphate. They keep glucokinase in the cytoplasm and active.
Phosphofructokinase is inhibited by ATP and citrate. (See 10/10/18 lecture at around 20 minutes). Phosphofructokinase is activated by ADP, AMP (AKA adenylate), and fructose-2,6-bisphosphate.
Pyruvate kinase is inhibited allosterically by ATP. Pyruvate kinase is inhibited by alanine. Pyruvate kinase is inhibited by phosphorylation (using PKA). Pyruvate kinase is dependent on lots of fructose-1,6-bisphosphate (pyruvate kinase can “see” what phosphofructokinase is doing; if phosphofructokinase is v. active and making a lot of fructose 1,6-bisphosphate, that’s “telling” pyruvate kinase to keep making pyruvate, which you don’t want if you’re trying to inhibit pyruvate kinase)
#activators#phosphofructokinase#hexokinase#biochemistry#glucokinase#inhibitors#regulators#pyruvate#pyruvate kinase#pyruvate kinase inhibition#inhibition#activation#enzymes
0 notes
Text
me: can spell ‘phosphofructokinase’ in an essay
also me: can’t spell ‘also’ on most days
4 notes
·
View notes
Text
Triose phosphate isomerases rapidly interconvert dihydroxyacetone phosphate and glyceraldehyde 3-phosphate in the plastid and cytosol (Table 8.6, reaction 1). The triose phosphate translocator – a protein complex in the inner membrane of the chloroplast envelope – exchanges chloroplast triose phosphates for cytosol phosphate (see Table 8.6, reaction 2). (...) Cytosolic fructose 6-phosphate can proceed to different destinations through:
Phosphorylation of carbon 1, which restores fructose 1,6-bisphosphate, catalyzed by two enzymes, phosphofructokinase and pyrophosphate-dependent phosphofructokinase (see Table 8.6, reactions 5a and b).
Phosphorylation of carbon 2, which yields fructose 2,6-bisphosphate, catalyzed by a unique bifunctional enzyme confined to the cytosol. Fructose 6-phosphate 2-kinase/fructose 2,6-bisphosphate phosphatase catalyzes both the incorporation and the hydrolysis of the phosphoryl group (see Table 8.6, reaction 5c and 6).
Isomerization, which produces glucose 6-phosphate, catalyzed by hexose phosphate isomerase (see Table 8.6, reaction 7).


In the cytosol, glucose 1-phosphate reacts with UTP to yield UDP-glucose and pyrophosphate in a reaction catalyzed by UDP-glucose pyrophosphorylase (see Table 8.6, reaction 9). (...) Sucrose 6^F-phosphate synthase (the superscript F indicates that sucrose is phosphorylated at carbon 6 of the fructose moiety) first catalyzes the formation of sucrose 6^F-phosphate from fructose 6-phosphate and UDP-glucose (see Table 8.6, reaction 10). Subsequently, sucrose 6^F-phosphate phosphatase releases inorganic phosphate from sucrose 6^F-phosphate, yielding sucrose (see Table 8.6, reaction 11).
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#phosphorylation#fructose#sucrose#phosphate#photosynthesis#photochemistry#photorespiration#organic chemistry
0 notes