#CO3
Text
The way you can tell this chapter is truly by monsterfuckers for monsterfuckers is it’s not like the tame mainstream tortured vampire and mildly bestial werewolf. Nope. We’re going full bug man and were-orca. Commitment.
#candela obscura#co3#candela obscura spoilers#rajan savarimuthu#elsie roberts#Zoe does stuff#cr adjacent#co spoilers#aabria iyengar#and co have blessed us again
35 notes
·
View notes
Text
"We're gonna wheel your ass into Oldfaire"
-Aabria Iyengar, "Creating Characters for Candela Obscura: Tide and Bone"
20 notes
·
View notes
Text
Insert Ouran High School Club opening song here

30 notes
·
View notes
Text
what if the “tide” part of their title comes from how tides are affected by the moon, and the doctor is also affected by it 🤔
3 notes
·
View notes
Text
Her Favorite Patient screening at Peekskill Film Festival
We’re excited to share that Her Favorite Patient will have its Hudson Valley premiere at the Paramount Hudson Valley Theater in competition at the Peekskill Film Festival on Saturday, June 25th at 5 pm EST. We’re looking forward to screening our film on the big screen at a landmark venue with over 1,000 seats. More info here.
We hope to see you there!
Directed by Reuben Hernandez
Written by Brady Evan Walker
Starring Barbara Miluski (Girls, The Marvelous Mrs. Maisel) and Isabelle Pierre
#her favorite patient#Her Favorite Patient#herfavoritepatient#barbaramiluski#barbara miluski#isabelle pierre#filmshop nyc#filmshop#The Filmshop#The Filmshop NYC#Jackson J. Eagan#Jackson Eagan#Jackson Jarvis Eagan#silver sound#silversound#CO3#Company 3#Company3#Jenny Montgomery#fil#thefilmshop#thefilmshopnyc
1 note
·
View note
Text


mMMMMmmMmmmmm Azurite she's so PRETTY!!!! Look at those BLUES!
93 notes
·
View notes
Text
bolehlah gw coba nulis bokep pake bhs indo
#mumpung lgi pada smut wars di co3#and ive been picking up random old books for inspo#some of which are indonesian#might aswell... right#nautical textposts
15 notes
·
View notes
Text
I was supposed to talk about Lustrum hours processing once upon a time, wasn't I?
#*brainpower seeping into the negatives*#tldr hours are actually an element so dense its metaphysically inverted itself.#you separate the rough ore by magnetic proerties and then send that mineral into large vats of water#and the vats give off O2 gas as the hours bond with H and form crystals and thats how you get refined hours#sskies#hire me failbetter#just googled and apparently K burns purple so im on the right track Hours are just some stupid high-density d shell element#Okay now im putting too much thought into it but Al and K burn purple so what if we pretended Hours was something like fucked up carbon#no no oxygen. so you have something like (K#(K-Hour) CH CO3 so its volatile and itll burn and its a magic stupid mineral with a magic stupid endmember with purple burning k#so the mineral you DONT want is K and they burn that and then send hours to be processed#ITS SO LATE. WHY AM I THINKING ABOUT THIS
4 notes
·
View notes
Text
CO2 Crisis Recap November 2023
The banana trees around Geneve, Suisse, are holding fine as the shortest days of the year leave them little light. Fifty years ago, there would have been one meter of snow on the ground. Switzerland, an elevated land, used to look like Sweden, but now, at least in the balmiest areas, it looks like Provence, with palm trees, pins parasols, and rows of cypresses. It’s eerie…
None of this surprises…

View On WordPress
0 notes
Text
It is an age-old question: "would putting pop rocks on your pussy feel like anything?" And while the spirit of adventure and curiosity means that in the Action Potentials¹ of my mind, this pushes me closer to vaginoplasty, I think we actually have the knowledge necessary to answer this question in the negative—I have seen a number of nsfw blog posts saying that they didn't feel anything at all when doing this, beyond maybe slight mechanical perturbation. But why?
I think the main reason is that pop rocks are basically tiny candy fragments with even tinier pockets of compressed CO2 gas. When the candy dissolves, the CO2 is released. "But Ananda," you say, "pop rocks taste sort of sourish!" Indeed, and I think a major component of why is that the CO2 dissolves in your spit, which has a pH of around 6.7²—nearly neutral. But vaginal secretions are typically far more acidic, at around 3.8-5.0³. The implication is that we should expect CO2 to be far, far less soluble in vaginal secretions. In particular, in this paper⁴ we expect the dominant dissolved species to be CO2, not HCO3 (-) (bicarbonate) or CO3 (2-) (carbonate). This means the effect on pH of the pop rocks should be quite low.
¹Metamorphosis of Prime Intellect style. IYKYK
²Baliga S, Muglikar S, Kale R. Salivary ph: A diagnostic biomarker. Journal of Indian Society of Periodontology. 2013;17(4):461. doi:10.4103/0972-124x.118317
³Lin Y-P, Chen W-C, Cheng C-M, Shen C-J. Vaginal ph value for clinical diagnosis and treatment of common vaginitis. Diagnostics. 2021;11(11):1996. doi:10.3390/diagnostics11111996
⁴König M, Vaes J, Klemm E, Pant D. Solvents and supporting electrolytes in the electrocatalytic reduction of CO2. iScience. 2019;19:135-160. doi:10.1016/j.isci.2019.07.014
97 notes
·
View notes
Text
new sexiest most intriguing man in cr history just dropped
#candela obscura#co3#candela obscura spoilers#co spoilers#rajan savarimuthu#cr adjacent#Zoe does stuff
11 notes
·
View notes
Note
Wait I’m confused. If Terzo and Copia are dating, what’s with omega? Is it like a three way relationship? Or does omega just join in for the pleasure? Or does he genuinely care for both Terzo and Copia, not just one?
The ghoul cares! It starts off out of fun first but Omega ends up falling for copia too
2 notes
·
View notes
Text
#this is literally nothing#biogeochemistry#eddie in the ocean#marine biology#marine chemistry#tumblr polls#polls#science shitpost
74 notes
·
View notes
Text
Calcite (CaCO3)


Calcite (CaCO3) is the most common carbonatic mineral on the Earth's surface. Carbonatic minerals are known for containing the CO3 compound in their structure, which then binds to an ion that is, in the calcite's case, calcium!
Carbonatic minerals are known primarily for forming in acquatic environments, be they in the ocean (carbonatic platforms) or on a continent (caves with stalagtites and stalagmites, lakes).


Calcite can take many different forms and shapes: the most well known variety is spatite, a completely transparent and flat crystal that is common in Iceland.


Sometimes it appears as an aggregate of long crystals that seem to all emerge from the same spot.

Often calcite will look colorless, white or a very dull light grey, but commonly it can take other colors: pink, yellow, green and blue





One of calcite's most remarkable properties is the double refraction effect, if you look at an image through a calcite crystal (most noticeable with spatite) the image will be shown twice!

Another remarkable thing about calcite is that by looking at a raw crystal's shape, you can assess if the water that it was formed in is rich or low in magnesium.
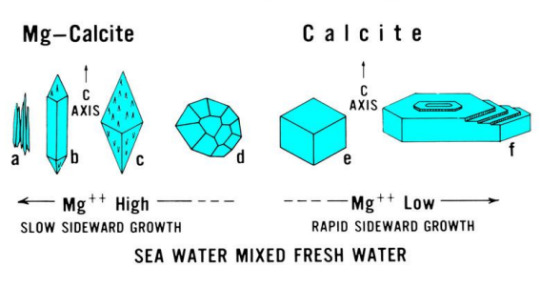
In general, the structure of a calcite crystal looks more or less like this, for those who are interested.
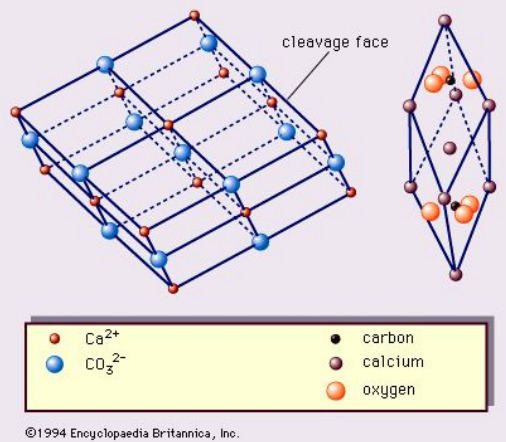
---
I wanted to talk about aragonite and dolomite too but I thought this was too long so maybe tomorrow I'll make a separate post about it👍
206 notes
·
View notes
Text
Tank update!

On plants:
I keep having to trim back the rotala rotundifolia because it grows at insane rates and covers all the other plants, the light gluttons. The cryptocorine wendtii are doing much better: one that had completely melted is growing back leaves at really quick pace for a crypt; the one I accidentally cut the leaves of (was trying to cut a bad leaf and accidentally cut that one and the only other healthy leaf the plant had) is coming back and growing a new leaf slowly but surely after I cut its bad-looking roots and replanted it.
I'm fiddling with the amount of potassium I should add as fertilizer, since many plants started showing signs of a potassium overdose - started at 5ml, now 3ml to see if any signs of a potassium deficit show up.
I've also been experimenting with the placement for the CO3 diffuser* (*see: a chunk of aquarium sponge pushed into hose, lol). I've now moved it to where the bubbles rise to the filter's waterfall, so it naturally pushes and distributes smaller bubbles around while bigger bubbles get broken into smaller bubbles. The first location I put it on, one of the corners, worked ok-ish but it wasted a lot of CO2. The second location was right underneath the filter intake, testing if the filter's impeller could maybe break the bubbles and dissolve the CO2 in the water before going back into the tank, but that not only didn't work but also put extra stress on the impeller which made it very noisy and annoying lol. Its current location as previously described seems to be doing the best out of the three, with the plants releasing a lot of oxygen! :3
Lastly, I'm still experimenting with the light fixture. Went from two 325lm 6500k bulbs and one 475lm 2700k bulb, to changing one of the 325lm bulbs for an 800ml 6500k one, two having two 800lm 6500k + one 475lm 2700k, to taking the 2700k out. The two 800lm bulbs have a good reach and colour temperature, buuuut they did leave a darker spot right in the middle where the hygrophilla angustifolia are, so I added one little 325lm 6500k bulb back. Right now, I'm using two 800lm bulbs and one 325lm bulb, all 6500k, with the dimmer one right in the middle.
On water chemistry:
I've gotten the nitrate levels down considerably, from 120ppm (very unsafe and bad) to 20ppm (good :3), turns out the food I was using to up the ammonia created inconmensurable and uncontrollable amounts of waste, gracias Shulet ni para ciclar acuarios servís.
Speaking of ammonia, I'd gotten the ammonia down to 0ppm but these last few days I've noticed it increase up to 0.25ppm - possibly due to a drop in pH caused by the DIY CO2, which could mean the "ammonia" detected is actually ammonium, much less toxic than ammonia, as the API Ammonia test detects both and has no way to distinguish between the two. As An Autistic Guy obsessed with numbers and data and accuracy I'm so happy that the numbers are inaccurate and the test is so vague, I love that so much, it doesn't make me want to pull my teeth out at all (I am in pain).
Despite the "ammonia" issues, things look good rn! Especially thanks to keeping nitrates under control at long last, the presence of visible algae has started to decrease. I've been taking the brown algae out with a stick, and have been dosing hydrogen peroxide locally to the harder-to-deal-with filament algae to weaken it enough for the snails to go at it. I'm happy to report that the hydrogen peroxide has weakened the filament algae to a point where the nerite snail is able to eat it.

(All the little dots floating in the water are planorbis snail larvae that hatched today!! Yippee!!!)
On stocking:
It's still just snails for now. A week or two ago I discovered a bunch of dead or half dead planorbis snails in the filter intake tube, victims of the siren call of all the brown algae within it - apparently - though two adults survived and have laid various egg sacks on the glass, on plants, etc, so their presence in the tank will make a triumphant comeback for sure. The one adult bladder snail I had also fell victim to the filter intake, though that one's babies had been crawling around for a few days before its untimely death; there'll be plenty of snails going around for everyone. Don't worry, I've fixed the issue now and snails with shells that are 2mm thick or more will be safe from now on. The nerite hasn't laid any eggs yet, though when it does I'll probably scrape them off (they don't hatch in freshwater).
I'll be gone from monday til thursday, and though I'm a little worried (as always) I am also confident in that things will be fine once I come back. I'm really excited, I should be able to add the tank's main attraction, a betta fish, very soon. :3
24 notes
·
View notes
