#deprotonation
Text
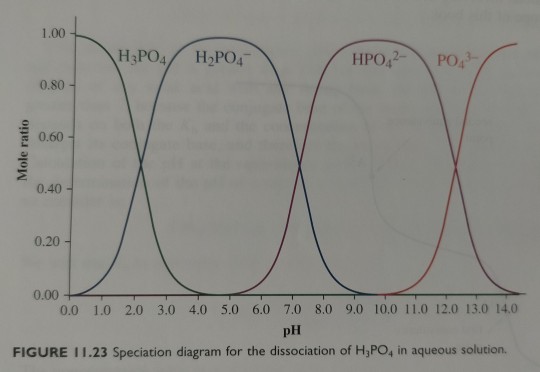
Figure 11.23 shows the speciation diagram for H3PO4, a triprotic acid, which yields anions H2PO4-, HPO4²- and PO4³- on successive deprotonations.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#triprotic acid#chemical reactions#phosphoric acid#anion#ionization#deprotonation#speciation#dissociation
0 notes
Text

This bugs me because it's wrong in the details but right overall. pKa, first of all, isn't the Acid dissociation constant: it's the negative base 10 logarithm of the acid dissociation constant, Ka, which is given by the following equation:

Where HA is the acid, H+ is the acidic protons it can give off, and a- is the conjugate base or the remainder of the molecule after the proton is given off.
(pH, to note, is also the negative base ten logarithm - in this case, of the amount of h+ ions in a particular solution, in the example springing off of this, the amount of H+ ions in monster energy).
If you have a solution that already has a ton of H+ protons in it, such as monster energy drink, and try to dissolve more acidic particles in it, well, the equilibrium is already very top heavy - so the H+ particles in HA, our acid, will be trying to squeeze into a crowded room, fail, and remain as the undissasociated acid, pushing the equilibrium constant to an even more tiny number than it already is for basically every organic acid. It's a much more complicated relationship than "pKa indicates roughly the pH around which acids can't dissociate" which unless I'm missing something is nonsense - you have to calculate the equilibria constants of these acids with the initial acidity factored it if you want to do anything relating these two values to each other.
It's frustrating because the overall conclusion is right!! for reasons not really related to what they said, the high pH of the monster energy does mean it's more difficult for the acid protons of these acids to dissociate!

This, however, is just flat out wrong. The lower the pKa value is, the easier it is for that proton to dissociate; this is how negative logarithms work. If you calculate the ratio up above and find that the amount of dissociated species to acid heavily favors the dissociated species, that is a very strong acid, and you will have pKa values that go negative. In most organic carboxylic acids (like those named here), we're looking at a pKa of around 5: meaning that if you dissolve the acid in pure neutral water, how many protons (and conjugate base ions) are in solution compared to the undissociated molecule, you'll get a ratio of about 1/100000 - in other words, a mixture that overwhelmingly does not dissociate.
For secondary/tertiary/so on deprotonations, it becomes progressively more difficult to get rid of those protons, because the conjugate base is already shouldering a negative charge and because the solution already has a concentration of protons. Thus, if you want to say "once this one dissociates then all of the other ones are already dissociated too" you really have to be looking at the highest pKa.

This part is just goofy. "The trapped acidity of the malic/citric/ascorbic acids is dumped into your mouth" that also happens when you eat the solid candy anyway...
#Like I get it you found the equilibrium esp acid equilibrium part of gen chem hard.#everyone did! but don't go calling yourself a chemist then say literally incorrect information just because you can find some pubmed links
3 notes
·
View notes
Text
okay so the wifi i'm on right now is shitting itself despite this being a popular study spot on campus but REGARDLESS i'm studying for my third organic chemistry midterm right now (my class grade is a 90.2% so i'm barely hovering around an A and i'd REALLY like to keep it that way as unrealistic as that may be) and as much as i am absolutely loving this class i'm struggling a little with remembering all of the mechanisms (final exam review sheet posted by professor indicates 40 different reactions we need to know).
granted, a lot of them share the same patterns, but i don't know what format i'll need to compare-contrast all of them in in order to have them down pat for may 2. so right now i'm just focusing on this exam, where we've gotta know
radical substitution of alkanes and allylic substitution, the funky alkyl halide ones with the fishhook arrows. the simplified mechanism looks like halogenation except these are done with heat/light. these go through three stages: initiation from nonradical to radical, propagation from radical to radical, and termination from radical to nonradical. you get your radical intermediate via homolytic cleavage, you react it with a hydrogen coming off of the most substituted carbon, you get your next radical intermediate once the hydrogen is gone, you react all your radicals together to tie things up nicely. if your radical is on a carbon that neighbors a double bond, you have a nice stable allylic radical (thank you resonance), but if your radical is on a double bond, that's a vinyl radical and we don't like those... you also gotta pay attention to where your alkene is because that'll affect your stability too. if you're happening to do your reaction with peroxides, you'll be reacting your intermediate with the less substituted area. a halogen? on MY terminal end?
elimination and substitution reactions Sn1/Sn2/E1/E2. these are silly and complicated and i need to make a chart to understand them better, but the substitution reactions will substitute a nucleophile in for the leaving group, and the elimination reactions will make an alkene out of an alkane using a base, and the 2-reactions are concerted with steric barriers while the 1- reactions have a carbocation intermediate with stability as the barrier.
alkyne reactions, including deprotonation (basically acid-base shit), alkyne formation (using acetylene, which makes good internal alkenes and looks like Sn2, or dibromides, which looks like two E2 reactions and can be done with bromides on the same carbon or on adjacent carbons), halogenation (nonregioselective concerted anti-addition of halogens using Br2/Cl2), hydrohalogenation (markovnikov addition of Br, can happen twice if you have two equivalents of HBr, carbocation intermediate), hydration and hydroboration-oxidation (markov and anti-markov reactions, respectively; adding a hydrogen and an OH, keto-enol tautomerism where you end up with the double bond on the oxygen), and three reduction reactions (Pd/C yields alkane, Lindlar/H2 yields a cis(z)alkene, Na/NH3 yields a trans(E) alkene).
anyway i have practice problems to do and the sn1/sn2/e1/e2 reaction comparison chart to make but i'm drinking my first monster energy (ultra paradise. it's good) and i'm getting dinner w at least one of my friends in 25 minutes... calc homework still due tonight but i think i'll be able to get it... exam in 3 hours... feeling good!
#a rare brain dump from puzzlehat but making notecards was not being an efficient way to study lawl#perhaps i should make a studyblr...
5 notes
·
View notes
Text

I drew this one at pH = 12.5, which is quite bitter, to represent Ed’s pain when he’s feeling like the kraken. The high pH causes the side chains for the lysine and arginine residues, as well as the amino terminus, to be deprotonated. This gives the peptide an overall charge of -2, instead of the +2 charge it has at physiological pH. It feels like the peptide is sad. Like Ed.
#ofmd kraken#ofmd fanart#ofmd#our flag means death#our flag means fanart#kraken#blackbeard#edward teach#biochemistry
23 notes
·
View notes
Text
baby r u histidine at a pH of 12 cuz im gonna deprotonate u 🫣😍 THREE TIMES
7 notes
·
View notes
Text
Nitrosamine Compounds
Nitrosamine are organic compounds with the chemical structure R2N−N=O, where R is usually an alkyl group They feature a nitroso group (NO+) bonded to a deprotonated amine. Most nitrosamines are carcinogenic in nonhuman animals.
Know more:- https://www.simsonpharma.com/promotions/nitrosamine-compounds
3 notes
·
View notes
Text
SODIUM ETHOXIDE DEPROTONATES AT THE ALPHA HYDROGEN POSITION SO A STRONG ACID CAN SHIMMY ITS WAY IN AND ATTACH THE MOLECULE AND REPROTONATE IT SO ITS STABLE OKAY GOT IT LETS JUST HOPE THATS ON THE FINAL IN FIVE HOURS 😭
#crys talks#personal#IM LOSING MY MIND#I’m hoping that if I write it here I’ll remember it later#go into the notes if you want to read my Orgo screeches
20 notes
·
View notes
Text


New insult dropped
F it *Become Deprotonated*
3 notes
·
View notes
Quote
Tryptophan (symbol Trp or W)[3] is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3.[4] It is encoded by the codon UGG.
Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–NH+
3; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38).[5]
Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. In 2023, the emission spectrum of tryptophan was discovered in the interstellar gas of the star cluster IC 348.[6]
Tryptophan - Wikipedia
0 notes
Text

Undergraduate scientific research project丨Revealing the interface structure: multi-technology teamed up to analyze the mystery of MXene
Two-dimensional (2D) transition metal carbides and nitrides (MXenes) combine the electronic and mechanical properties of 2D inorganic crystals with chemically modified surfaces, providing an ideal platform for fundamental and applied studies of interfaces. The functionalization of MXenes with small inorganic ligands has made good progress, but the covalent bonding of various organic groups to the surface of MXenes has been relatively less studied. Here, a class of hybrid MXenes (h-MXenes) was synthesized by reacting halogen-terminated MXenes with deprotonated organic amines to form amine and imine linkages between organic and inorganic moieties. The resulting hybrid structures combine the tailorability of organic molecules with electronic connectivity and other properties of inorganic 2D materials. Describing the structure of h-MXenes requires integrating the concepts of coordination chemistry, self-assembled monolayers, and surface science. The optical properties of h-MXenes reveal the coherent coupling between organic and inorganic components, and h-MXenes also possess excellent stability against hydrolysis.
A new method for attaching organic molecules to the surface of 2D MXene materials. MXene is a class of layered metal carbides or nitrides with good electrical conductivity and mechanical properties. However, the surface atoms of MXene are very active and easily react with water, which affects its stability.
The subject will use an organic molecule called an alkylamine, and through some chemical reactions, the nitrogen atoms of these organic molecules will form chemical bonds with the titanium atoms on the surface of the MXene. In this way, two different organic groups can be attached on the surface of MXene, one is amide group and the other is imine group.
Then, many advanced experimental techniques such as solid-state NMR were used to study the connection of these two groups with MXene. The calculated results show that the imine group has a very strong linking force, which helps to improve the stability of MXene. In addition, the arrangement of the alkyl chains is very special, and they are inserted between the MXene layers at an oblique angle, just like a self-assembled monolayer film.
MXene materials modified with these organic groups exhibit some very interesting new properties. For example, the appearance of Fano resonance in the infrared light absorption spectrum indicates that the organic part and the inorganic part have undergone coherent coupling. By designing dual organic ligands, MXene can be stripped to form a colloidal solution. Most importantly, the organic groups significantly improved the hydrolytic stability of MXene.
1 note
·
View note
Text
What Is Papain Enzyme And How Is It Made?
What Is Papain Enzyme And How Is It Made?
Overview Of Papain Enzyme
Papain, also known as papaya proteinase I, is a cysteine protease (EC 3.4.22.2) enzyme present in papaya (Carica papaya) and mountain papaya (Vasconcellea cundinamarcensis). Papain is taken from the fruit of the papaya tree. It is used to make medicine.
Some people take papain by mouth for pain and swelling (inflammation) and to remove extra fluid following trauma and surgery. It is also taken by mouth to help with digestion, to treat parasitic worms, inflammation of the throat and pharynx, shingles (herpes zoster) symptoms, sore muscles, diarrhea, hay fever, runny nose, and a skin condition called psoriasis. Papain is also taken by mouth to treat the side effects of radiation therapy, or it may be used in combination with other therapies to treat tumors.
Some people apply papain directly to the skin to treat insect or animal bites, infected wounds, sores, and ulcers.
In manufacturing, papain is used in cosmetics, toothpaste, contact lens cleaners, meat tenderizers, and meat products.
Structure Of Papain Enzyme
The papain precursor protein contains 345 amino acid residues and consists of a signal sequence (1-18), a propeptide (19-133) and the mature peptide (134-345). The amino acid numbers are based on the mature peptide. The protein is stabilised by three disulfide bridges. Its three-dimensional structure consists of two distinct structural domains with a cleft between them. This cleft contains the active site, which contains a catalytic diad that has been likened to the catalytic triad of chymotrypsin. The catalytic triad is made up of the amino acids – cysteine-25 (from which it gets its classification) and histidine-159. Aspartate-158 was thought to play a role analogous to the role of aspartate in the serine protease catalytic triad, but that has since been disproved.
Function Of Papain Enzyme
The mechanism by which papain breaks peptide bonds involves the use of a catalytic triad with a deprotonated cysteine. Asn-175 helps to orient the imidazole ring of His-159 to allow it to deprotonate the catalytic Cys-25. This cysteine then performs a nucleophilic attack on the carbonyl carbon of a peptide backbone. This forms a covalent acyl-enzyme intermediate and frees the amino terminus of the peptide. The enzyme is deacylated by a water molecule and releases the carboxyl-terminal portion of the peptide. In immunology, papain is known to cleave the Fc (crystallisable) portion of immunoglobulins (antibodies) from the Fab (antigen-binding) portion.
Papain is a relatively heat-resistant enzyme, with an optimal temperature range of 60 and 70 °C. Papain prefers to cleave at (hydrophobic)- (Arg or Lys)- cleaves here – (not Val). Hydrophobic is Ala, Val, Leu, Ile, Phe, Trp, or Tyr.
Uses Of Papain Enzyme
Papain breaks down tough meat fibers and has been used for thousands of years to tenderise meat eaten in its native South America. Meat tenderisers in powder form with papain as an active component are widely sold.
Papain can be used to dissociate cells in the first step of cell culture preparations. A ten-minute treatment of small tissue pieces (less than 1 mm cubed) will allow papain to begin cleaving the extracellular matrix molecules holding the cells together. After ten minutes, the tissue should be treated with a protease inhibitor solution to stop the protease action. Left untreated, papain activity will lead to complete lysis of the cells. The tissue must then be triturated (passed quickly up and down through a Pasteur pipette) to break up the pieces of tissue into a single cell suspension.
It is also used as an ingredient in various enzymatic debriding preparations, notably Accuzyme. These are used in the care of some chronic wounds to clean up dead tissue.
Papain is added to some toothpaste and mint sweets as a tooth whitener. Its whitening effect is, however, minimal because the papain is present in low concentrations and is quickly diluted by saliva. It would take several months of use to have a noticeable effect.
Papain is the main ingredient of Papacarie, a gel used for chemomechanical dental caries removal. It does not require drilling and does not interfere in the bond strength of restorative materials to dentin.
Papain has been known to interfere with urine drug tests for cannabinoids. It is found in some drug detox products.
Recently, it has been demonstrated that papain can be used to assemble thin films of titania used in photovoltaic cells.
Benefits Of Papain Enzyme
1. Stimulates Digestion
One of the key areas in which papain serves the body is in the realm of its protein-digestive properties. One case study found that when a male patient with gluten intolerance ate a gluten-free diet, he still experienced diarrhea, but when he additionally took 1800 mg of papain for one month, he had fewer loose stools and less malabsorption. This is just one study, and more research needs to be conducted.
2. Aids Skin and Wound Healing
Due to papain’s beneficial capacities, people have used it for many years as a topical application to burns, ulcers, irritations, bedsores and other wounds, and to assist recovery from sports injuries. Some practitioners have used it dental cavities. Papain’s enzymatic action is very specific, and it does not harm healthy skin. Traditional cultures in Hawaii and Tahiti made poultices out of the skins of papaya, as this part of the fruit has a particularly high concentration of papain. Traditional healers applied this substance to the skin to heal wounds, burns, rashes and insect stings.
3. Digests Mucus
Studies have found that papain digests sinus mucin, a glycoprotein found in mucus, and hence may have beneficial effects for people having sinus issues. Papain makes mucus less viscous, or runnier, and hence better able to be eliminated. Because of this feature, some researchers are studying how papain can help deliver nanoparticle medicines to the body so that they can get through the body’s natural mucosal barrier in the gut. Using papain with nanoparticles may not be the best for your health.
4. Supports Immune System Function
Studies have found that papain may have anti-cell proliferation properties. Some studies have shown papain delivers a strong effect while others found no difference between papain and controls. A review article found strong evidence for the overall immune function properties of papaya.
5. Resists Redness and Irritation
Studies confirm that the papain enzyme offers powerful resistance to redness and irritation. Papain helps aid the absorption of another beneficial substance, quercetin. One study found that when papain and bromelain were given along with quercetin, it helped swelling symptoms associated with prostate health.
6. Acts as an Antioxidant
Papain holds compounds that may aid in protecting the body from cellular damage caused by free radicals, which makes it an antioxidant. The compounds in papaya juice effectively scavenge, or counteract, highly reactive hydroxyl (OH-) free radicals, as well as super-oxides. Papain has an antioxidant level on par with Vitamins E and C. In one study, the Sunrise Solo cultivar (a type of papaya) was more effective as an antioxidant than two other cultivars.
7. Prevents Food Spoilage
Since research has shown papain has antifungal and antibacterial properties, and it is sometimes used to preserve foods naturally. It is a powerful agent commonly used in food preservation, reducing bacterial infestations and spoilage due to oxidation.
How Does Papain Tenderize Meat?
The most important characteristics of meat quality are tenderness, juiciness, and flavour. Consumers consider tenderness as the most important factor in determining eating satisfaction of beef (Issanchou, 1996, Boleman, Miller, Taylor, Cross, Wheeler, Koohmaraie, Johnson and Savell, 1997). Tenderness is defined as the ease of mastication, which involves the initial ease of penetration by the teeth, the ease with which the meat breaks into fragments and the amount of residue remaining after mastication (Lawrie, 1998).
Meat tenderness depends on spices, breed, age, sex and individual skeletal muscle tissue of the animal. Tenderness originates in structural and biochemical properties of scheletal muscle fibers, especially myofibrils and intermediate filaments, and of the intramuscular connective tissue, the endomysium and perimysium, which are composed of collagen fibrils and fibers (Takahashi, 1996). The mechanical stability of collagen fibrils increased markedly with chronological aging.
Meat produced from an old animal is tough and has a lower eating quality. Improvement of meat tenderness of aged cattle is necessary to increase functional properties and value of the meat (Shiba, 2004). Meat can be tenderized in different ways: mechanical methods (mincing or hitting to crush the conjunctive and muscle tissue), chemical methods by injecting in the muscle solutions with chemical substances (salt, sodium chloride, sodium polyphosphate, potassium lactate, sodium diacetate all dissolved into water, R.K., 2003), enzymatic methods used proteolytic enzymes like papain, bromelain or ficin.
Enzymatic tenderisation can be made in a humid way (by injecting in the muscle solutions with enzymatic preparations) or in a dry way (pressing tender mixtures on the meat surface). The end of the tenderisation process and the method employed for aging and thermal processing should be established to get an optimum sensorial quality of the meat.
Injection of beef cuts with papain cause an important improvement of functional properties of adult beef. Papain is a powerful proteases preparation, with great under-layer specificity, catalysing the breaking of the peptidic bonds in the protein molecules and their degradation products to amino acids. The increase of the papain level, from injection brine, as well as the increase of the time of ageing, determined a significant weakening of the meat structure. Papain tenderisation of the adult beef determined the improvement of tenderness, flavour, and juiciness. It is recommended to use papain doses as low as possible, in order to avoid advanced tenderisation and obtaining meats with soft structure, with a very low resistance to mastication and paste texture. Application of this technology could assist beef producers and processors to obtain meat products that can satisfy consumer’s expectation.
0 notes
Text
Une nouvelle méthode de synthèse de l'artémisinine pourrait conduire à un traitement rentable du paludisme
See on Scoop.it - EntomoNews
Les traitements antipaludiques les plus efficaces utilisent l’artémisinine, un médicament dérivé de la plante d’absinthe douce, Artemisia annuelle. Cependant, le processus d’isolement de l’artémisinine de la plante prend du temps et les rendements des cultures sont sensibles aux conditions météorologiques, aux insectes nuisibles et à d’autres facteurs. Malgré les avancées scientifiques dans les méthodes de traitement, le coût de l’artémisinine pèse toujours sur les pays les plus touchés par le paludisme.
par Ma Clinique
3 mai 2023
dans Actualités médicales
"« Nous avons pu développer une nouvelle façon de synthétiser l’artémisinine qui imite la façon dont elle est fabriquée dans la nature. Notre méthode imite la voie de biosynthèse de la fabrication de l’artémisinine dans la plante d’où elle provient, Artemisia annuelle. Nous avons étudié les composés intermédiaires le long de cette voie, puis avons utilisé la chimie pour créer ces mêmes intermédiaires et recréer la voie. »
Dr Shawn Blumberg, chercheur scientifique principal du SwRI"
-------
NDÉ
L'étude
Site-Selective Functionalization of Unactivated Allylic C–H Bonds via Direct Deprotonation with KTMP: Application to the Formal Total Synthesis of (+)-Artemisinin from Amorphadiene | Organic Letters, 02.01.2023 https://pubs.acs.org/doi/10.1021/acs.orglett.2c04145
[Image] Abstract
Bernadette Cassel's insight:
'artémisinine' in EntomoNews | 10 scoops
https://www.scoop.it/topic/entomonews/?&tag=art%C3%A9misinine
0 notes
Text
Chromophore Structure in an Inactive State of a Novel Photosensor Protein Opn5L1: Resonance Raman Evidence for the Formation of a Deprotonated Adduct at the 11th Carbon Atom
http://dlvr.it/SkDd5C
0 notes
Text
Chromophore Structure in an Inactive State of a Novel Photosensor Protein Opn5L1: Resonance Raman Evidence for the Formation of a Deprotonated Adduct at the 11th Carbon Atom
http://dlvr.it/SkCyyy
0 notes
Text
Nitrosamine Compounds are organic compounds most nitrosamines are carcinogenic in nonhuman animals.
Chemical Structure R2N−N=O,
R : an alkyl group
NO+ : bonded to a deprotonated amine
https://www.simsonpharma.com/promotions/nitrosamine-compounds
0 notes
Text

Atomic Development Commission Hopes Deprotonation Will Draw More People To City Center
0 notes